Abstract
Spinal cord ischemia/reperfusion injury is a stress injury to the spinal cord. Our previous studies using differential proteomics identified 21 differentially expressed proteins (n > 2) in rabbits with spinal cord ischemia/reperfusion injury. Of these proteins, stress-related proteins included protein disulfide isomerase A3, stress-induced-phosphoprotein 1 and heat shock cognate protein 70. In this study, we established New Zealand rabbit models of spinal cord ischemia/reperfusion injury by abdominal aorta occlusion. Results demonstrated that hind limb function initially improved after spinal cord ischemia/reperfusion injury, but then deteriorated. The pathological morphology of the spinal cord became aggravated, but lessened 24 hours after reperfusion. However, the numbers of motor neurons and interneurons in the spinal cord gradually decreased. The expression of protein disulfide isomerase A3, stress-induced-phosphoprotein 1 and heat shock cognate protein 70 was induced by ischemia/reperfusion injury. The expression of these proteins increased within 12 hours after reperfusion, and then decreased, reached a minimum at 24 hours, but subsequently increased again to similar levels seen at 6–12 hours, showing a characterization of induction-inhibition-induction. These three proteins were expressed only in cytoplasm but not in the nuclei. Moreover, the expression was higher in interneurons than in motor neurons, and the survival rate of interneurons was greater than that of motor neurons. It is assumed that the expression of stress-related proteins exhibited a protective effect on neurons.
Keywords: neural regeneration, spinal cord ischemia/reperfusion injury, protein disulfide isomerase A3, stress-induced-phosphoprotein 1, heat shock cognate protein 70, neuron, necrosis, apoptosis, grants-supported paper, neuroregeneration
Research Highlights
-
(1)
We investigated the temporal and spatial changes in the expression of protein disulfide isomerase A3, stress-induced-phosphoprotein 1 and heat shock cognate protein 70 in rabbit spinal cords after ischemia/reperfusion injury.
-
(2)
Motor neurons became more vulnerable than interneurons after spinal cord ischemia/reperfusion injury. Increased expression of protein disulfide isomerase A3, stress-induced-phosphoprotein 1 and heat shock cognate protein 70 could protect neurons against injury.
-
(3)
Hind limb functions were not positively associated with neuronal number within 48 hours after spinal cord ischemia/reperfusion injury. Edema was most obvious at 12 hours, which was induced by downregulation of various protein expressions.
-
(4)
Elevating the expression of stress-related protein in neurons could be a new target for prevention and treatment of spinal cord ischemia/reperfusion injury.
INTRODUCTION
After surgery, the symptoms of patients with cervical spinal stenosis, thoracic spinal stenosis, and fracture dislocation of the cervical and thoracic spine are lessened for a short period, following which they then become aggravated. This phenomenon is believed to be caused by spinal cord ischemia/reperfusion injury. Spinal cord ischemia/reperfusion injury can damage the neuronal function of the spinal cord. At present, there is no gold standard for prevention and treatment of spinal cord ischemia/reperfusion injury[1,2,3,4]. Because of the severe lesion caused by this injury in patients, studies investigating the mechanism by which it occurs are particularly important.
Our previous studies using differential proteomics identified 21 differentially expressed proteins (n > 2) in rabbits with spinal cord ischemia/reperfusion injury. Of these 21 proteins, stress-related proteins included protein disulfide isomerase A3 (PDIA3), stress-induced-phosphoprotein 1 (STIP1) and heatshock cognate protein 70 (Hsc70)[5]. Previous studies have demonstrated that heat shock protein (Hsp) 70 has important protective effects on cerebral, spinal cord and myocardial ischemia/reperfusion injuries[6,7,8,9]. STIP1, an important accessory molecule of Hsp70, coordinates the functions of Hsp70 and Hsp90 in protein folding[10,11]. STIP1 resists various kinds of stress in nervous system disease, and exerts effects on survival and differentiation of neuronal and glial cells[12,13,14]. Hsc70 is an essential member of the Hsp70 family. A previous study confirmed that Hsc70 reduced oxidative stress[15], removed abnormal proteins, and was neuroprotective[16,17,18,19]. Both PDIA3 and Hsp70 function in protein folding and can resist endoplasmic reticulum stress[20,21,22,23,24,25,26]. Spinal cord ischemia/reperfusion injury is a stress injury to the spinal cord. Thus, it is important to investigate the changes in stress-related protein expression after spinal cord ischemia/reperfusion injury to determine the responsible pathological mechanisms. This will enable the development of new strategies for prevention and treatment.
RESULTS
Quantitative analysis of experimental animals
A total of 36 New Zealand rabbits were equally and randomly assigned to six groups: sham surgery group (I0R0), 30-minute ischemia group (I30R0), 30-minute ischemia 6-hour (I30R6), 12-hour (I30R12), 24-hour (I30R24), and 48-hour (I30R48) reperfusion groups. The I0R0 group only received surgery to expose the abdominal aorta, without occlusion. In the I30R0, I30R6, I30R12, I30R24 and I30R48 groups, rabbit abdominal aortas were blocked for 30 minutes to induce spinal cord ischemia. Reperfusion for 6, 12, 24 and 48 hours was conducted in the I30R6, I30R12, I30R24 and I30R48 groups. All rabbits were included in the final analysis.
Changes in hind limb function in rabbits with spinal cord ischemia/reperfusion injury
After spinal cord ischemia/reperfusion injury, rabbit hind limb function was inactive and unresponsive to pain stimulus, revealing flaccid paralysis. With increased reperfusion time, movement in bilateral hind limbs gradually recovered and animals were responsive to pain stimulus. In the I30R6, I30R12, I30R24 and I30R48 groups, bilateral hind limbs displayed weakness in backward extension, excessive forward protrusion, lameness and gait instability. In the I30R24 group, one rabbit suffered from inconsistent hind limb paralysis. Hind limb function gradually improved after spinal cord ischemia/reperfusion injury, with the highest level of function achieved at 24 hours after reperfusion, similar to the early stages of spinal cord ischemia/reperfusion injury. Moreover, Tarlov's score was significantly greater than that at 6 hours after reperfusion (P < 0.05), and reduced at 48 hours (Figure 1).
Figure 1.
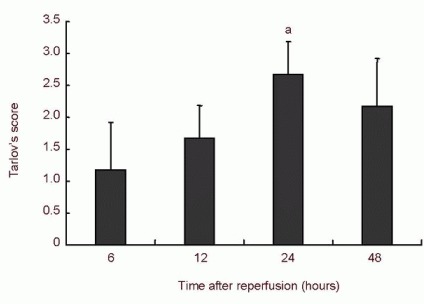
Bilateral hind limb function (Tarlov's score) after spinal cord ischemia/reperfusion injury.
Tarlov scoring ranged from 0 to 4 points. The higher score indicated better hind limb function. Data are expressed as mean ± SD. aP < 0.05, vs. 6 hours after ischemia/reperfusion (one-way analysis of variance, Tukey-Kramer test).
