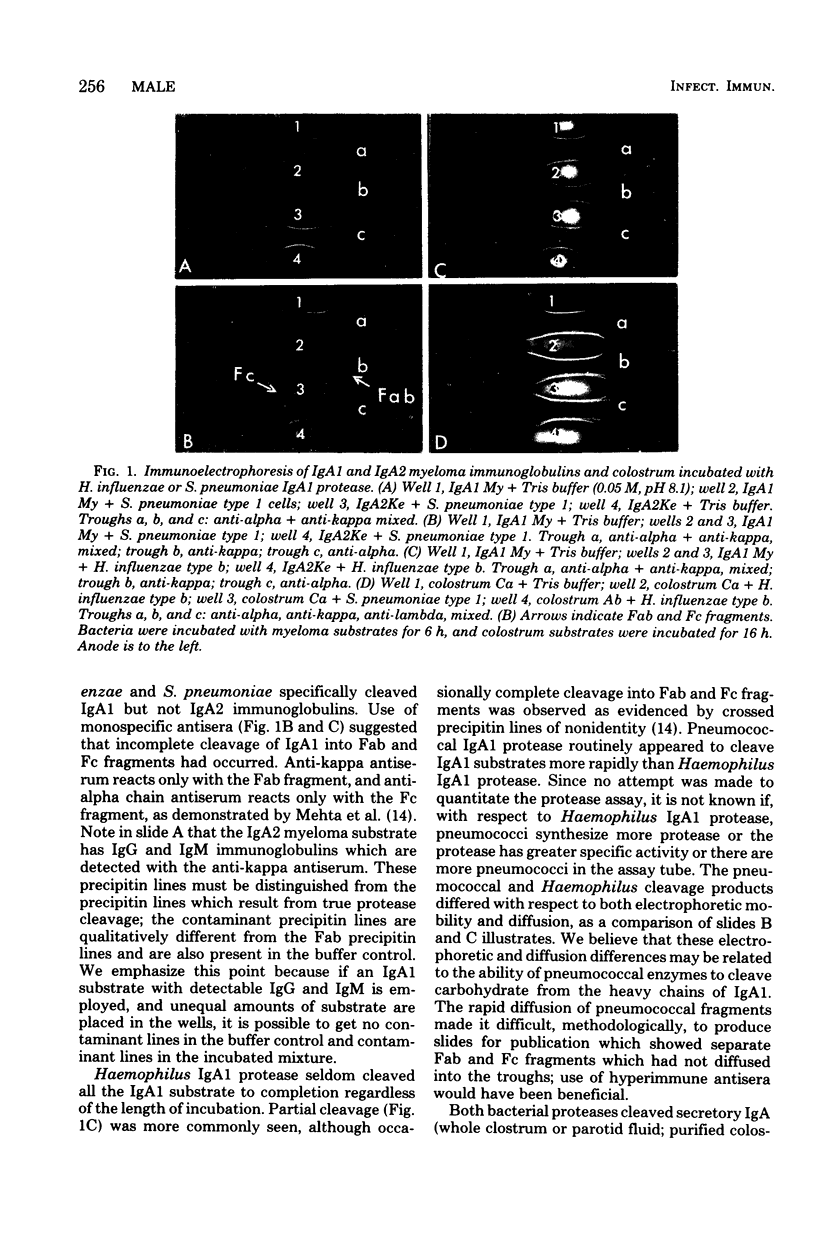
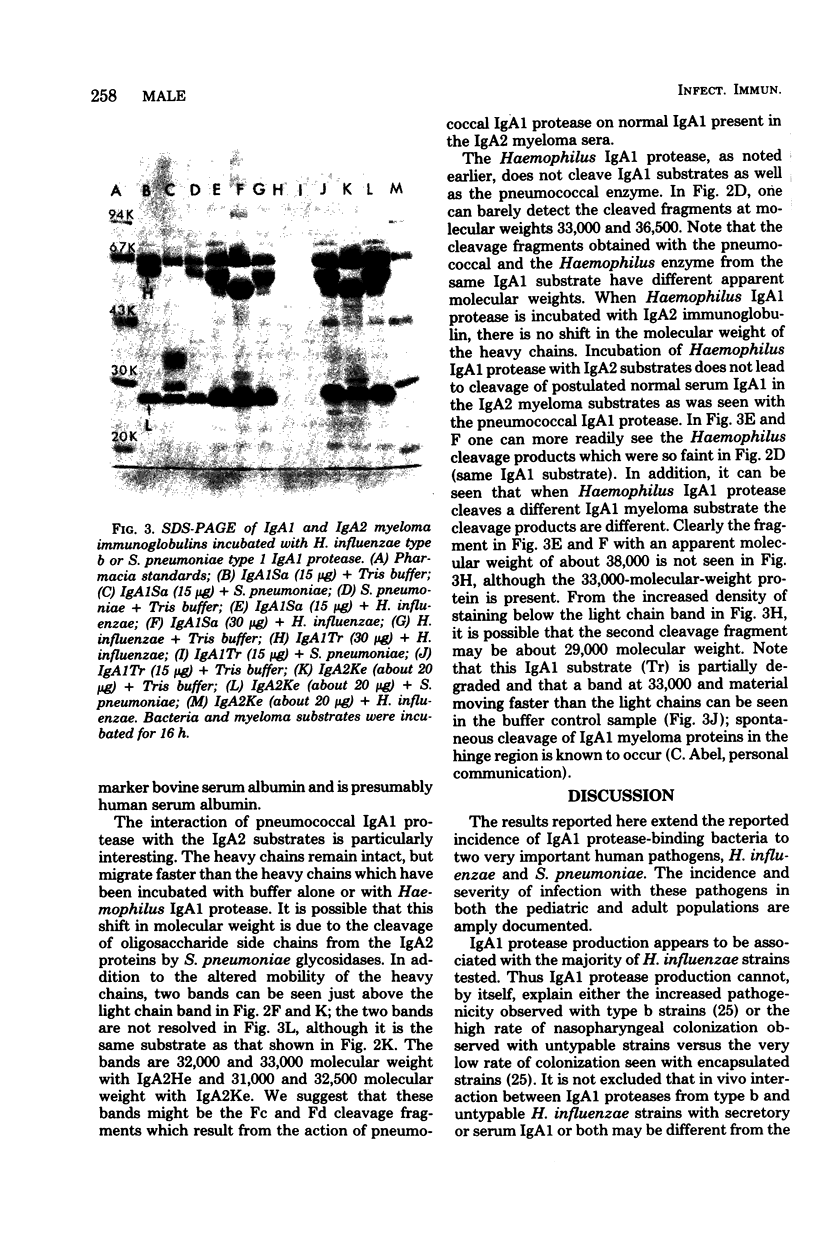
Abstract
Bacterial strains of Haemophilus species and Streptococcus pneumoniae were examined for synthesis of the enzyme immunoglobulin A1 (IgA1) protease. Of 36 H. influenzae strains examined, 35 produced IgA1 protease; strains included all six capsular types, unencapsulated variants of types b and d, and untypable H. influenzae. Eight Haemophilus strains (non-H. influenzae) were studied, and two produced IgA1 protease. All 10 strains of S. pneumoniae produced IgA1 protease; these strains included 9 different capsular polysaccharide types and 1 untypable strain. Both IgA1 proteases cleaved myeloma IgA1 and secretory IgA but not myeloma IgA2, IgM, or IgG as determined by immunoelectrophoresis. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis showed that both enzymes cleaved IgA1 myeloma sera, but not IgA2, into two fragments. The apparent molecular weight of the cleaved fragments was dependent both on the apparent molecular weight of the cleaved fragments was dependent both on the specific IgA1 protease assayed and the specific IgA1 substrate utilized. It is postulated that both carbohydrate variation between the IgA1 substrates studied and the ability of S. pneumoniae glycosidases to cleave carbohydrates from glycoprotein offer an explanation for the different fragment sizes observed.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abel C. A., Spiegelberg H. L., Grey H. M. The carbohydrate contents of fragments and polypeptide chains of human gamma-G-myeloma proteins of different heavy-chain subclasses. Biochemistry. 1968 Apr;7(4):1271–1278. doi: 10.1021/bi00844a004. [DOI] [PubMed] [Google Scholar]
- Baenziger J., Kornfeld S. Structure of the carbohydrate units of IgA1 immunoglobulin. I. Composition, glycopeptide isolation, and structure of the asparagine-linked oligosaccharide units. J Biol Chem. 1974 Nov 25;249(22):7260–7269. [PubMed] [Google Scholar]
