Abstract
Mice carrying mutant amyloid-β precursor protein and presenilin-1 genes (APP/PS1 double transgenic mice) have frequently been used in studies of Alzheimer's disease; however, such studies have focused mainly on hippocampal and cortical changes. The severity of Alzheimer's disease is known to correlate with the amount of amyloid-β protein deposition and the number of dead neurons in the locus coeruleus. In the present study, we assigned APP/PS1 double transgenic mice to two groups according to age: young mice (5–6 months old) and aged mice (16–17 months old). Age-matched wild-type mice were used as controls. Immunohistochemistry for tyrosine hydroxylase (a marker of catecholaminergic neurons in the locus coeruleus) revealed that APP/PS1 mice had 23% fewer cells in the locus coeruleus compared with aged wild-type mice. APP/PS1 mice also had increased numbers of cell bodies of neurons positive for tyrosine hydroxylase, but fewer tyrosine hydroxylase-positive fibers, which were also short, thick and broken. Quantitative analysis using unbiased stereology showed a significant age-related increase in the mean volume of tyrosine droxylase-positive neurons in aged APP/PS1 mice compared with young APP/PS1 mice. Moreover, the mean volume of tyrosine hydroxylase-positive neurons was positively correlated with the total volume of the locus coeruleus. These findings indicate that noradrenergic neurons and fibers in the locus coeruleus are predisposed to degenerative alterations in APP/PS1 double transgenic mice.
Keywords: neural regeneration, presenilin-1, Alzheimer's disease, tyrosine hydroxylase, locus coeruleus, no-radrenergic neuron, β-amyloid, senile plaques, grants-supported paper, neuroregeneration
Research Highlights
-
(1)
Degenerative alterations were found in noradrenergic neurons and fibers in the locus coeruleus of amyloid-β precursor protein and presenilin-1 double transgenic mice.
-
(2)
The double transgenic mice had fewer noradrenergic neurons than wild types, because of death of noradrenergic neurons; the surviving noradrenergic neurons developed hypertrophy in the locus coeruleus.
-
(3)
This study highlights the utility of amyloid-β precursor protein and presenilin-1 double transgenic mice in investigating catecholaminergic systems in Alzheimer's disease.
INTRODUCTION
Animal models of Alzheimer's disease can be induced by physical injury, organic drug and biological polypeptide injury, autoimmune injury, and transgenes. However, there is as yet no single model that can completely reflect the pathology of Alzheimer's disease. Transgenes can interact in model animals to closely simulate the natural progression of Alzheimer's disease in humans.
Amyloid-β deposition, senile plaques, and activation and proliferation of microglia and astrocytes have been found 2–3 months after birth in the cortex and hippocampus of double transgenic mice carrying mutant amyloid-β precursor protein (APPSwe) and presenilin-1 (PS1)-A246E genes[1,2]. These pathological changes exacerbate gradually with age, accompanied by changes in neurological function, such as cholinergic dysfunction and learning and memory alterations[3,4,5,6]. Therefore, they are widely used as models for Alzheimer's disease.
A large number of studies have described the pathogenic mechanism and treatments for Alzheimer's disease[7,8,9,10]. Death of neurons in the dorsal raphe nucleus and locus coeruleus is a pathological characteristic of the disease. In one study, the number of locus coeruleus neurons in patients with Alzheimer's disease was 54.5% lower than that in healthy subjects[11].
Another study showed a 60% decrease in the number of locus coeruleus neurons in patients with either Alzheimer's or Parkinson's disease[12]. The locus coeruleus is the principal site for brain synthesis of noradrenaline and noradrenaline synthetase. Noradrenergic axons project from the locus coeruleus to many locations, including the hippocampus, entorhinal cortex, and prefrontal cortex, and noradrenergic terminals are found adjacent to cell bodies, astrocytes, microglia, and endothelial cells.