Pathomorphological changes in rabbit spinal cord after spinal cord ischemia/reperfusion injury
Hematoxylin-eosin staining revealed that in the sham surgery group, the structure of the gray and white matter of the spinal cord of rabbits was intact. Nerve fibers and intercellular components were uniformly distributed. The neuronal membrane was intact. Abundant Nissl bodies were visible in cytoplasm. Large, round nuclei, with heterochromatin (weak staining) in some cells, and clear nucleoli were also observed. Glial cells were uniformly distributed surrounding neurons (Figure 2A). After spinal cord ischemia, the morphology of rabbit spinal cord tissue did not alter under a light microscope (Figure 2B). At 6 hours after spinal cord ischemia/reperfusion, sporadic tiny vacuoles appeared in the spinal cord gray matter, some neurons became irregular, there were decreases in the number of processes, and some nuclei were pyknotic and irregular. Cellular swelling and lightly stained cytoplasm are visible in Figure 2C. At 12 hours after reperfusion, a large number of vacuoles of different sizes appeared in the gray matter. Neurons presented with pyknosis, breakage, and dissolution. Cell bodies became small and deformed and Nissl bodies disappeared. Some neurons had abundant vacuoles in their cytoplasm. The cytoplasm was weakly stained and nuclei were absent. Cell remnants were apparent after neuronal necrosis, and the area was surrounded by inflammatory cell infiltration (Figure 2D). At 24 hours, the number of vacuoles in the gray matter decreased, the number of neuronal cells diminished and the number of motor neurons in the IX region was significantly reduced. Neuronal nuclei became spindle-shaped, with the presence of noticeable nuclear deviation. Nuclei were noticeably smaller and the cytoplasm was darkly stained. A few nuclei were dissolved, with the presence of vacuole-like neurons and necrotic neuron remnants (Figure 2E).
Figure 2.
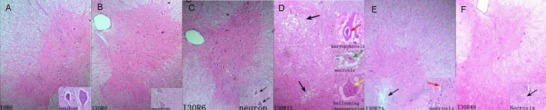
Pathomorphology of rabbit spinal cord after spinal cord ischemia/reperfusion injury (hematoxylin-eosin staining, light microscope × 40; neuron, light microscope, × 400).
Spinal cord edema and neuronal necrosis gradually aggravated after reperfusion. Injury was worst at 12 hours. Edema and necrosis gradually lessened.
(A) In the sham surgery group, structures of the gray and white matters were normal, and the structure and staining of neurons were normal.
(B) In the I30R0 group, structures of the gray and white matters were normal, and shape and staining of neurons were normal.
(C) In the I30R6 group, widely scattered small vacuoles appeared in the gray matter, some neurons were irregular; the number of processes reduced; pyknosis and nuclear deviation are shown by black arrows.
(D) In the I30R12 group, the numbers and size of vacuoles increased (black arrow). Neuronal pyknosis, smaller and deformed cell bodies (red arrow); vacuolar degeneration, lightly stained cytoplasm (green arrow); dissolved nuclei, Nissl body disappearance, and ballooning and degeneration of the neuron body were observed (yellow arrow).
(E) In the I30R24 group, vacuoles reduced (black arrow). Cell death occurred in some neurons and nuclei dissolved. Lightly stained cytoplasm (red arrow) was also observed.
(F) In the I30R48 group, vacuoles were further decreased; there was increased neuronal death and inflammatory cell infiltration. Neuronal pyknosis and cytoplasmic weak staining (black arrow) were visible.
At 48 hours, vacuoles disappeared, the number of neurons was reduced, glial cell density increased, and inflammatory cells infiltrated in gray matter. Neuronal pyknosis, breakage, dissolution, dark staining of cytoplasm, and necrotic neuronal remnants can be observed in Figure 2F.
Gradually reduction in the numbers of motor neurons and interneurons in the rabbit spinal cord after spinal cord ischemia/reperfusion injury
At 30 minutes after ischemia, there was no obvious change in the numbers of surviving motor neurons and interneurons in the spinal cord (P > 0.05). With increasing reperfusion time, the numbers of survival spinal cord motor neurons and interneurons gradually reduced (P < 0.05; Table 1), with the proportion of dead interneurons being less than that of motor neurons (Figure 3).
Table 1.
Effects of spinal cord ischemia/reperfusion injury on the numbers of motor neurons and interneurons (/400-fold visual field)
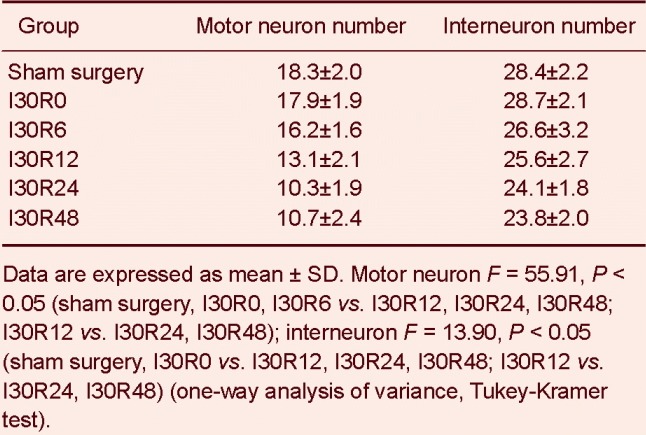
Figure 3.
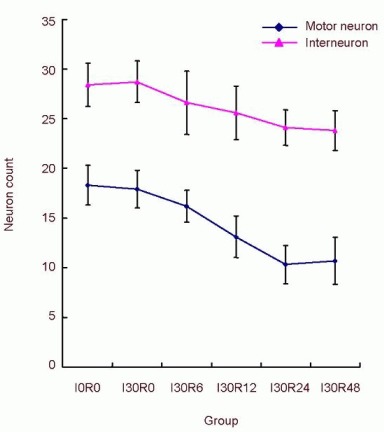
Effects of spinal cord ischemia/reperfusion injury on the numbers of motor neurons and interneurons.
The number change curve of motorneurons and interneurons. The mortality of interneurons was lower than that of motorneurons (P < 0.05, chi-square test).
Changes in the expression of PDIA3, STIP1 and Hsc70 in rabbit spinal cord after spinal cord ischemia/reperfusion injury
In the sham surgery group, PDIA3 and STIP1 were lightly expressed in gray matter interneurons matter and anterior horn motor neurons, but negatively expressed in posterior horn neurons. Hsc70 was lightly expressed in the cytoplasm of small interneurons in the middle of the gray matter, but negatively expressed in large neurons of the spinal cord anterior horn. Hsc70 expression was visible in the nuclear membrane of glial cells. After spinal cord ischemia alone, no significant changes in PDIA3 and STIP1 expressions were detected in interneurons of the central part of the gray matter and motor neurons of the anterior horn. Low expression of PDIA3 and STIP1 was observed in cytoplasm of neurons of the posterior horn. Hsc70 expression was visible in the neuronal cytoplasm throughout the spinal cord gray matter, and was enhanced in glial cell nuclei. At 6 hours after reperfusion, PDIA3 and STIP1 expressions were enhanced in the neuronal cytoplasm of the spinal cord gray matter, but there was no significant change in Hsc70 expression.
