Abstract
We selected 106 hemiplegic patients with shoulder pain hospitalized after stroke from three hospitals in Nanjing, China between February 2007 and January 2012. All patients had complete clinical data sets and accounted for 45.5% of the inpatients because of stroke. Results showed that the number of patients with hemiplegic shoulder pain post stroke increased yearly, attacking mainly males 50–69 years of age. Of 106 patients, there were 60 cases (56.6%) of adhesive capsulitis, 19 (17.9%) of shoulder subluxation, 14 (13.2%) of complex regional pain syndrome, and 13 (12.6%) of central pain. The main symptoms were shoulder pain (100%), limit of shoulder mobility (98.1%), and adhesion of the scapula (56.6%). MRI of the shoulder showed tendon and ligament lesions (57.1%) and rotator cuff tear (38.1%). 53.8% of central pain was related to the thalamus, in addition to the basal ganglia, brain stem, and cerebellopontine angle. Shoulder pain, upper limb motor function, and function independence were significantly improved after comprehensive rehabilitation. In particular, electroacupuncture based on basic physical therapy exhibited efficacy on shoulder tion and complex regional pain syndrome. Multiple linear regression results showed a negative relationship of efficacy of pain management with the attack period of shoulder pain, involvement of the posterior limb of the internal capsule, and duration between onset and rehabilitation treatment, but a positive correlation with pain-related education, pain regression period, and pain diagnosis.
Keywords: neural regeneration, brain injury, hemiplegia post stroke, shoulder pain, adhesive capsulitis, shoulder subluxation, complex regional pain syndrome, grants-supported paper, neuroregeneration
Research Highlights
-
(1)
The incidence of shoulder pain post stroke was high. Thus, it is clinically significant to study the onset characteristics and pain management.
-
(2)
We analyzed yearly onset condition, age distribution, classification diagnosis, onset period, major symptoms and signs, imaging examinations, misdiagnosis, rehabilitation treatment, and factors influencing curative effects.
-
(3)
Involvement of the posterior limb of internal capsule and early onset of shoulder pain can reduce the efficacy of pain management, while pain-related education before treatment and early pain regression increased the efficacy of pain management. In addition, diagnosis type of shoulder pain can influence the efficacy of pain management.
INTRODUCTION
Shoulder pain is a common complication of hemiplegic patients post stroke[1]. It is classified into adhesive capsulitis[2], shoulder subluxation, complex regional pain syndrome, and central pain according to the causes. Hemiplegic shoulder pain post stroke prolongs rehabilitation of affected limbs and hospital stay, thereby affecting activities of daily living, and rehabilitation of upper limb and hand function[3,4,5,6]. There is a wide reported range of the incidence of hemiplegic shoulder pain post stroke because of different study methods, period, activity environment, stroke foci, and pain reaction[7,8,9,10]. Furthermore, there are only a few studies of hemiplegic shoulder pain post stroke in China. Although 15 341 patients have been reported between 1999 and 2011, a summary and systematic retrospective analysis of its clinical characteristics remain lacking.
Thus, in this retrospective study, we analyzed clinical data of stroke patients admitted to three hospitals of Nanjing, China between February 2007 and January 2012 to investigate the characteristics of hemiplegic shoulder pain post stroke to provide reference for its diagnosis, prevention and treatment.
RESULTS
Quantitative analysis of participants
A total of 223 stroke patients from three hospitals of Nanjing, China between February 2007 and January 2012 were investigated, and 106 with complete clinical data sets were included in the final analysis after screening (Figure 1).
Figure 1.
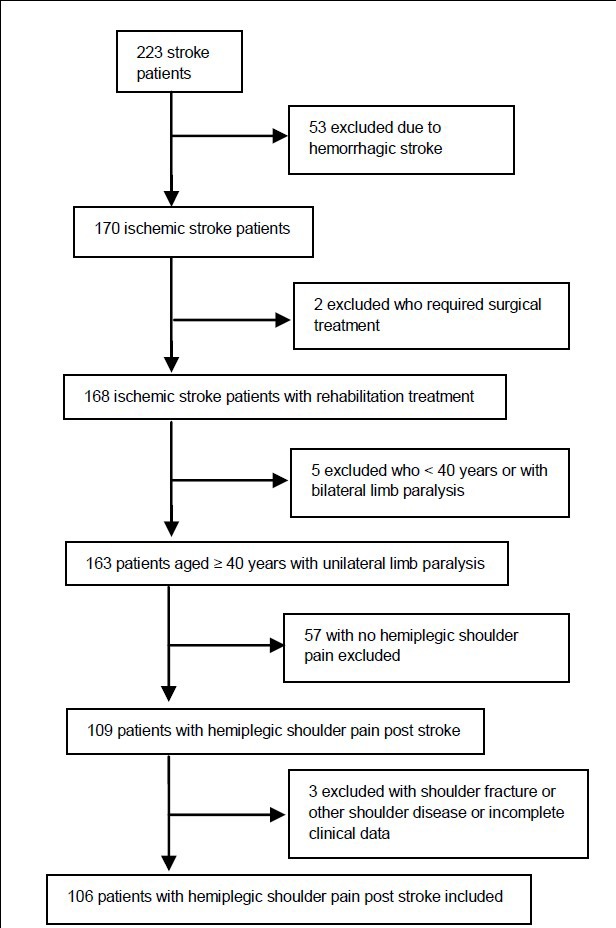
Flowchart of participant screening.
General data of stroke patients with hemiplegic shoulder pain
A total of 106 hospitalized cases with complete clinical data in 223 stroke patients were included. Of these, 106 (45.5%) suffered hemiplegic shoulder pain post stroke, with 65 males and 41 females (ratio of 1.59:1), and a mean age of 57.6 ± 8.8 years (range 40–97 years). The onset age was at 50–59 years in 47.2% of patients and 60–69 years in 35.8% of patients (Figure 2). 74.5% of patients (n = 79) were from town, and 25.5% (n = 27) from the countryside. Of 78 patients recorded with occupational characteristics, 36 (46.1%) were office workers, 25 (32.1%) were physical workers, and 17 (21.8%) were both.