- Baenziger J., Kornfeld S. Structure of the carbohydrate units of IgA1 immunoglobulin. II. Structure of the O-glycosidically linked oligosaccharide units. J Biol Chem. 1974 Nov 25;249(22):7270–7281. [PubMed] [Google Scholar]
- Blake M. S., Swanson J. Studies on gonococcus infection. XVI. Purification of Neisseria gonorrhoeae immunoglobulin A1 protease. Infect Immun. 1978 Nov;22(2):350–358. doi: 10.1128/iai.22.2.350-358.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake M., Holmes K. K., Swanson J. Studies on gonococcus infection. XVII. IgA1-cleaving protease in vaginal washings from women with gonorrhea. J Infect Dis. 1979 Jan;139(1):89–92. doi: 10.1093/infdis/139.1.89. [DOI] [PubMed] [Google Scholar]
- Crowle A. J., Cline L. J. An improved stain for immunodiffusion tests. J Immunol Methods. 1977;17(3-4):379–381. doi: 10.1016/0022-1759(77)90122-3. [DOI] [PubMed] [Google Scholar]
- Despont J. P., Abel C. A. Glycopeptides of heavy chains from human IgA myeloma proteins. J Immunol. 1974 May;112(5):1623–1627. [PubMed] [Google Scholar]
- Genco R. J., Plaut A. G., Moellering R. C., Jr Evaluation of human oral organisms and pathogenic Streptococcus for production of IgA protease. J Infect Dis. 1975 May;131 (Suppl):S17–S21. doi: 10.1093/infdis/131.supplement.s17. [DOI] [PubMed] [Google Scholar]
- Koide N., Muramatsu T. Endo-beta-N-acetylglucosaminidase acting on carbohydrate moieties of glycoproteins. Purification and properties of the enzyme from Diplococcus pneumoniae. J Biol Chem. 1974 Aug 10;249(15):4897–4904. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mehta S. K., Plaut A. G., Calvanico N. J., Tomasi T. B., Jr Human immunoglobulin A: production of an Fc fragment by an enteric microbial proteolytic enzyme. J Immunol. 1973 Oct;111(4):1274–1276. [PubMed] [Google Scholar]
- Mulks M. H., Plaut A. G. IgA protease production as a characteristic distinguishing pathogenic from harmless neisseriaceae. N Engl J Med. 1978 Nov 2;299(18):973–976. doi: 10.1056/NEJM197811022991802. [DOI] [PubMed] [Google Scholar]