However, Hsc70 expression was increased in glial cell nuclei and Hsc70 detectable in Schwann cell nuclei in the white matter. At 12 hours, the expression of PDIA3 and STIP1 were increased in neuronal cytoplasm of the spinal cord gray matter and in interneurons. The expression of Hsc70 was decreased in gray matter neurons, but remained high in glial and Schwann cell nuclei. At 24 hours, the expression of PDIA3, STIP1 and Hsc70 were decreased in anterior horn motor neurons of the spinal cord gray mater, and reduced in interneurons, but were still higher in interneurons than in motor neurons. PDIA3 expression was not detected in posterior horn neurons. At 48 hours, the expression of PDIA3 and STIP1 were increased in gray matter neurons. The expression of PDIA3 was similar to the levels at 6 hours and the expression of STIP1 was similar to the levels at 12 hours. The expression of PDIA3 and STIP1 were noticeably increased in interneurons of the central part of the gray matter. The expression of Hsc70 was increased in the neuronal cytoplasm of gray matter and was higher in interneurons of the central part of the gray matter than that in motor neurons of the anterior horn. The expression of Hsc70 remained high in glial and Schwann cell nuclei. The expression of PDIA3 and STIP1 were not detected in glial cells of spinal cord gray matter after reperfusion. The expression of PDIA3, STIP1 and Hsc70 were greater in small interneurons in the gray matter than that in large motor neurons in the cytoplasm after reperfusion (Figure 4).
Figure 4.
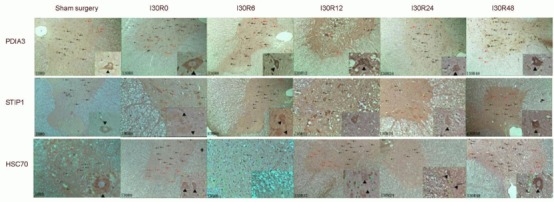
Expression of protein disulfide isomerase A3 (PDIA3), stress-induced-phosphoprotein 1 (STIP1) and heat shock cognate protein 70 (Hsc70) in rabbit spinal cord after spinal cord ischemia/reperfusion injury (immunohistochemical staining, light microscope, × 40; local magnification, × 400).
Red arrows show motor neurons of the spinal cord anterior horn; black arrows indicate interneurons; green arrows show glial cells; orange arrows represent Schwann cells; black triangles indicate the intensity of immunoreactivity. Tawny cytoplasm or nuclei indicate mild immunoreactivity. Brown staining shows moderate immunoreactivity. Brownish-black or black exhibit severe immunoreactivity. Local magnification reveals neurons.
Western blot assay verified the changes in PDIA3, STIP1 and Hsc70 levels. The expression characteristics of these three proteins were induction-inhibition-induction during spinal cord ischemia/reperfusion injury and were lowest at 24 hours after reperfusion (Figure 5).
Figure 5.
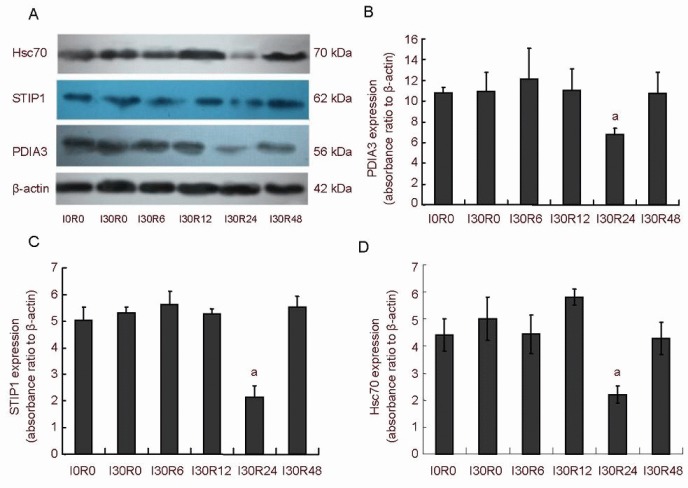
Effects of spinal cord ischemia/reperfusion injury on the expression of PDIA3 (PDIA3), stress-induced-phosphoprotein 1 (STIP1) and heat shock cognate protein 70 (Hsc70) in rabbit spinal cord.
(A) Western blot assay showing the expression of PDIA3, STIP1 and Hsc70 in the rabbit spinal cord after ischemia/reperfusion injury.
(B–D) Effects of spinal cord ischemia/reperfusion injury on the expression of PDIA3, STIP1 and Hsc70 in rabbit spinal cord, respectively. Compared with the other groups, the absorbance values of PDIA3, STIP1 and Hsc70 in the rabbit spinal cord were significantly lower in the I30R24 group (aP < 0.01, vs. other groups), showing a characteristic of increase–decrease–increase. Intergroup comparison was performed using one-way analysis of variance and Tukey-Kramer test.
DISCUSSION
Relationship of ischemia/reperfusion time, nerve function and neuron number
Reece et al[27] confirmed that after 25–30 minutes of spinal cord ischemia, the damage to nerve function is a moderate progressive lesion.
In the I30R6, I30R12, I30R24 and I30R48 groups, hind limb function gradually recovered after consciousness. At 24 hours after reperfusion, hind limb functional recovery was highest. Subsequently, bilateral hind limb function deteriorated. Studies have demonstrated that the score of bilateral hind limb function is not consistent with changes in the number of motor neurons in the spinal cord anterior horn in early stages of spinal cord ischemia/reperfusion injury. This is the reason for the gradual recovery of bilateral hind limb function within the first 24 hours.
However, the number of surviving motor neurons in the spinal cord anterior horn gradually reduced within the first 24 hours. At 24–48 hours after reperfusion, spinal cord function worsened further while the number of motor neurons in the spinal cord anterior horn was not obviously diminished. Thus, it is assumed that the spinal cord function is not only associated with neuron number, but also with neuronal function. After spinal cord ischemia/reperfusion injury, neurons with normal morphology can have abnormal function. Kurita et al[28] found that the number of motor neurons in the spinal cord was significantly positively associated with spinal cord function after spinal cord ischemia/reperfusion injury, which is inconsistent with results of this study. This may be due to different ischemia and/or observation time periods.
At 6 hours after spinal cord ischemia/reperfusion injury, sporadic tiny vacuoles appeared in spinal cord gray matter. At 12 hours, abnormal morphology was apparent. Abundant gray matter vacuoles of different sizes were detected in the spinal cord gray matter. With increasing reperfusion time, the number of gray matter vacuoles gradually decreased. At 48 hours, the density and size of the vacuoles recovered to the level at 6 hours. At 12 hours, abundant cavitation suggested that vascular permeability increased, inflammatory cell infiltration had occurred, water-electrolyte metabolism was unbalanced, resulting in gray matter edema and hemorrhage[29,30].
At this time, the spinal cord had severe metabolic disturbances, it could not remove excessive water, and protein synthesis had decreased.