Figure 2.
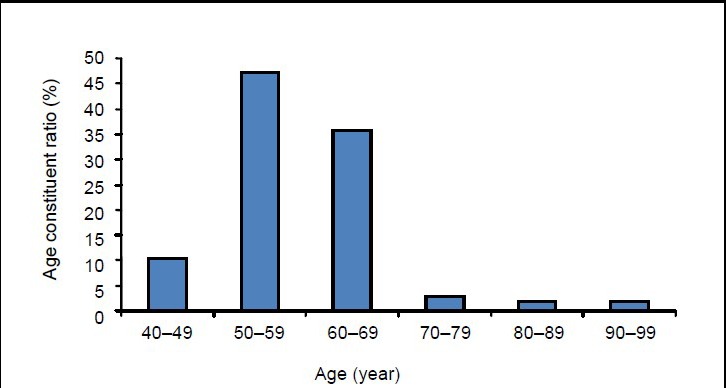
Distribution of age constituent ratio (%) in 106 included patients with hemiplegic shoulder pain post stroke.
The course of disease ranged from 10 days to 1 year, including 2 cases (1.9%) of 1 month, 19 (17.9%) of 1–3 months, 35 (33.0%) of 3–6 months, and 50 (47.2%) of 6 months to 1 year. Of 106 patients, there were 60 cases (56.6%) of adhesive capsulitis, 19 (17.9%) of shoulder subluxation, 14 (13.2%) of complex regional pain syndrome, and 13 (12.6%) of central pain (Figure 3).
Figure 3.
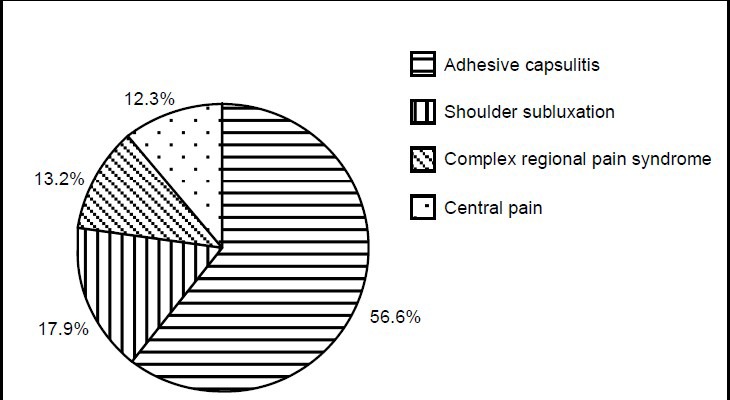
Constituent ratio (%) of classification of hemiplegic shoulder pain in 106 stroke patients.
Adhesive capsulitis occurred in 15.0% of 60 patients in 1 month post stroke, in 75.0% of patients in 1–3 months, and 10.0% of patients in 3–6 months. Among the 19 patients with shoulder subluxation, 84.2% occurred in 1 month post stroke and 15.8% in 1–3 months. Among the 14 patients with complex regional pain syndrome, 23.1% occurred in 1 month post stroke and 76.9% in 1–3 months. Central pain occurred in 57.1% of 13 patients in 1–3 months post stroke and 42.9% in 3–6 months (Figure 4).
Figure 4.
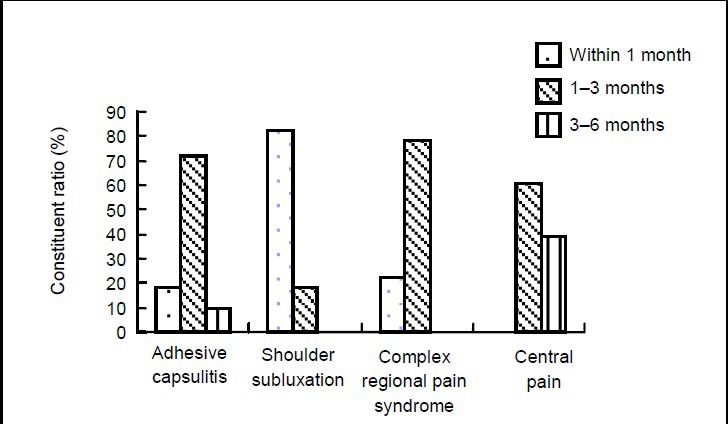
Constituent ratio (%) of onset period of hemiplegic shoulder pain in 106 stroke patients. As only a few patients developed shoulder pain after 6 months, they were not included in the statistical analysis.
The pain disappeared in 23.3% of patients with adhesive capsulitis in 1–6 months, while 76.7% disappeared after 6 months. The pain was attenuated in 52.6% of patients with shoulder subluxation in 1 month, and 47.4% disappeared after 1 month. The pain disappeared in 15.4% of patients with complex regional pain syndrome in 1–6 months, and 84.6% after 6 months. The pain in 7.1% of patients with central pain disappeared in 6 months, while 92.9% felt pain after 6 months.
Yearly treatment of patients
Yearly treatment of stroke patients and shoulder pain patients post stroke in three hospitals of Nanjing, China between February 2007 and January 2012 was analyzed (Figure 5). Over the past 5 years, the number of hospitalizations because of stroke or shoulder pain post stroke gradually increased, as did the constituent ratio of shoulder pain post stroke (1.95- and 1.81-fold higher in 2010 and 2011, respectively, than in 2007).
Figure 5.