- O'Toole R. D., Goode L., Howe C. Neuraminidase activity in bacterial meningitis. J Clin Invest. 1971 May;50(5):979–985. doi: 10.1172/JCI106591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaut A. G., Gilbert J. V., Artenstein M. S., Capra J. D. Neisseria gonorrhoeae and neisseria meningitidis: extracellular enzyme cleaves human immunoglobulin A. Science. 1975 Dec 12;190(4219):1103–1105. doi: 10.1126/science.810892. [DOI] [PubMed] [Google Scholar]
- Plaut A. G., Gilbert J. V., Heller I. Assay and properties of IgA protease of Streptococcus sanguis. Adv Exp Med Biol. 1978;107:489–495. doi: 10.1007/978-1-4684-3369-2_55. [DOI] [PubMed] [Google Scholar]
- Plaut A. G., Gilbert J. V., Wistar R., Jr Loss of antibody activity in human immunoglobulin A exposed extracellular immunoglobulin A proteases of Neisseria gonorrhoeae and Streptococcus sanguis. Infect Immun. 1977 Jul;17(1):130–135. doi: 10.1128/iai.17.1.130-135.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaut A. G., Wistar R., Jr, Capra J. D. Differential susceptibility of human IgA immunoglobulins to streptococcal IgA protease. J Clin Invest. 1974 Dec;54(6):1295–1300. doi: 10.1172/JCI107875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reading C. L., Penhoet E. E., Ballou C. E. Carbohydrate structure of vesicular stomatitis virus glycoprotein. J Biol Chem. 1978 Aug 25;253(16):5600–5612. [PubMed] [Google Scholar]
- Toraño A., Tsuzukida Y., Liu Y. S., Putnam F. W. Location and structural significance of the oligosaccharides in human Ig-A1 and IgA2 immunoglobulins. Proc Natl Acad Sci U S A. 1977 Jun;74(6):2301–2305. doi: 10.1073/pnas.74.6.2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virella G., Coelho I. M. Unexpected mobility of human lambda chains in sodium dodecyl sulphate-polyacrylamide gel electrophoresis. Immunochemistry. 1974 Mar;11(3):157–160. doi: 10.1016/0019-2791(74)90213-4. [DOI] [PubMed] [Google Scholar]
- Ward J. I., Gorman G., Phillips C., Fraser D. W. Hemophilus influenzae type b disease in a day-care center. Report of an outbreak. J Pediatr. 1978 May;92(5):713–717. doi: 10.1016/s0022-3476(78)80134-6. [DOI] [PubMed] [Google Scholar]