Temporal and spatial changes in the expression of PDIA3, STIP1 and Hsc70 in rabbit spinal cord after spinal cord ischemia/reperfusion injury
Studies suggest that PDIA3 is expressed in interneurons and anterior horn motor neurons in the spinal cord in the sham surgery group, indicating that PDIA3 is physiologically indispensable. STIP1 and Hsc70 are only expressed in interneurons of the control group. After ischemic stress, the expression of PDIA3, STIP1 and Hsc7 was increased in the cytoplasm of motor neurons and interneurons. Expression of these was stronger in interneurons than in anterior horn motor neurons. Hsc70 was also expressed in glial and Schwann cell nuclei, but negatively expressed in neuronal nuclei. At 12 hours after reperfusion, the expression of the three proteins was intensified, following which they then diminished. Their expression levels reached the lowest at 24 hours, and subsequently increased. Their reduction was more significant in anterior horn motor neurons than in interneurons. These three stress-related proteins were possibly expressed higher in interneurons because the mortality of motor neurons was higher.
Previous studies confirmed that the ability of interneurons to resist stress injury was stronger than that of motor neurons, which could possibly be associated that the ability of interneurons to resist calcium overload, excitatory amino acid toxicity, and mitochondrial inhibition[31,32,33].
The expression of PDIA3, STIP1 and Hsc70 diminished at 12 hours after reperfusion, was lowest at 24 hours, and then increased. These changes were associated with pathological changes of the spinal cord. At 6 hours, sporadic tiny vacuoles appeared in spinal cord gray matter. At 12 hours, abnormal morphology was noticeable, and many vacuoles of different sizes were observed in spinal cord gray matter. With increased reperfusion time, the number of gray matter vacuoles gradually diminished. At 48 hours, these changes became identical to those at 6 hours. Vacuolization in the spinal cord gray matter was associated with an increase in vascular permeability and a disorder in the water-electrolyte balance. When severe pathological changes appeared in the spinal cord, these changes would affect the synthesis of proteins, resulting in a decrease in the expression of PDIA3, STIP1 and Hsc70. At this time, the water-electrolyte balance maintained normal membrane potential. At 24 hours, when edema in the spinal cord was lessened, the expression of stress-related proteins increased. Protein expression at 48 hours became identical to that at 6–12 hours.
Effects of PDIA3 on spinal cord ischemia/reperfusion injury
PDIA3 is an important molecular chaperone in the intracavity space of the endoplasmic reticulum, and a key regulatory substance for correctly protein synthesis and maintaining cell function under normal growth conditions[34,35,36]. Upregulation of PDIA3 expression was mainly induced by decreases in intracellular glucose concentration and oxygen content, protein transport and secretion defects in endoplasmic reticulum, and an imbalance in intracellular Ca2+ levels[37]. Immunohistochemistry and western blot assay revealed that PDIA3 expression was abundant in neurons in the control group, suggesting that PDIA3 plays an important role in normal physiological activities in neurons. Yamauchi et al[38] confirmed that the vulnerability of anterior horn motor neurons is associated with endoplasmic reticulum stress, and that increased PDIA3 expression can maintain correct protein folding in endoplasmic reticulum, thereby reducing stress[20,21,24,26,39]. After spinal cord ischemia/reperfusion injury, a large amount of abnormal protein unfolding or misfolding occurred in neurons. PDIA3 diminished the accumulation of these mis/unfolded proteins, and protected neurons against the cytotoxic effect of unfolded proteins[22,23,25,40,41]. Yamauchi et al[42] confirmed that the number of unfolded protein was higher in anterior horn motor neurons than that in interneurons after spinal cord ischemia/reperfusion injury. Thus, the authors assumed that relative insufficient expression of PDIA3 was a reason for the damage to anterior horn motor neurons. The high expression of PDIA3 protected neural cells against scrapie prion- and endoplasmic reticulum stress-mediated cell apoptosis[22,43], and diminished methamphetamine-mediated oxidative stress[44], suggesting a neuroprotective effect of PDIA3. High expression of PDIA3 in interneurons could promote the high survival of interneurons by lessening endoplasmic reticulum stress after spinal cord ischemia/reperfusion injury.
Effects of STIP1 on spinal cord ischemia/reperfusion injury
STIP1 coordinates the activities of Hsp70 and Hsp90 in protein folding[10,11,45,46]. A previous study demonstrated that Hsp70 has positive protective effects on spinal cord ischemia/reperfusion injury, reduced abnormal protein accumulation induced by spinal cord ischemia/reperfusion injury, and induced normal folding of abnormal proteins[47,48,49,50]. STIP1 has an essential interactor effect on protein folding for Hsp70[47,48,49,50]. STIP1 interacts with prion proteins in cells of the nervous system, contributes to differentiation and renewal of nerve cells[12], promotes axonal growth[13,51], and elevates the survival ability of glial cells[14,52]. STIP1 independently regulates retinal cell differentiation, proliferation and death[53]. In this study, STIP1 expression increased in neurons after spinal cord ischemia/reperfusion injury, and its expression was associated with neuronal survival to some extent. Therefore, it is assumed that increased expression of STIP1 can inhibit the stress caused by spinal cord ischemia/reperfusion injury to a certain degree.
Effects of Hsc70 on spinal cord ischemia/reperfusion injury
Hsc70, a member of the Hsp70 family, protects cells from physical and chemical injuries[15,18,54]. Hsc70 is steadily expressed in cells and its upregulation has a positive effect on elevating the tolerance of the heart to ischemia[55]. It also plays an important role in adjusting myocardial immunoreactivity and myocardial function after ischemia[56]. The expression of Hsc70 in nervous tissue was significantly higher than in other tissues, where it has been shown to maintain a normal synaptic function under stress conditions[17,19,57]. A previous study verified that Hsc70 was associated with neuronal survival during neural tube formation, and Hsc70 was a protective protein for the neuroepithelium and neural precursor cells[58]. Exogenous Hsc70 could resist oxidative stress[18] and reduce traumatic injury-induced neuronal apoptosis[59]. Ischemic preconditioning elevated Hsc70 expression, blocked neuronal apoptosis, and elevated the tolerance of neurons to toxicity caused by excitatory amino acids. Binding of Hsc70 to caspase 3 also blocked cell apoptosis via the caspase pathway[60]. After cerebral ischemia, Hsc70 expression is upregulated, indicating that it has positive effects in the protection and repair of the injured nervous system[16,61].
This study demonstrated that interneurons express Hsc70 under physiological conditions. Hsc70 expression was significantly increased in interneurons after spinal cord ischemia/reperfusion injury and could also be detected in motor neurons. Low mortality of interneurons suggests that Hsc70 has neuroprotective effects on spinal cord ischemia/reperfusion injury. Hsc70 expression was detectable in the nuclei of glial cells of the spinal cord gray matter and white matter Schwann cells after spinal cord ischemia/reperfusion injury. High expression of Hsc70 was only observed in the cytoplasm of neurons. Previous studies showed that Hsc70 accumulates in cell nuclei when cells are subjected to heat and oxidative stresses[62,63]. A large number of abnormal proteins were detected in the cytoplasm of motor neurons after spinal cord ischemia/reperfusion injury. Hsc70 was greatly consumed because of accessory protein folding, following which it was translocated into cytoplasm, and could not be transported back into nuclei[38,42]. Because Hsc70 was consumed so greatly, its anti-apoptotic effect was weakened[38,42]. Thus, neuronal apoptosis increased after spinal cord ischemia/reperfusion injury[38,42]. Abundant levels of Hsc70 translocated into the nuclei of glial cells and Schwann cells, where it repaired abnormal folded protein, prevented DNA damage, interacted with caspase 3, blocked apoptosis, and reduced spinal cord ischemia/reperfusion injury[62,63].