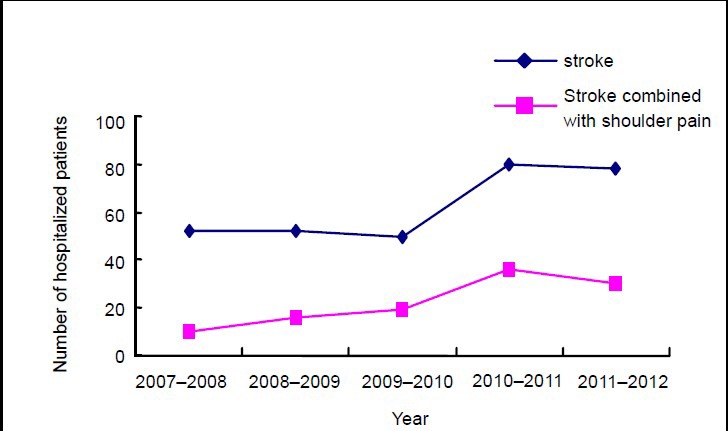
Number (n) of hospitalized patients due to stroke or shoulder pain post stroke between February 2007 and January 2012.
Major symptoms and signs of patients with post-stroke shoulder pain
The main symptoms were described as shoulder pain (100%), limit of shoulder mobility (98.1%), adhesion of scapula (56.6%), depression inferior to acromion (17.9%), swelling in the arm, wrist and fingers of the affected side (17.0%), abnormal skin temperature and hyperhidrosis (19.8%), altered skin color (13.2%), and limit of finger flexion (92.4%).
Imaging manifestations of patients with shoulder pain post stroke
MRI of head showed that the shoulder pain in 12 (11.3%) of 106 stroke patients was related to the thalamus, 7 (7/13, 53.8%) subjects suffered central pain, 4 (4/60, 6.7%) suffered adhesive capsulitis, and 1 (1/14, 7.1%) suffered complex regional pain syndrome. The pain in 75 of patients (70.8%) was related to the basal ganglia, and 47 (47/60, 78.3%) of them suffered adhesive capsulitis, 14 (14/19, 73.7%) suffered shoulder subluxation, 11 (11/14, 78.6%) had complex regional pain syndrome, and 3 (3/13, 23.1%) suffered central pain. MRI of the head of patients with central pain showed that pain was related to the internal capsule in 85.7% of patients. The pain of 19 patients (17.9%) was related to other sites, including 9 cases (9/60, 15.0%) of adhesive capsulitis, 5 (5/19, 26.3%) of shoulder subluxation, 2 (2/14, 14.3%) of complex regional pain syndrome, and 3 (3/13, 23.1%) of central pain (1 related to the cerebellopontine angle and 2 related to the brain stem and cerebellopontine angle).
MRI of the shoulder of 42 stroke patients with hemiplegic shoulder pain showed tendon and ligament lesions (57.1%) and rotator cuff tear (38.1%). Of 22 patients with adhesive capsulitis, 8 and 7 suffered rotator cuff tear, 14 and 6 suffered tendon and ligament lesions, and 9 and 8 suffered intra-articular hemorrhage prior to and following treatment, respectively. Of 9 patients with shoulder subluxation, 4 and 3 suffered rotator cuff tear, 5 and 1 suffered tendon and ligament lesions, and 6 and 4 suffered intra-articular hemorrhage prior to and following treatment, respectively. Of 7 patients with complex regional pain syndrome, 3 (42.9%) and 1 (50.0%) suffered rotator cuff tear, 4 (57.1%) and 1 (50.0%) suffered tendon and ligament lesions, and 1 (14.3%) and 0 suffered intra-articular hemorrhage prior to and following treatment, respectively. Of 4 patients with central pain, 1 had myotenositis and was cured after treatment for 4 weeks. However, rotator cuff tear and intra-articular hemorrhage were not significantly improved.
Misdiagnosis
In 223 stroke patients, 126 were complicated with shoulder pain, 5 suffered adhesive capsulitis at the healthy side which was misdiagnosed as shoulder pain post stroke, 3 had upper humeral fracture and shoulder dislocation due to falling in hospital, which was misdiagnosed as shoulder pain post stroke, and 12 with shoulder subluxation were misdiagnosed as complex regional pain syndrome.
Rehabilitation treatment
Of 106 patients with shoulder pain, 51 were subjected to pain education prior to treatment. The 60 patients with adhesive capsulitis were treated with joint mobilization, including 24 patients (40.0%) additionally subjected to regional electroacupuncture and 29 (48.3%) subjected to manipulation. The 19 patients with shoulder subluxation were treated by correcting scapular posture, stimulating the tension and activity of muscles surrounding the shoulder, and passive activities of the shoulder joint in a pain-free range, including 9 (47.4%) additional subjected to regional electroacupuncture and 10 (52.6%) subjected to manipulation. The 14 patients with complex regional pain syndrome were treated with desensitization, wax therapy, and joint motion, including 7 (53.8%) additional subjected to regional electroacupuncture and 5 (38.5%) subjected to manipulation. The 13 patients with central pain were subjected to pain management and joint motion, as well as antidepressant drugs if necessary, including 2 (14.3%) additional treated with manipulation. All the treatments were conducted once daily, 30–45 minutes for each treatment, with 4 weeks as one course of treatment.