In addition, synaptic structures are missing between noradrenergic axons and many cells, indicating that noradrenaline may serve a paracrine function. Noradrenaline can regulate the regional microenvironment, and functions both as a neurotransmitter and as an endogenous anti-inflammatory factor[13,14]. The death of noradrenergic neurons in the locus coeruleus and noradrenergic axons in projection areas in Alzheimer's models and patients results in a decrease in noradrenaline. The inflammatory reaction in a brain with Alzheimer's disease can induce a loss of noradrenergic neurons in the locus coeruleus, which, in turn, worsens the inflammatory reaction. It has been shown that the number of dead neurons in the locus coeruleus is positively correlated with beta-amyloid deposition, neurofibrillary tangles and the degree of dementia[13].
However, what are the pathological changes in noradrenergic neurons in the locus coeruleus of a transgenic mouse model of Alzheimer's disease? Furthermore, what are the morphological changes, cell volume, total number of neurons and locus coeruleus volume, and how are they associated with age progression?
In the present study, we investigated the pathological changes in noradrenergic neurons in the locus coeruleus in β-amyloid precursor protein and PS1 (APP/PS1) double transgenic mice. We studied the morphology of noradrenergic neurons in the locus coeruleus using tyrosine hydroxylase immunohistochemistry as a specific marker of catecholaminergic neurons, and quantitatively analyzed changes in the number of tyrosine hydroxylase-positive neurons and fibers using unbiased stereology.
RESULTS
Quantitative analysis of experimental animals
Six young (5–6 months) and six aged (16–17 months) APP/PS1 double transgenic mice and 12 age-matched wild types were used. All 24 rats were included in the final analysis.
Morphology and quantity of noradrenergic neurons in locus coeruleus
Immunohistochemistry showed that tyrosine hydroxylase-positive neurons in the locus coeruleus were multipolar neurons with a round or oval cell body and thin, interlacing processes in both young and aged wild-type mice, as well as in young, double transgenic mice.
However, in aged double transgenic mice, tyrosine hydroxylase-positive neurons had large cell bodies and short, thick, broken processes, accompanied by a few tyrosine hydroxylase-positive fibers (Figure 1). Quantitative analysis of unbiased stereology revealed that the total quantity of tyrosine hydroxylase-positive neurons in the locus coeruleus was 23% lower in aged double transgenic mice than in aged wild-type mice (P < 0.05; Figure 1).
Figure 1.
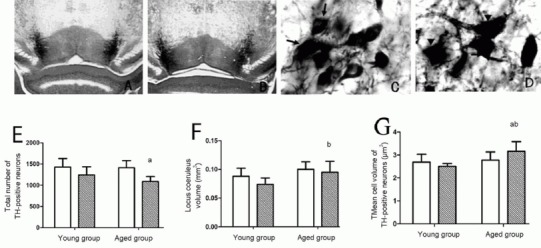
Pathological changes in locus coeruleus of wild-type and amyloid-β precursor protein and presenilin-1 double transgenic mice
(A, C) Tyrosine hydroxylase (TH) staining of noradrenergic neurons in the locus coeruleus of aged wild-type mice; (B, D) TH staining of noradrenergic neurons in the locus coeruleus of aged double transgenic mice. (E) Number of TH-positive neurons in unilateral locus coeruleus; (F) total volume of unilateral locus coeruleus; (G) mean volume of TH-positive cells in unilateral locus coeruleus.
Compared with wild-type mice, the total number of TH-positive neurons in the locus coeruleus (E) and total volume of the locus coeruleus were lower (F) in the aged groups, and the volume of cell bodies higher (D, G) in the aged groups. A, B: × 40; C, D: × 600; arrows: normal TH-positive cells; arrowheads: abnormal TH-positive cells. aP < 0.05, vs. aged wild-type group (aged group); bP < 0.05, vs. young double transgenic group (young group). Data are expressed as mean ± SD of six mice in each group (paired t-test). Black bars in E, F, G: double transgenic mice; white bar: wild-type mice.
Correlation between age and changes in volume of tyrosine hydroxylase-positive neurons in double transgenic mice
Quantitative stereological analysis showed that the total volume of the locus coeruleus and mean volume of tyrosine hydroxylase-positive neurons were larger in aged double transgenic mice than in young double transgenic mice (P < 0.05; Figure 1).