In summary, the upregulation of PDIA3, STIP1 and Hsc70 in motor neurons and interneurons can decrease neuronal death after spinal cord ischemia/reperfusion injury. Therefore, increasing the expression of stress-related proteins in neurons could be a new target for the prevention and treatment of spinal cord ischemia/reperfusion injury.
MATERIALS AND METHODS
Design
A randomized, controlled animal study.
Time and setting
Experiments were performed at the Animal Experiment Center of Jilin University and Central Laboratory of China-Japan Union Hospital of Jilin University in China from September to December 2012.
Materials
Clean adult New Zealand rabbits of both genders and weighing 2.5–3.0 kg were supplied by the Center for Experimental Animal Basic Research, Jilin University, China (license No. SCXK (Ji) 2010-0006). The protocols were conducted in accordance with the Guidance Suggestions for the Care and Use of Laboratory Animals, formulated by Ministry of Science and Technology of China[64]. All animals were housed at 22°C in individual cages, and allowed free access to food and water.
Methods
Establishment of models of spinal cord ischemia/reperfusion injury
Rabbits were intraperitoneally anesthetized with 10% chloral hydrate 300 mg/kg. A median incision was made in the peritoneal cavity, and the abdominal aorta was dissociated at 0.5 cm inferior to the branch of the left renal artery. A total of 5 mL heparin (25 U/mL) was injected in blood vessels. After the abdominal aorta was blocked for 30 minutes, blood flow was restored. Gentamicin 8.0 × 104 U was intraperitoneally injected. The incision was sutured gradually[65]. In the sham surgery group, the abdominal aorta was exposed for 30 minutes, and L3–5 segments were obtained in vivo. In the I30R0 group, the abdominal aorta was blocked for 30 minutes with an artery clamp, and the L3–5 segments were obtained in vivo. After establishing models of spinal cord ischemia/reperfusion injury, an adjustable heating pad was placed under the animal. They were housed at 22°C in individual cages with good ventilation. For the rabbits with defecation disorders, hand compression was used on the abdomen to accelerate defecation.
Observation of neurological function
In accordance with the modified Tarlov scale[66], motor function of the rabbit hind limb was scored by three specified persons with single-blind method, and the average score was recorded. 0 = complete paraplegia, 1 = slight lower limb movement, 2 = hind limb movement, without the ability to walk or hop, 3 = walk or hop, but with obvious ataxia, 4 = normal gait of hind limb. In the sham surgery and I30R0 groups, the rabbits were sacrificed when they were not conscious, so their hind limb function was not scored.
Sample collection
The rabbits in each group were intraperitoneally injected with 10% chloral hydrate 300 mg/kg. Under deep anesthesia, the L3–5 segments of the spinal cord were washed in precooled saline at 4°C. Tissues were cut into pieces on ice cubes. 0.8-cm-long L3–4 segments of the spinal cord were immersed in 4% neutral paraformaldehyde for 24 hours, and embedded in paraffin. The remaining samples were stored at –80°C.
Hematoxylin-eosin staining of rabbit spinal cord morphology
Cranial, caudal and middle spinal cord was sliced into 5-μm-thick sections. The sections were stained with hematoxylin and eosin, and observed with a light microscope (BX51WI-DPMC; Olympus, Tokyo, Japan). The number of neurons in Rexed IX layer was considered as the number of motor neurons. The number of neurons in Rexed VII and VIII layers was considered as the number of interneurons. Three sections of each rabbit were obtained to calculate the average value. The number of neurons was quantified by two pathologists blind to the conditions, and the average value was calculated. Neuronal pyknosis, cell body deformation, Nissl body disappearance, dark staining, vacuolization and nuclear disappearance were visible, indicating neuronal death. Normal staining and the presence of Nissl bodies in cytoplasm were considered as surviving neurons[42].
Immunohistochemical staining for the expression of PDIA3, STIP1 and Hsc70 in rabbit spinal cord
Spinal cord tissue was dewaxed, hydrated, and sliced (immersed in xylene and alcohol). The sections were incubated in 3% H2O2 deionized water for 5–10 minutes to block endogenous peroxidase, followed by antigen retrieval: the sections were placed in citric acid at 98°C, cooled to room temperature for 30 minutes, and washed three times with PBS for 5 minutes each. The sections were incubated in rabbit anti-PDIA3, anti-STIP1 and anti-Hsc70 polyclonal antibodies (1:150; Beijing Biosynthesis Biotechnology Co., Ltd., Beijing, China) at 4°C overnight, followed by 3 × 2 minute washes in PBS. The sections were incubated in goat anti-rabbit IgG antibody-horse-radish peroxidase (1:1 000; Beijing Biosynthesis Biotechnology Co., Ltd.) at room temperature or 37°C for 20–30 minutes, followed by 3 × 2 minute washes in PBS. The sections were incubated in peroxidase complex at room temperature for 30 minutes, and washed with PBS, 3 × 5 minutes. The sections were visualized with 3,3’-diaminobenzidine kit (Santa Cruz Biotechnology, Santa Cruz, CA, USA). Under the microscope, the cytoplasm presented with a tawny color. The sections were counterstained with hematoxylin for 1 minute, differentiated with 1% acidic alcohol, and then terminated by washing with water. The sections were treated with weak ammonia liquor, washed with distilled water, mounted with neutral resin, and observed under a microscope (BX51WI-DPMC; Olympus). Positive results showed brown or tawny particles, and nuclei were counterstained blue with hematoxylin.
Western blot assay for the expression of PDIA3, STIP1 and Hsc70 in rabbit spinal cord
Spinal cord tissue was homogenized with lysis buffer, supplemented with 0.1 mol/L NaCl, 0.01 mol/L Tris-HCl, pH 7.5, 1 mmol/L ethylenediamine tetraacetic acid, and 1 μg/mL protease inhibitor. The homogenate was centrifuged at 4°C and 10 000 × g for 5 minutes. The supernatant was stored in 0.5 mL centrifuge tubes in a refrigerator at –20°C. Protein concentration was analyzed using the Bradford method[38]. The samples were electrophoresed in a 10% polyacrylamide gel. Protein samples were boiled in 2.5% sodium dodecyl sulfate and 5% β-mercaptoethanol. A total of 20 μg protein samples and markers (MagicMark XP Western Standard; Invitrogen, Carlsbad, CA, USA) of each group were electrophoresed at 20 mA for 90 minutes. Electrophoresis buffer contained 25 mmol/L tris(hydroxymethyl)aminomethane, 250 mmol/L glycine and 0.1% sodium dodecyl sulfate. Proteins on the gel were transferred onto polyvinylidene fluoride membrane (LC2002; Invitrogen) using transmembrane buffer and 10% methanol. The membrane was incubated with rabbit anti-PDIA3, anti-STIP1, anti-Hsc70, and anti-β-actin polyclonal antibodies (1:1 000; Beijing Biosynthesis Biotechnology Co., Ltd.) at room temperature for 1 hour, washed with PBS, and then incubated with horseradish peroxidase-labeled goat anti-rabbit IgG (1:1 000; Beijing Biosynthesis Biotechnology Co., Ltd.) at room temperature for 90 minutes. The samples were visualized with ECL Plus Kit (Amersham Biosciences, Piscataway, NJ, USA). Absorbance values were analyzed using NIH ImageJ software (National Institutes of Health, USA).