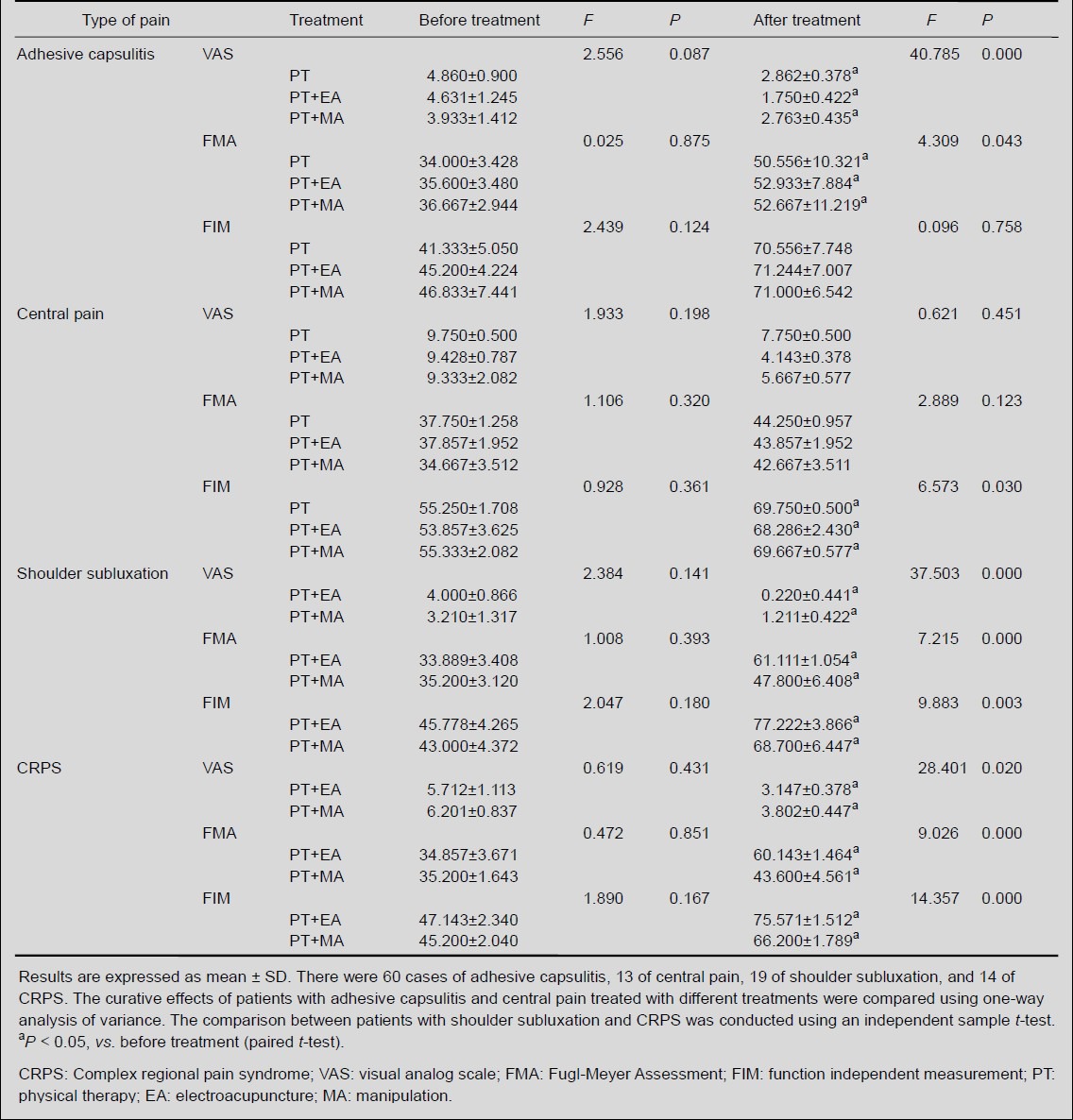
Following 4 weeks of rehabilitation treatment, the shoulder pain, upper limb motion, and function independence were significantly improved (Table 1). Analysis of variance showed that upper limb motor function and pain were significantly improved in patients with adhesive capsulitis treated with electroacupuncture compared with those treated with simple physical therapy and manipulation (P < 0.05). Independent sample t-test showed that for patients with shoulder subluxation or complex regional pain syndrome, physical therapy in combination with electroacupuncture significantly ameliorated upper limb motion and pain compared with physical therapy in combination with manipulation (P < 0.05). Analysis of variance showed that physical therapy alone or in combination with electroacupuncture and manipulation exhibited similar effects on upper limb motion and pain of patients with central pain (P > 0.05), but physical therapy in combination with electroacupuncture significantly improved function independence. In addition, 61.5% (n = 8) of patients with central pain received antidepressant drugs 3 months after shoulder pain.
Table 1.
Pain, upper limb function, and function independence in patients with shoulder pain post stroke prior to and following various treatments
Factors related to curative effects of hemiplegic shoulder pain
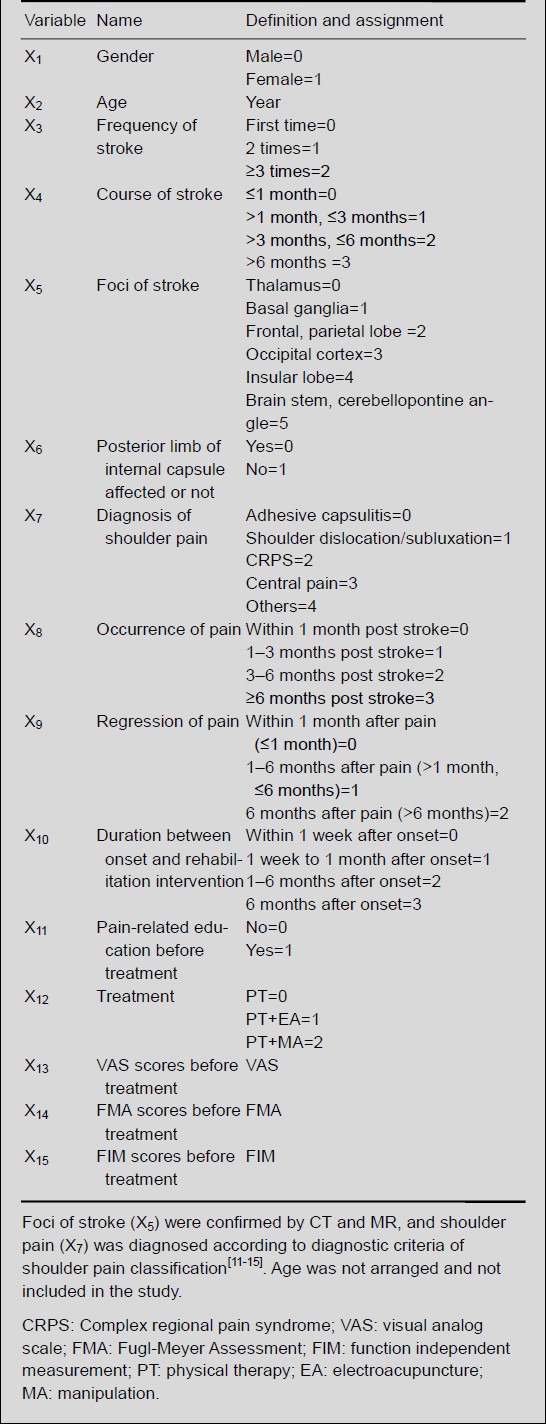
Pain management included an evaluation system of pain, formulation of therapy for pain, management of general condition of patients, pain relief, and pain-related education. To understand the influential factors in pain management, we assigned the related factors in the investigation as described in Table 2.