Moreover, the mean volume of tyrosine hydroxylase-positive neurons correlated positively with the total volume of the locus coeruleus (r = 0.694, P < 0.05; Figure 2).
Figure 2.
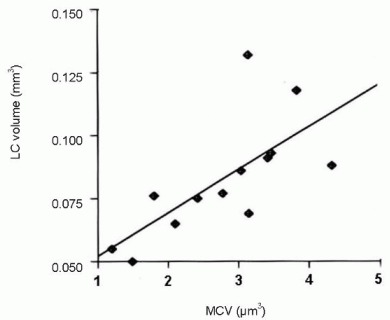
Positive correlation between mean cell volume (MCV) of tyrosine hydroxylase-positive neurons and volume of locus coeruleus (LC) in aged amyloid-β protein precursor and presenilin-1 double transgenic mice (r = 0.694, P < 0.05).
DISCUSSION
The locus coeruleus is located in the posterior area of the rostral pons in the lateral floor of the fourth ventricle. It is the major site to secrete the catecholamine neurotransmitter, noradrenaline, and is therefore also known as a noradrenergic cell group[15]. The locus coeruleus has over 50% of the noradrenergic neurons in the entire central nervous system, and is the principal site for brain synthesis of noradrenaline. The nucleus has widespread projections that innervate the spinal cord, cerebellum, hypothalamus, thalamic relay nuclei, amygdala, basal telencephalon, and cortex.
Previous studies of Alzheimer's disease pathology have mainly focused on learning and memory and the hippocampus, but have largely neglected the locus coeruleus. The noradrenaline released from neurons in the locus coeruleus has an excitatory effect on the hippocampus and cortex, so this region is also important for cognitive functions such as learning and memory[16]. The loss of neurons in the locus coeruleus and thalamic relay nuclei has been demonstrated to be a pathological characteristic of Alzheimer's disease.
Another study reported degeneration and enlarged cell bodies, and a significant reduction (50%) in cell number, of locus coeruleus cells in human brains post Alzheimer's disease compared with those without Alzheimer's disease[17]. Transgenic mice co-expressing mutant human presenilin 1 and amyloid-β precursor protein mimic major neuropathological processes in patients with Alzheimer's disease[18], so they are regarded as a good animal model for studying the disease. Noradrenergic neurons can be visualized by tyrosine hydroxylase, a marker of catecholaminergic neurons.
We used immunohistochemistry and quantitative analysis of unbiased stereology to study the morphology of noradrenergic neurons and numbers of tyrosine hydroxylase-positive neurons and fibers in the locus coeruleus, respectively. The total volume of the locus coeruleus did not differ between the two groups of aged mice, but the total quantity of tyrosine hydroxylase-positive neurons was 23% lower in aged double transgenic mice compared with aged wild-type mice.
Moreover, the surviving tyrosine hydroxylase-positive neurons had enlarged cell bodies and thickened, shortened dendrites. This indicates that some noradrenergic neurons died, while others developed hypertrophia, in the double transgenic mice. These changes were consistent with pathological alterations in locus coeruleus neurons in the brains of patients with Alzheimer's disease, demonstrating that this model can simulate closely this pathological process observed clinically.
The degeneration of tyrosine hydroxylase-positive neurons in the locus coeruleus of APP/PS1 mice and patients with Alzheimer's disease may be attributed to several factors. (1) A large amount of amyloid-β protein deposition and senile plaque formation has previously been observed in this model. These are highly neurotoxic and could have directly damaged the neurons. In addition, amyloid-β and senile plaques can injure nerve endings, and dystrophic neurites have previously been found in or adjacent to senile plaques[19,20,21]. This pathological change may result in retrograde injury and neuronal degeneration in the locus coeruleus. (2) During the progression of Alzheimer's disease, amyloid-β protein deposition and senile plaque formation activate microglia and astrocytes and release pro-inflammatory cytokines, leading to chronic inflammatory reactions in the brain. These inflammatory reactions may be associated with the loss of noradrenergic neurons and axonal degeneration in the locus coeruleus[22,23,24].