Statistical analysis
Data were expressed as mean ± SD and analyzed using SPSS 19.0 software (SPSS, Chicago, IL, USA). Intergroup differences of neurological score, counting of motor neurons and interneurons, and quantitative analysis of western blot assay were compared using one-way analysis of variance and Tukey-Kramer test. A value of P < 0.05 was considered statistically significant.
Footnotes
Funding: This study was supported by the National Natural Science Foundation of China, No. 30872609.
Conflicts of interest: None declared.
Ethical approval: This study was approved by the Animal Ethics Committee, China-Japan Union Hospital of Jilin University in China.
(Reviewed by Wallace M, Yajima W, Zheng XY, Li JT)
(Edited by Yu J, Qiu Y, Li CH, Song LP, Liu WJ, Zhao M)
REFERENCES
- [1].Rovira M, Torrent O, Ruscalleda J. Some aspects of the spinal cord circulation in cervical myelopathy. Neuroradiology. 1975;9(4):209–214. doi: 10.1007/BF00346149. [DOI] [PubMed] [Google Scholar]
- [2].Chiba K, Toyama Y, Matsumoto M, et al. Segmental motor paralysis after expansive open-door laminoplasty. Spine (Phila Pa 1976) 2002;27(19):2108–2115. doi: 10.1097/00007632-200210010-00006. [DOI] [PubMed] [Google Scholar]
- [3].Nassr A, Eck JC, Ponnappan RK, et al. The incidence of C5 palsy after multilevel cervical decompression procedures: a review of 750 consecutive cases. Spine (Phila Pa 1976) 2012;37(3):174–178. doi: 10.1097/BRS.0b013e318219cfe9. [DOI] [PubMed] [Google Scholar]
- [4].Chin KR, Seale J, Cumming V. “White cord syndrome” of acute tetraplegia after anterior cervical decompression and fusion for chronic spinal cord compression: a case report. Case Rep Orthop 2013. 2013 doi: 10.1155/2013/697918. 697918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Gao Q, Liang YH, Yang XY, et al. Differential protein expression in spinal cord tissue of a rabbit model of spinal cord ischemia/reperfusion injury. Neural Regen Res. 2012;7(20):1534–1539. doi: 10.3969/j.issn.1673-5374.2012.20.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Lee KS, Chung JH, Oh BH, et al. Increased plasma levels of heat shock protein 70 in patients with vascular mild cognitive impairment. Neurosci Lett. 2008;436(2):223–226. doi: 10.1016/j.neulet.2008.03.025. [DOI] [PubMed] [Google Scholar]
- [7].Hamilton KL, Powers SK, Sugiura T, et al. Short-term exercise training can improve myocardial tolerance to I/R without elevation in heat shock proteins. Am J Physiol Heart Circ Physiol. 2001;281(3):H1346–1352. doi: 10.1152/ajpheart.2001.281.3.H1346. [DOI] [PubMed] [Google Scholar]
- [8].Chiu PY, Ko KM. Schisandrin B protects myocardial ischemia-reperfusion injury partly by inducing Hsp25 and Hsp70 expression in rats. Mol Cell Biochem. 2004;266(1-2):139–144. doi: 10.1023/b:mcbi.0000049151.79238.30. [DOI] [PubMed] [Google Scholar]
- [9].van der Weerd L, Lythgoe MF, Badin RA, et al. Neuroprotective effects of HSP70 overexpression after cerebral ischaemia--an MRI study. Exp Neurol. 2005;195(1):257–266. doi: 10.1016/j.expneurol.2005.05.002. [DOI] [PubMed] [Google Scholar]
- [10].Gonçalves DC, Gava LM, Ramos CH. Human Hsp70/Hsp90 organizing protein (Hop) D456G is a mixture of monomeric and dimeric species. Protein Pept Lett. 2010;17(4):492–498. doi: 10.2174/092986610790963708. [DOI] [PubMed] [Google Scholar]
- [11].Carrigan PE, Sikkink LA, Smith DF, et al. Domain:domain interactions within Hop, the Hsp70/Hsp90 organizing protein, are required for protein stability and structure. Protein Sci. 2006;15(3):522–532. doi: 10.1110/ps.051810106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Santos TG, Silva IR, Costa-Silva B, et al. Enhanced neural progenitor/stem cells self-renewal via the interaction of stress-inducible protein 1 with the prion protein. Stem Cells. 2011;29(7):1126–1136. doi: 10.1002/stem.664. [DOI] [PubMed] [Google Scholar]
- [13].Santos TG, Beraldo FH, Hajj GN, et al. Laminin-γ1 chain and stress inducible protein 1 synergistically mediate PrPC-dependent axonal growth via Ca2+ mobilization in dorsal root ganglia neurons. J Neurochem. 2013;124(2):210–223. doi: 10.1111/jnc.12091. [DOI] [PubMed] [Google Scholar]
- [14].Hartmann CA, Martins VR, Lima FR. High levels of cellular prion protein improve astrocyte development. FEBS Lett. 2013;587(2):238–244. doi: 10.1016/j.febslet.2012.11.032. [DOI] [PubMed] [Google Scholar]
- [15].Ekimova IV, Nitsinskaya LE, Romanova IV, et al. Exogenous protein Hsp70/Hsc70 can penetrate into brain structures and attenuate the severity of chemically-induced seizures. J Neurochem. 2010;115(4):1035–1044. doi: 10.1111/j.1471-4159.2010.06989.x. [DOI] [PubMed] [Google Scholar]
- [16].Chen A, Liao WP, Lu Q, et al. Upregulation of dihydropyrimidinase-related protein 2, spectrin alpha II chain, heat shock cognate protein 70 pseudogene 1 and tropomodulin 2 after focal cerebral ischemia in rats--a proteomics approach. Neurochem Int. 2007;50(7-8):1078–1086. doi: 10.1016/j.neuint.2006.11.008. [DOI] [PubMed] [Google Scholar]
- [17].Jinwal UK, O’Leary JC, 3rd, Borysov SI, et al. Hsc70 rapidly engages tau after microtubule destabilization. J Biol Chem. 2010;285(22):16798–16805. doi: 10.1074/jbc.M110.113753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Robinson MB, Taylor AR, Gifondorwa DJ, et al. Exogenous Hsc70, but not thermal preconditioning, confers protection to motoneurons subjected to oxidative stress. Dev Neurobiol. 2008;68(1):1–17. doi: 10.1002/dneu.20550. [DOI] [PubMed] [Google Scholar]
- [19].Bauer PO, Goswami A, Wong HK, et al. Harnessing chaperone-mediated autophagy for the selective degradation of mutant huntingtin protein. Nat Biotechnol. 2010;28(3):256–263. doi: 10.1038/nbt.1608. [DOI] [PubMed] [Google Scholar]