Table 2.
Definition and assignment of related factors influencing recovery of hemiplegic shoulder pain post stroke
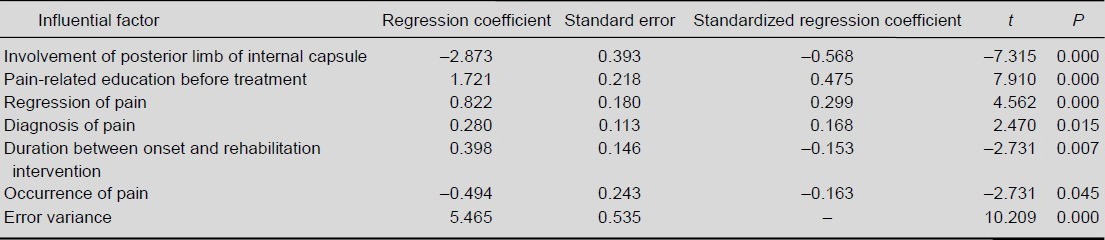
Multiple linear regression showed that the efficacy of pain management was negatively correlated with involvement of the posterior limb of the internal capsule, time of shoulder pain, and duration between onset and rehabilitation intervention, but positively correlated with pain-related education before treatment, regression of pain, and diagnosis of pain (Table 3). R2 = 0.736 indicates that 73.6% of patients were in accordance with the statistical results: Y1= 5.465 – 2.873X6 + 1.721X11 + 0.822X9 + 0.280X7 + 0.398X10 – 0.494X8.
Table 3.
Multiple linear regression analysis of factors influencing effectiveness of treatments for hemiplegic shoulder pain post stroke
DISCUSSION
Onset characteristics of hemiplegic shoulder pain post stroke
The present study retrospectively reviewed the diagnosis, treatment, and yearly treatment of 310 stroke patients with hemiplegic shoulder pain from three hospitals in Nanjing, China. Several characteristics were found, as follows:
(1) Incidence gradually increased: Pinedo et al[16] retrospectively analyzed the incidence of complications at 1 year post stroke and found that the incidence of shoulder pain was the highest, accounting for 40%. In the present study, the increase in the number of patients with hemiplegic shoulder pain may be associated with the increase of stroke incidence, diagnostic criteria of shoulder pain and modified study methods, or increased attention of physician on hemiplegic shoulder pain post stroke. In addition, 74.5% of patients were from the town where the study was conducted, 67.9% of whom were office or physical workers, indicating that environment is associated with the incidence of hemiplegic shoulder pain post stroke.
(2) Adhesive capsulitis was the main cause: Of 106 patients with shoulder pain, the incidence of adhesive capsulitis was 56.6%, shoulder subluxation was 17.9%, complex regional pain syndrome was 13.2%, and central pain was 12.3%. In another study, 50% of stroke patients suffered adhesive capsulitis, 44% had shoulder subluxation, and 16% had complex regional pain syndrome[17]. Kendall[18] reported that rotator cuff tear was the major cause, with 50–81% of stroke patients developing shoulder subluxation. In another study of 114 stroke patients with shoulder pain, 71 suffered shoulder subluxation, 70 had complex regional pain syndrome, 70 had shoulder impingement syndrome, 49 suffered adhesive capsulitis, and 10 had thalamic pain[19]. Compared with these studies, the incidence of shoulder subluxation was lower in the present study, possibly due to early appropriate nursing and rehabilitation intervention.
(3) Significant differences in rehabilitation of shoulder pain resulted from different causes: MRI suggested that rotator cuff tendon and ligament lesions were significantly improved after 4 weeks of comprehensive rehabilitation treatment, while rotator cuff tear in patients with adhesive capsulitis remained unchanged. Yamamoto et al[20] reported that 20.7% of people at a mean age of 57.9 years suffered full-thickness tear of the rotator cuff. Other studies reported proliferation of fibroblast and thickening of vessel wall in the articular capsule of the rotator cuff[21,22]. Thus, the remaining rotator cuff tear following 4 weeks of treatment may result from a large number of fibroblasts and type III collagen, which promoted scar formation and reduced tissue elasticity. In addition, the adhesive capsulitis always complicates with tendon and ligament lesions. A MRI examination showed thickening of the coracohumeral ligament and the joint capsule in the rotator cuff interval, as well as the subcoracoid triangle sign, in patients with periarthritis of the shoulder[23].
In the present study, the incidence of hemorrhage in the joint capsule was higher than rotator cuff tear and tendon and ligament lesions following 4 weeks of treatment, indicating that the comprehensive treatment could not control hemorrhage in the joint capsule when compared with shock wave therapy, which could promote resorption of the hemorrhage in the joint capsule[24,25].