Recent evidence indicates that noradrenaline is an endogenous anti-inflammatory substance. It can regulate immunity, inhibit or reduce the formation of senile plaques and neurofibrillary tangles, suppress glial cell activity and inhibit production of pro-inflammatory cytokines. Similarly, in a brain with Alzheimer's disease, the loss of noradrenergic neurons can promote the formation of senile plaques and neurofibrillary tangles, and activate glial cells. These changes can further increase the loss of noradrenergic neurons[13,25,26]. The number of dead noradrenergic neurons in the locus coeruleus is positively correlated with amyloid-β deposition, neurofibrillary tangles, and the degree of dementia[27,28].
In the present study, the total volume of the locus coeruleus and mean volume of tyrosine hydroxylase-positive neurons were larger in aged double transgenic mice compared with young double transgenic mice, and the mean volume of tyrosine hydroxylase-positive neurons was positively correlated with the total volume of the locus coeruleus. In addition, the mean volume of tyrosine hydroxylase-positive neurons was greater, and there were fewer neurons, in aged double transgenic mice compared with aged wild-type mice. This may result from surviving noradrenergic neurons becoming hypertrophic to compensate for the degenerative loss of other noradrenergic neurons in the locus coeruleus. The pathological alterations observed here reflect those in human brains with Alzheimer's disease after death, further suggesting that APP/PS1 double transgenic mice can closely model pathological changes in Alzheimer's disease.
Because of the complex pathogenesis of Alzheimer's disease, further studies are necessary to investigate the roles of various transmitter systems in its etiology. This study provides experimental evidence in favor of studying specific neurotransmitter pathways in APP/PS1 double transgenic mice as a model for Alzheimer's disease.
MATERIALS AND METHODS
Design
A randomized, controlled animal study.
Time and setting
The experiments were conducted in the Laboratory of Human Anatomy and Neurobiology, Central South University, China from June 2010 to September 2011.
Materials
Six young (5–6 months old) and six aged (16–17 months old) APP/PS1 double transgenic mice were selected. They were housed individually for 1 week at 20–24°C, 45–55% humidity, natural illumination, and allowed free access to food and water. The animals were provided by the Gerontology Research Center of the National Institutes of Health, USA.
Experimental procedures were conducted in accordance with the Guidance Suggestions for the Care and Use of Laboratory Animals, formulated by the Ministry of Science and Technology of China[29].
Methods
Sampling and sectioning of the locus coeruleus in brainstem of mice
The mice were anesthetized by intraperitoneal injection with 3% pentobarbital sodium (30 mg/kg), subjected to left ventricle-ascending aorta cannulation, and perfused with normal saline followed by 4% paraformaldehyde (pH 7.4) at 4°C. The brains were harvested, postfixed for 6 hours, immersed in gradient sucrose solution (15%, 30%), and 25 μm thick serial coronal sections of the locus coeruleus and surrounding area in the brainstem were cut using a freezing microtome (Shandon, UK), according to the principal of Systematic-Uniform-Random sampling[30,31,32,33].
The sections were collected using 12-well plates, and one section was selected for every five cuts. Four sets of sections were obtained from each mouse, and each set contained nine sections that included the locus coeruleus. One set was used for immunohistochemistry, and the others were placed in anti-freeze buffer and stored at –80°C.
Immunohistochemistry for detecting morphology of noradrenergic neurons in the locus coeruleus
The sections were rinsed in 0.01 mol/L PBS (pH 7.4) three times, mixed with 3% H2O2 at room temperature for 30 minutes to eliminate endogenous peroxidase, blocked in normal 5% goat serum containing 0.3% Triton X-100 (Vector, USA) for 60 minutes, and incubated with rabbit anti-tyrosine hydroxylase polyclonal antibody (1:200; Chemicon Internation, Temecual, CA, USA) overnight at 4°C. The sections were then incubated with biotinylated goat anti-rabbit IgG (1:200; Vector Laboratories, Burlingame, CA, USA) at room temperature for 2 hours, mixed with avidin-biotin complex solution (1:200) at room temperature for 60 minutes, followed by freshly prepared 3,3’-diaminobenzidine (Sigma, St Louis, MO, USA) at room temperature. The length of time to attain an appropriate degree of staining was controlled under a microscope. The sections were washed with 0.01 mol/L PBS to terminate the reaction, coated with gelatin, air dried, counterstained with Nissl solution (0.1% cresyl violet solution), dehydrated through an alcohol gradient, cleared in xylene, and mounted with neutral gum.