- [20].Corazzari M, Lovat PE, Armstrong JL, et al. Targeting homeostatic mechanisms of endoplasmic reticulum stress to increase susceptibility of cancer cells to fenretinide-induced apoptosis: the role of stress proteins ERdj5 and ERp57. Br J Cancer. 2007;96(7):1062–1071. doi: 10.1038/sj.bjc.6603672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Lovat PE, Corazzari M, Armstrong JL, et al. Increasing melanoma cell death using inhibitors of protein disulfide isomerases to abrogate survival responses to endoplasmic reticulum stress. Cancer Res. 2008;68(13):5363–5369. doi: 10.1158/0008-5472.CAN-08-0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Hetz C, Russelakis-Carneiro M, Wälchli S, et al. The disulfide isomerase Grp58 is a protective factor against prion neurotoxicity. J Neurosci. 2005;25(11):2793–2802. doi: 10.1523/JNEUROSCI.4090-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Erickson RR, Dunning LM, Olson DA, et al. In cerebrospinal fluid ER chaperones ERp57 and calreticulin bind beta-amyloid. Biochem Biophys Res Commun. 2005;332(1):50–57. doi: 10.1016/j.bbrc.2005.04.090. [DOI] [PubMed] [Google Scholar]
- [24].Dukes AA, Van Laar VS, Cascio M, et al. Changes in endoplasmic reticulum stress proteins and aldolase A in cells exposed to dopamine. J Neurochem. 2008;106(1):333–346. doi: 10.1111/j.1471-4159.2008.05392.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Kim-Han JS, O’Malley KL. Cell stress induced by the parkinsonian mimetic, 6-hydroxydopamine, is concurrent with oxidation of the chaperone, ERp57, and aggresome formation. Antioxid Redox Signal. 2007;9(12):2255–2264. doi: 10.1089/ars.2007.1791. [DOI] [PubMed] [Google Scholar]
- [26].Akazawa YO, Saito Y, Nishio K, et al. Proteomic characterization of the striatum and midbrain treated with 6-hydroxydopamine: alteration of 58-kDa glucose-regulated protein and C/EBP homologous protein. Free Radic Res. 2010;44(4):410–421. doi: 10.3109/10715760903536349. [DOI] [PubMed] [Google Scholar]
- [27].Reece TB, Tribble CG, Okonkwo DO, et al. Early adenosine receptor activation ameliorates spinal cord reperfusion injury. J Cardiovasc Med (Hagerstown) 2008;9(4):363–367. doi: 10.2459/JCM.0b013e3282eee836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Kurita N, Kawaguchi M, Kakimoto M, et al. Reevaluation of gray and white matter injury after spinal cord ischemia in rabbits. Anesthesiology. 2006;105(2):305–312. doi: 10.1097/00000542-200608000-00013. [DOI] [PubMed] [Google Scholar]
- [29].Sader AA, Barbieri-Neto J, Sader SL, et al. The protective action of chlorpromazine on the spinal cord of rabbits submitted to ischemia and reperfusion is dose-dependent. J Cardiovasc Surg (Torino) 2002;43(6):827–831. [PubMed] [Google Scholar]
- [30].Takigawa T, Yonezawa T, Yoshitaka T, et al. Separation of the perivascular basement membrane provides a conduit for inflammatory cells in a mouse spinal cord injury model. J Neurotrauma. 2010;27(4):739–751. doi: 10.1089/neu.2009.1111. [DOI] [PubMed] [Google Scholar]
- [31].Diao ZY, Shen Y, Fan DS, et al. Establishment of the organotypic model of amyotrophic lateral sclerosis from the SD rats’ spinal cord. Beijing Da Xue Xue Bao. 2005;37(2):134–138. [PubMed] [Google Scholar]
- [32].Li B, Dong H, Li Z, et al. Lipopolysacchride-induced motor neuron injury in the anterior horn of spinal cord. Xibao Shengwu Xue Zazhi. 2008;30(1):114–120. [Google Scholar]
- [33].Xiao XJ, Wang XJ, Liu WG, et al. Establishment of rat organotypic culture model for selective motor neuron death. Jichu Yixue yu Linchuang. 2004;24(6):687–691. [Google Scholar]
- [34].Diedrich G, Bangia N, Pan M, et al. A role for calnexin in the assembly of the MHC class I loading complex in the endoplasmic reticulum. J Immunol. 2001;166(3):1703–1709. doi: 10.4049/jimmunol.166.3.1703. [DOI] [PubMed] [Google Scholar]
- [35].Sadasivan B, Lehner PJ, Ortmann B, et al. Roles for calreticulin and a novel glycoprotein, tapasin, in the interaction of MHC class I molecules with TAP. Immunity. 1996;5(2):103–114. doi: 10.1016/s1074-7613(00)80487-2. [DOI] [PubMed] [Google Scholar]
- [36].Garbi N, Tanaka S, Momburg F, et al. Impaired assembly of the major histocompatibility complex class I peptideloading complex in mice deficient in the oxidoreductase ERp57. Nat Immunol. 2006;7(1):93–102. doi: 10.1038/ni1288. [DOI] [PubMed] [Google Scholar]
- [37].Lee AS. The glucose-regulated proteins: stress induction and clinical applications. Trends Biochem Sci. 2001;26(8):504–510. doi: 10.1016/s0968-0004(01)01908-9. [DOI] [PubMed] [Google Scholar]
- [38].Yamauchi T, Sakurai M, Abe K, et al. Impact of the endoplasmic reticulum stress response in spinal cord after transient ischemia. Brain Res. 2007;1169:24–33. doi: 10.1016/j.brainres.2007.06.093. [DOI] [PubMed] [Google Scholar]
- [39].Rao RV, Bredesen DE. Misfolded proteins, endoplasmic reticulum stress and neurodegeneration. Curr Opin Cell Biol. 2004;16(6):653–662. doi: 10.1016/j.ceb.2004.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Yu ZF, Mattson MP. Dietary restriction and 2-deoxyglucose administration reduce focal ischemic brain damage and improve behavioral outcome: evidence for a preconditioning mechanism. J Neurosci Res. 1999;57(6):830–839. [PubMed] [Google Scholar]
- [41].Hoozemans JJ, Veerhuis R, Van Haastert ES, et al. The unfolded protein response is activated in Alzheimer's disease. Acta Neuropathol. 2005;110(2):165–172. doi: 10.1007/s00401-005-1038-0. [DOI] [PubMed] [Google Scholar]
- [42].Yamauchi T, Sakurai M, Abe K, et al. Ubiquitin-mediated stress response in the spinal cord after transient ischemia. Stroke. 2008;39(6):1883–1889. doi: 10.1161/STROKEAHA.106.455832. [DOI] [PubMed] [Google Scholar]