(4) Onset of shoulder pain of different types was varied: A perspective study showed that 34% of stroke patients had shoulder pain, 28% of which occurred in 2 weeks, and 87% of which occurred in 2 months[26]. In addition, 80% of shoulder pain regressed after 6 months. The occurrence of shoulder pain may be associated with muscle spasm of the shoulder joint post stroke. In the present study, shoulder subluxation occurred within 1 month post stroke, possibly because of reduced rotator cuff muscle force. Adhesive capsulitis and complex regional pain syndrome always appeared in 1–3 months, while central pain was found at 3 months post stroke (57.1% in 1–3 months, and 42.9% in 3–6 months). 53.8% of central pain was related to the thalamus, 23.1% to basal ganglia, and 23.1% to the brain stem or cerebellopontine angle. There are few reports regarding correlation between shoulder pain and stroke foci. Nevertheless, the thalamus is a relay station of sensory information transfer, and any lesion in the spinothalamic pathway or cortical projection fibers was reported to attenuate central inhibition and result in central pain[27,28,29].
Effectiveness of pain management
In the present study, the degree of shoulder pain, upper limb function, and function independence in 106 patients were significantly improved following comprehensive rehabilitation treatment. Physical therapy plus regional electroacupuncture exhibited better effects on pain due to shoulder subluxation and complex regional pain syndrome compared with the other therapies, consistent with previous results[30,31]. Functional neuromuscular electrical stimulation can significantly relieve hemiplegic shoulder pain post stroke compared with body surface electrical stimulation[32]. High voltage pulse can effectively improve shoulder subluxation and relieve pain[33]. Physical therapy, electroacupuncture, and manipulation produced a significant improvement in upper limb function and function independence. Functional electrical stimulation effectively improved upper limb function in patients with hemiplegic shoulder pain[34,35,36]. Electrical nerve stimulation can promote recovery of muscles surrounding the shoulder pain and relieve the should pain[37]. Botulinus toxin in combination with physical therapy has been shown to attenuate hemiplegic shoulder pain[38]. Segmental neuromyotherapy could also relieve hemiplegic shoulder pain and arm function[39].
In the present study, the effectiveness of pain management was negatively correlated with involvement of the posterior limb of the internal capsule and time of shoulder pain, and the duration between onset and rehabilitation intervention, but positively correlated with pain-related education before treatment, regression of pain, and diagnosis of pain. Stroke patients with an affected internal capsule mainly suffered central pain, which occurred at 3 months post stroke. As the pathogenesis is complex, there is no effective analgesia method. Moreover, the later that shoulder pain occurred, the more difficult the diagnosis. Thus, pain management was negatively correlated with involvement of the posterior limb of the internal capsule and time of shoulder pain. Pain-related education prior to treatment can provide a better understand of pain cause, characteristics, treatment protocols, course of treatment, cost, and prognosis, and promote the active participation of patients in pain management. Thus, outcomes were positively correlated with pain- related education before treatment. In this study, shoulder pain, not central pain, disappeared in 76.7% of patients. The longer the duration from shoulder pain onset, the milder the pain. Diagnosis of pain is beneficial to formulating treatment protocols and prevention of re-injury, and can promote efficacy of pain management.
Several factors can influence pain management, including diagnosis of pain, time of onset, regression of pain, and involvement of the posterior limb of the internal capsule. Therefore, the type of shoulder pain and time of onset can provide reference for prevention of shoulder pain and reduce the rate of misdiagnosis. Moreover, they can influence the prognosis and effectiveness of pain management in the early period of shoulder pain.
In conclusion, to our knowledge there are no previous reports on the incidence and morbidity of hemiplegic shoulder pain post stroke in China. The present study selected stroke patients over 5 years from three hospital of Nanjing, China. However, a large-scale retrospective study and multi-center clinical randomized controlled trails are needed to study characteristics of hemiplegic shoulder pain post stroke. In addition, the differences in our study compared with those outside China may be attributed to regional and cultural differences. Thus, multicenter epidemiological investigations based on different populations are necessary to determine the incidence of hemiplegic shoulder pain post stroke in China, to search for potential etiological factors, and to further our understanding of hemiplegic shoulder pain post stroke.
SUBJECTS AND METHODS
Design
Retrospective case analysis.
Time and setting
The study was conducted in the Department of Acupuncture Rehabilitation, Jiangsu Province Hospital of Traditional Chinese Medicine, Ruihaibo Medical Rehabilitation Center, and Department of Acupuncture Rehabilitation, Jiangsu Province Second Hospital of Traditional Chinese Medicine between February 2007 and January 2012.
Subjects
Stroke patients admitted to the Department of Acupuncture Rehabilitation, Jiangsu Province Hospital of Traditional Chinese Medicine, Ruihaibo Medical Rehabilitation Center, and Department of Acupuncture Rehabilitation, Jiangsu Province Second Hospital of Traditional Chinese Medicine between February 2007 and January 2012 were selected. Patients were diagnosed according to the criteria of clinical manifestations and diagnosis of hemiplegic shoulder pain of Chinese Standards for Diagnosis and Treatment of Rehabilitation Medicine, formulated by the Department of Medical Administration, Ministry of Health, China in 1999[11]. Adhesive capsulitis was diagnosed according to the Diagnostic Standard of Traditional Chinese Medicine Syndrome, formulated by the State Administration of Traditional Chinese Medicine[12] and confirmed by special examination and imaging evidence[13]. Shoulder subluxation was diagnosed based on related clinical manifestations and diagnosis of Chinese Standards for Diagnosis and Treatment of Rehabilitation Medicine[15]. Complex regional pain syndrome was diagnosed according to the standard of the International Association for the Study of Pain: important nerve injury as complex regional pain syndrome II; no important nerve injury as complex regional pain syndrome I[14]. The diagnostic criteria of central pain included several aspects: (1) central nervous system disease exists with possible lesion and functional disturbance at any level of the neural axis; (2) pain appears immediately or later after onset of the disease; (3) the pain may be constant, intermittent, or paroxysmal, or present in a hyperalgesia manner; (4) the pain appears in different types at one or different sites; (5) the intensity of pain is different, and the pain can be induced in response to various internal and external stimuli, such as touching, cold, or sudden excitation; (6) somatic sensation is abnormal, manifested by abnormal pain, hyperesthesia, or parasthesia, dysesthetic pain; (7) the presence or absence of non-sensory neurologic symptoms and signs (no association between central pain and incoordination); (8) the presence or absence of psychological and mental disturbances (the majority of patients with central pain are normal); (9) the pain should not be psychological; (10) the diagnosis of some central pain should be confirmed using clinical background and diagnostic standards based on laboratory examinations (quantitative sensory test, somatosensory evoked potential, cerebrospinal fluid, electromyogram, CT and MRI)[40] to differentiate that the pain is not nociception or from a peripheral nerve[14].