PBS instead of primary antibody was used as a negative control. Sections were observed by light microscope (Olympus, Tokyo, Japan).
Unbiased stereology for quantifying tyrosine hydroxylase-positive neurons and volume of locus coeruleus
The total quantity and volume of tyrosine hydroxylase-positive neurons, and locus coeruleus volume, were determined according to a previously described method[30,31,32,33].
Briefly, the brain tissues were dehydrated, embedded, and cut into serial frozen sections (30 μm thick), and sections containing locus coeruleus were collected. One section was selected from every five sections for tyrosine hydroxylase staining. The stained sections were mounted and dried.
Results were obtained using investigator software (NIH, USA) with the following parameters: frame area, 50%; frame height, 11 μm; frame pitch, 80 μm. Total quantity and size of positive neurons, and volume of locus coeruleus tissues, were calculated based on calculus principles.
Statistical analysis
Results were expressed as mean ± SD and analyzed using SPSS 17.0 software (SPSS, Chicago, IL, USA). Intergroup differences in mean value were compared by paired t-test. The total volume of locus coeruleus and mean volume of tyrosine hydroxylase-positive neurons in locus coeruleus were subjected to linear correlation analysis. A value of P < 0.05 was considered statistically significant.
Acknowledgments:
We thank the Gerontology Research Center, National Institutes of Health, United States for kindly providing experimental animals.
Footnotes
Funding: This study was supported by the National Natural Science Foundation of China, No. 81100663; the Scientific Research Funds of the Health Department of Hunan Province, No.120303; Hunan Provincal Natural Science Foundation of China, No. 13JJ3058 and a grant from the Scientific Research Program of Hunan Provincial Higher Education Institutes, No. 11C0829.
Conflicts of interest: None declared.
Ethical approval: This study received permission from the Animal Ethics Committee of Central South University in China.
(Reviewed by Robens J, Stow A, Zhang JJ, Li J)
(Edited by Wang J, Su LL, Li CH, Song LP, Liu WJ, Zhao M)
REFERENCES
- [1].McGaugh JL, Roozendaal B. Drug enhancement of memory consolidation: historical perspective and neurobiological implications. Psychopharmacology (Berl) 2009;202(1-3):3–14. doi: 10.1007/s00213-008-1285-6. [DOI] [PubMed] [Google Scholar]
- [2].Sultana R, Robinson RA, Di Domenico F, et al. Proteomic identification of specifically carbonylated brain proteins in APP(NLh)/APP(NLh) × PS-1(P264L)/PS-1(P264L) human double mutant knock-in mice model of Alzheimer disease as a function of age. J Proteomics. 2011;74(11):2430–2440. doi: 10.1016/j.jprot.2011.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Borchelt DR, Thinakaran G, Eckman CB, et al. Familial Alzheimer's disease-linked presenilin 1 variants elevate Abeta1-42/1-40 ratio in vitro and in vivo. Neuron. 1996;17(5):1005–1013. doi: 10.1016/s0896-6273(00)80230-5. [DOI] [PubMed] [Google Scholar]
- [4].Price DL, Wong PC, Markowska AL, et al. The value of transgenic models for the study of Neurodegenerative disease. Ann N Y Acad Sci. 2000;920:179–191. doi: 10.1111/j.1749-6632.2000.tb06920.x. [DOI] [PubMed] [Google Scholar]
- [5].Wang H, He J, Zhang R, et al. Sensorimotor gating and memory deficits in an APP/PS1 double transgenic mouse model of Alzheimer's disease. Behav Brain Res. 2012;233(1):237–243. doi: 10.1016/j.bbr.2012.05.007. [DOI] [PubMed] [Google Scholar]