- [43].Hetz CA, Soto C. Stressing out the ER: a role of the unfolded protein response in prion-related disorders. Curr Mol Med. 2006;6(1):37–43. doi: 10.2174/156652406775574578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Pendyala G, Ninemire C, Fox HS. Protective role for the disulfide isomerase PDIA3 in methamphetamine neurotoxicity. PLoS One. 2012;7(6):e38909. doi: 10.1371/journal.pone.0038909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Chang HC, Nathan DF, Lindquist S. In vivo analysis of the Hsp90 cochaperone Sti1 (p60) Mol Cell Biol. 1997;17(1):318–325. doi: 10.1128/mcb.17.1.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Chen S, Prapapanich V, Rimerman RA, et al. Interactions of p60, a mediator of progesterone receptor assembly, with heat shock proteins hsp90 and hsp70. Mol Endocrinol. 1996;10(6):682–693. doi: 10.1210/mend.10.6.8776728. [DOI] [PubMed] [Google Scholar]
- [47].Cizkova D, Carmel JB, Yamamoto K, et al. Characterization of spinal HSP72 induction and development of ischemic tolerance after spinal ischemia in rats. Exp Neurol. 2004;185(1):97–108. doi: 10.1016/j.expneurol.2003.09.020. [DOI] [PubMed] [Google Scholar]
- [48].Kyrou IE, Papakostas JC, Ioachim E, et al. Early ischaemic preconditioning of spinal cord enhanced the binding profile of heat shock protein 70 with neurofilaments and promoted its nuclear translocation after thoraco-abdominal aortic occlusion in pigs. Eur J Vasc Endovasc Surg. 2012;43(4):408–414. doi: 10.1016/j.ejvs.2011.12.028. [DOI] [PubMed] [Google Scholar]
- [49].Selimoglu O, Ugurlucan M, Basaran M, et al. Efficacy of remote ischaemic preconditioning for spinal cord protection against ischaemic injury: association with heat shock protein expression. Folia Neuropathol. 2008;46(3):204–212. [PubMed] [Google Scholar]
- [50].Awad H, Suntres Z, Heijmans J, et al. Intracellular and extracellular expression of the major inducible 70kDa heat shock protein in experimental ischemia-reperfusion injury of the spinal cord. Exp Neurol. 2008;212(2):275–284. doi: 10.1016/j.expneurol.2008.03.024. [DOI] [PubMed] [Google Scholar]
- [51].Beraldo FH, Arantes CP, Santos TG, et al. Role of alpha7 nicotinic acetylcholine receptor in calcium signaling induced by prion protein interaction with stress-inducible protein 1. J Biol Chem. 2010;285(47):36542–36550. doi: 10.1074/jbc.M110.157263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Arantes C, Nomizo R, Lopes MH, et al. Prion protein and its ligand stress inducible protein 1 regulate astrocyte development. Glia. 2009;57(13):1439–1449. doi: 10.1002/glia.20861. [DOI] [PubMed] [Google Scholar]
- [53].Arruda-Carvalho M, Njaine B, Silveira MS, et al. Hop/STI1 modulates retinal proliferation and cell death independent of PrPC. Biochem Biophys Res Commun. 2007;361(2):474–480. doi: 10.1016/j.bbrc.2007.07.038. [DOI] [PubMed] [Google Scholar]
- [54].Smith DF, Whitesell L, Katsanis E. Molecular chaperones: biology and prospects for pharmacological intervention. Pharmacol Rev. 1998;50(4):493–514. [PubMed] [Google Scholar]
- [55].Kingma JG., Jr Cardiac adaptation to ischemia-reperfusion injury. Ann N Y Acad Sci. 1999;874:83–99. doi: 10.1111/j.1749-6632.1999.tb09227.x. [DOI] [PubMed] [Google Scholar]
- [56].Zou N, Ao L, Cleveland JC, Jr, et al. Critical role of extracellular heat shock cognate protein 70 in the myocardial inflammatory response and cardiac dysfunction after global ischemia-reperfusion. Am J Physiol Heart Circ Physiol. 2008;294(6):H2805–2813. doi: 10.1152/ajpheart.00299.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Chen S, Brown IR. Translocation of constitutively expressed heat shock protein Hsc70 to synapse-enriched areas of the cerebral cortex after hyperthermic stress. J Neurosci Res. 2007;85(2):402–409. doi: 10.1002/jnr.21124. [DOI] [PubMed] [Google Scholar]
- [58].Rubio E, Valenciano AI, Segundo C, et al. Programmed cell death in the neurulating embryo is prevented by the chaperone heat shock cognate 70. Eur J Neurosci. 2002;15(10):1646–1654. doi: 10.1046/j.1460-9568.2002.01998.x. [DOI] [PubMed] [Google Scholar]
- [59].Tidwell JL, Houenou LJ, Tytell M. Administration of Hsp70 in vivo inhibits motor and sensory neuron degeneration. Cell Stress Chaperones. 2004;9(1):88–98. doi: 10.1379/CSC-9R.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].McLaughlin B, Hartnett KA, Erhardt JA, et al. Caspase 3 activation is essential for neuroprotection in preconditioning. Proc Natl Acad Sci U S A. 2003;100(2):715–720. doi: 10.1073/pnas.0232966100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Muranyi M, He QP, Fong KS, et al. Induction of heat shock proteins by hyperglycemic cerebral ischemia. Brain Res Mol Brain Res. 2005;139(1):80–87. doi: 10.1016/j.molbrainres.2005.05.023. [DOI] [PubMed] [Google Scholar]
- [62].Kodiha M, Chu A, Lazrak O, et al. Stress inhibits nucleocytoplasmic shuttling of heat shock protein hsc70. Am J Physiol Cell Physiol. 2005;289(4):C1034–1041. doi: 10.1152/ajpcell.00590.2004. [DOI] [PubMed] [Google Scholar]
- [63].Shiota M, Kusakabe H, Izumi Y, et al. Heat shock cognate protein 70 is essential for Akt signaling in endothelial function. Arterioscler Thromb Vasc Biol. 2010;30(3):491–497. doi: 10.1161/ATVBAHA.109.193631. [DOI] [PubMed] [Google Scholar]
- [64].The Ministry of Science and Technology of the People's Republic of China. Guidance Suggestions for the Care and Use of Laboratory Animals. 2006 Sep 30; [Google Scholar]
- [65].Zivin JA, DeGirolami U. Spinal cord infarction: a highly reproducible stroke model. Stroke. 1980;11(2):200–202. doi: 10.1161/01.str.11.2.200. [DOI] [PubMed] [Google Scholar]
- [66].Fehlings MG, Tator CH. The relationships among the severity of spinal cord injury, residual neurological function, axon counts, and counts of retrogradely labeled neurons after experimental spinal cord injury. Exp Neurol. 1995;132(2):220–228. doi: 10.1016/0014-4886(95)90027-6. [DOI] [PubMed] [Google Scholar]