Inclusion criteria
The patients were diagnosed in accordance with cerebral infarction criteria of Cerebrovascular Disease Diagnosis Points, formulated by the Fourth National Conference on Cardiovascular Disease in 1995[41] and confirmed by CT or MRI. They were aged ≥ 40 years and admitted to our hospital within 1 month after onset. All patients exhibited unilateral limb paralysis, with no audiovisual disturbance or psychiatric history. Patients cooperated well with the study.
Exclusion criteria
Patients were excluded if they had brain trauma or cerebral hemorrhage induced by coagulation disorders, aneurysm, arteriovenous malformation, or brain tumor; if they suffered upper limb or shoulder fracture due to accident at 3 months before or after onset; or if they exhibited shoulder subluxation, periarthritis of the shoulder, or long-period of pain before admission. In addition, patients with incomplete clinical data were not included.
A total of 106 stroke patients with hemiplegic shoulder pain were included. They all underwent physical therapy, and additional manipulation or acupuncture if they wanted.
Methods
Investigation methods
The questionnaire was self-designed, and was filled out by professional physicians. For patients treated several times, they filled the hospitalization data for the first time, and the other clinical data were regarded as follow-up data. The questionnaire included several items: (1) general items of stroke patients: gender, age, occupation, and course of disease; (2) classification and treatment of stroke and shoulder pain: clinical type of stroke (hemorrhagic, ischemic), staging (frequency of stroke, course of disease; period of shoulder pain onset and regression), type of shoulder pain, course of stroke and shoulder pain, major symptoms and signs, auxiliary examinations, evaluation methods, diagnosis methods, differential diagnosis, and treatment (drugs, curative effects, and outcomes).
Evaluation methods
The pain degree of 106 patients was assessed by rehabilitation physicians at 1 week before treatment and 1 week before discharge using visual analogue scales (0–10 scores, 0: no pain; 10: severe pain[42]). Fugl-Meyer Assessment was used to assess the upper limb motor function (66 scores in total; higher scores represent better function)[43]. Function independence was assessed using function independent measures (18 items in total, each item scored from 7–1, with highest scores of 126; higher scores represent better independence)[44].
Statistical analysis
Data were analyzed using descriptive statistics. Numeration data were expressed as rate, and measurement data as mean ± SD. The curative effects of patients with adhesive capsulitis and central pain treated with different treatments were compared using a one-way analysis of variance. The comparison between patients with shoulder subluxation and complex regional pain syndrome was conducted using an independent sample t-test. The comparison between before and after treatment was conducted using a paired t-test. General condition and treatment-related information of 106 cases were analyzed using multiple linear stepwise regression to explore influential factors of pain management. A value of P < 0.05 was considered statistically significant.
Footnotes
Funding: This study was supported by the Qinglan Engineering of Higher Institutes Foundation for Outstanding Young Teachers of Jiangsu Province in China.
Conflicts of interest: None declared.
Ethical approval: This study received permission from the Ethics Committee of Nanjing University of Traditional Chinese Medicine, China.
(Reviewed by Dean J, Wysong S, Yang W, Sui NB)
(Edited by Wang LM, Su LL, Li CH, Song LP, Liu WJ, Zhao M)
REFERENCES
- 1.Suethanapornkul S, Kuptniratsaikul PS, Kuptniratsaikul V, et al. Post stroke shoulder subluxation and shoulder pain: a cohort multicenter study. J Med Assoc Thai. 2008;91(12):1885–1892. [PubMed] [Google Scholar]
- 2.Bentley S, Dalgleish A, Taylor W, editors. Auckland: New Zealand Guidelines Group; 2011. The Diagnosis and Management of Soft Tissue Shoulder Injuries and Related Disorders: Best Practice Evidence-Based Guideline. [Google Scholar]
- 3.Roosink M, Geurts AC, Ijzerman MJ. Defining post-stroke pain: diagnostic challenges. Lancet Neurol. 2010;9(4):344–345. doi: 10.1016/S1474-4422(10)70072-7. [DOI] [PubMed] [Google Scholar]
- 4.Lindgren I, Jönsson AC, Norrving B, et al. Shoulder pain after stroke: a prospective population-based study. Stroke. 2007;38(2):343–348. doi: 10.1161/01.STR.0000254598.16739.4e. [DOI] [PubMed] [Google Scholar]
- 5.Walsh K. Management of shoulder pain in patients with stroke. Postgrad Med J. 2001;77(912):645–649. doi: 10.1136/pmj.77.912.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smith M. Management of hemiplegic shoulder pain following stroke. Nurs Stand. 2012;26(44):35–44. doi: 10.7748/ns2012.07.26.44.35.c9191. [DOI] [PubMed] [Google Scholar]