- [6].Woo RS, Lee JH, Yu HN, et al. Expression of ErbB4 in the neurons of Alzheimer's disease brain and APP/PS1 mice, a model of Alzheimer's disease. Anat Cell Biol. 2011;44(2):116–127. doi: 10.5115/acb.2011.44.2.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Stone JG, Casadesus G, Gustaw-Rothenberg K, et al. Frontiers in Alzheimer's disease therapeutics. Ther Adv Chronic Dis. 2011;2(1):9–23. doi: 10.1177/2040622310382817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Funke SA, Liu H, Sehl T, et al. Identification and characterization of an aβ oligomer precipitating peptide that may be useful to explore gene therapeutic approaches to Alzheimer disease. Rejuvenation Res. 2012;15(2):144–157. doi: 10.1089/rej.2011.1262. [DOI] [PubMed] [Google Scholar]
- [9].Huang Y, Mucke L. Alzheimer mechanisms and therapeutic strategies. Cell. 2012;148(6):1204–1222. doi: 10.1016/j.cell.2012.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Panza F, Frisardi V, Solfrizzi V, et al. Immunotherapy for Alzheimer's disease: from anti-β-amyloid to tau-based immunization strategies. Immunotherapy. 2012;4(2):213–238. doi: 10.2217/imt.11.170. [DOI] [PubMed] [Google Scholar]
- [11].Mann D. The locus coeruleus and its possible role in ageing and degenerative disease of the human central nervous system. Mech Ageing Dev. 1983;23(1):73–94. doi: 10.1016/0047-6374(83)90100-8. [DOI] [PubMed] [Google Scholar]
- [12].German DC, Manaye KF, White CL, III, et al. Disease-specific patterns of locus coeruleus cell loss. Ann Neurol. 1992;32:667–676. doi: 10.1002/ana.410320510. [DOI] [PubMed] [Google Scholar]
- [13].Feinstein DL, Heneka MT, Gavrilyuk V, et al. Noradrenergic regulation of inflammatory gene expression in brain. Neurochem Int. 2002;41(5):357–365. doi: 10.1016/s0197-0186(02)00049-9. [DOI] [PubMed] [Google Scholar]
- [14].Heneka MT, Ramanathan M, Jacobs AH, et al. Locus ceruleus degeneration promotes Alzheimer pathogenesis in amyloid precursor protein 23 transgenic mice. J Neurosci. 2006;26(5):1343–1354. doi: 10.1523/JNEUROSCI.4236-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Bouret S, Sara SJ. Network reset: a simplified overarching theory of locus coeruleus noradrenaline function. Trends Neurosci. 2005;28(11):574–582. doi: 10.1016/j.tins.2005.09.002. [DOI] [PubMed] [Google Scholar]
- [16].German DC, Nelson O, Liang F, et al. The PDAPP mouse model of Alzheimer's disease: locus coeruleus neuronal shrinkage. J Comp Neurol. 2005;492(4):469–476. doi: 10.1002/cne.20744. [DOI] [PubMed] [Google Scholar]
- [17].Busch C, Bohl J, Ohm TG, et al. Spatial, temporal and numeric analysis of Alzheimer changes in the nucleus coeruleus. Neurobiol Aging. 1997;18(4):401–406. doi: 10.1016/s0197-4580(97)00035-3. [DOI] [PubMed] [Google Scholar]
- [18].Dineley KT, Xia X, Bui D, et al. Accelerated plaque accumulation, associative learning deficits, and up-regulation of alpha 7 nicotinic receptor protein in transgenic mice co-expressing mutant human presenilin 1 and amyloid precursor proteins. J Biol Chem. 2002;277(25):22768–22780. doi: 10.1074/jbc.M200164200. [DOI] [PubMed] [Google Scholar]
- [19].Dhawan G, Combs CK. Inhibition of Src kinase activity attenuates amyloid associated microgliosis in a murine model of Alzheimer's disease. J Neuroinflammation. 2012;9:117. doi: 10.1186/1742-2094-9-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Busche MA, Chen X, Henning HA, et al. Critical role of soluble amyloid-β for early hippocampal hyperactivity in a mouse model of Alzheimer's disease. Proc Natl Acad Sci U S A. 2012;109(22):8740–8745. doi: 10.1073/pnas.1206171109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Poduslo JF, Howell KG, Olson NC, et al. Alzheimer's disease amyloid β-protein mutations and deletions that define neuronal binding/internalization as early stage nonfibrillar/fibrillar aggregates and late stage fibrils. Biochemistry. 2012;51(19):3993–4003. doi: 10.1021/bi300275g. [DOI] [PubMed] [Google Scholar]
- [22].Sastre M, Klockgether T, Heneka MT. Contribution of inflammatory processes to Alzheimer's disease: molecular mechanisms. Int J Dev Neurosci. 2006;24(2-3):167–176. doi: 10.1016/j.ijdevneu.2005.11.014. [DOI] [PubMed] [Google Scholar]
- [23].Jardanhazi-Kurutz D, Kummer MP, Terwel D, et al. Distinct adrenergic system changes and neuroinflammation in response to induced locus ceruleus degeneration in APP/PS1 transgenic mice. Neuroscience. 2011;176:396–407. doi: 10.1016/j.neuroscience.2010.11.052. [DOI] [PubMed] [Google Scholar]
- [24].Heneka MT, Galea E, Gavriluyk V, et al. Noradrenergic depletion potentiates beta-amyloid-induced cortical inflammation: implications for Alzheimer's disease. J Neurosci. 2002;22(7):2434–2442. doi: 10.1523/JNEUROSCI.22-07-02434.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Jardanhazi-Kurutz D, Kummer MP, Terwel D, et al. Induced LC degeneration in APP/PS1 transgenic mice accelerates early cerebral amyloidosis and cognitive deficits. Neurochem Int. 2010;57(4):375–382. doi: 10.1016/j.neuint.2010.02.001. [DOI] [PubMed] [Google Scholar]
- [26].Carnevale D, De Simone R, Minghetti L. Microglia-neuron interaction in inflammatory and degenerative diseases: role of cholinergic and noradrenergic systems. CNS Neurol Disord Drug Targets. 2007;6(6):388–397. doi: 10.2174/187152707783399193. [DOI] [PubMed] [Google Scholar]
- [27].Morrissette DA, Parachikova A, Green KN, et al. Relevance of transgenic mouse models to human Alzheimer disease. J Biol Chem. 2009;284(10):6033–6037. doi: 10.1074/jbc.R800030200. [DOI] [PubMed] [Google Scholar]
- [28].Marchetti C, Marie H. Hippocampal synaptic plasticity in Alzheimer's disease: what have we learned so far from transgenic models. Rev Neurosci. 2011;22(4):373–402. doi: 10.1515/RNS.2011.035. [DOI] [PubMed] [Google Scholar]
- [29].The Ministry of Science and Technology of the People's Republic of China. Guidance Suggestions for the Care and Use of Laboratory Animals. 2006 Sep 30; [Google Scholar]
- [30].O’Neil JN, Mouton PR, Tizabi Y, et al. Catecholaminergic neuronal loss in locus coeruleus of aged female dtg APP/PS1 mice. J Chem Neuroanat. 2007;34(3-4):102–107. doi: 10.1016/j.jchemneu.2007.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Long JM, Kalehua AN, Muth NJ, et al. Stereological analysis of astrocyte and microglia in aging mouse hippocampus. Neurobiol Aging. 1998;19(5):497–503. doi: 10.1016/s0197-4580(98)00088-8. [DOI] [PubMed] [Google Scholar]
- [32].Kelley CM, Perez SE, Overk CR, et al. Effect of neocortical and hippocampal amyloid deposition upon galaninergic and cholinergic neurites in AβPPswe/PS1ΔE9 mice. J Alzheimers Dis. 2011;25(3):491–504. doi: 10.3233/JAD-2011-102097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Riise J, Pakkenberg B. Stereological estimation of the total number of myelinated callosal fibers in human subjects. J Anat. 2011;218(3):277–284. doi: 10.1111/j.1469-7580.2010.01333.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