- 7.Teasell RW, Foley NC, Bhogal SK, et al. An evidence- based review of stroke rehabilitation. Top Stroke Rehabil. 2003;10(1):29–58. doi: 10.1310/8YNA-1YHK-YMHB-XTE1. [DOI] [PubMed] [Google Scholar]
- 8.Klit H, Finnerup NB, Overvad K, et al. Pain following stroke: a population-based follow-up study. PLoS One. 2011;6(11):e27607. doi: 10.1371/journal.pone.0027607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Oliveira RA, de Andrade DC, Machado AG, et al. Central poststroke pain: somatosensory abnormalities and the presence of associated myofascial pain syndrome. BMC Neurol. 2012;12:89. doi: 10.1186/1471-2377-12-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Snels IA, Dekker JH, van der Lee JH, et al. Treating patients with hemiplegic shoulder pain. Am J Phys Med Rehabil. 2002;81(2):150–160. doi: 10.1097/00002060-200202000-00013. [DOI] [PubMed] [Google Scholar]
- 11.Chinese Journal of Rehabilitation. 2nd ed. Beijing: Huaxia Press; 1999. Department of Medical Administration, Ministry of Health, People's Republic of China. [Google Scholar]
- 12.Nanjing: Nanjing University Press; 1994. The State Administration of Traditional Chinese Medicine. The Doctor Of Traditional Chinese Medicine Syndrome Diagnosis Effect Standard. [Google Scholar]
- 13.Hu YP, Diao X, Yang YK. Frozen shoulder clinical curative effect evaluation methods profile. Jiangxi Zhongyiyao. 2007;9(4):63–66. [Google Scholar]
- 14.Harden RN, Bruehl SP. Diagnosis of complex regional pain syndrome: signs, symptoms, and new empirically derived diagnostic criteria. Clin J Pain. 2006;22(5):415–419. doi: 10.1097/01.ajp.0000194279.36261.3e. [DOI] [PubMed] [Google Scholar]
- 15.Wasner G. Central pain syndromes. Curr Pain Headache Rep. 2010;14(6):489–496. doi: 10.1007/s11916-010-0140-8. [DOI] [PubMed] [Google Scholar]
- 16.Pinedo S, de la Villa FM. Complications in the hemiplegic patient in the first year after the stroke. Rev Neurol. 2001;32(3):206–209. [PubMed] [Google Scholar]
- 17.Lo SF, Chen SY, Lin HC, et al. Arthrographic and clinical findings in patients with hemiplegic shoulder pain. Arch Phys Med Rehabil. 2003;84(12):1786–1791. doi: 10.1016/s0003-9993(03)00408-8. [DOI] [PubMed] [Google Scholar]
- 18.Kendall R. Musculoskeletal problems in stroke survivors. Top Stroke Rehabil. 2010;17(3):173–178. doi: 10.1310/tsr1703-173. [DOI] [PubMed] [Google Scholar]
- 19.Barlak A, Unsal S, Kaya K, et al. Poststroke shoulder pain in Turkish stroke patients: relationship with clinical factors and functional outcomes. Int J Rehabil Res. 2009;32(4):309–315. doi: 10.1097/MRR.0b013e32831e455f. [DOI] [PubMed] [Google Scholar]
- 20.Yamamoto A, Takagishi K, Osawa T, et al. Prevalence and risk factors of a rotator cuff tear in the general population. J Shoulder Elbow Surg. 2010;19(1):116–120. doi: 10.1016/j.jse.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 21.Kilian O, Kriegsmann J, Berghäuser K, et al. The frozen shoulder. Arthroscopy, histological findings and transmission electron microscopy imaging. Chirurg. 2001;72(11):1303–1308. doi: 10.1007/s001040170036. [DOI] [PubMed] [Google Scholar]
- 22.Omari A, Bunker TD. Open surgical release for frozen shoulder: surgical findings and results of the release. J Shoulder Elbow Surg. 2001;10(4):353–357. doi: 10.1067/mse.2001.115986. [DOI] [PubMed] [Google Scholar]
- 23.Mengiardi B, Pfirrmann CW, Gerber C, et al. Frozen shoulder: MR arthrographic findings. Radiology. 2004;233(2):486–492. doi: 10.1148/radiol.2332031219. [DOI] [PubMed] [Google Scholar]
- 24.Engebretsen K, Grotle M, Bautz-Holter E, et al. Supervised exercises compared with radial extracorporeal shock-wave therapy for subacromial shoulder pain: 1-year results of a single-blind randomized controlled trial. Phys Ther. 2011;91(1):37–47. doi: 10.2522/ptj.20090338. [DOI] [PubMed] [Google Scholar]
- 25.Tornese D, Mattei E, Bandi M, et al. Arm position during extracorporeal shock wave therapy for calcifying tendinitis of the shoulder: a randomized study. Clin Rehabil. 2011;25(8):731–739. doi: 10.1177/0269215510396740. [DOI] [PubMed] [Google Scholar]
- 26.Gamble GE, Barberan E, Laasch HU, et al. Poststroke shoulder pain: a prospective study of the association and risk factors in 152 patients from a consecutive cohort of 205 patients presenting with stroke. Eur J Pain. 2002;6(6):467–474. doi: 10.1016/s1090-3801(02)00055-1. [DOI] [PubMed] [Google Scholar]
- 27.Klit H, Finnerup NB, Jensen TS. Central post-stroke pain: clinical characteristics, pathophysiology, and management. Lancet Neurol. 2009;8(9):857–868. doi: 10.1016/S1474-4422(09)70176-0. [DOI] [PubMed] [Google Scholar]
- 28.Kumar B, Kalita J, Kumar G, et al. Central poststroke pain: a review of pathophysiology and treatment. Anesth Analg. 2009;108(5):1645–1657. doi: 10.1213/ane.0b013e31819d644c. [DOI] [PubMed] [Google Scholar]
- 29.Demasles S, Peyron R, Garcia Larrea L, et al. Central post-stroke pain. Rev Neurol (Paris) 2008;164(10):825–831. doi: 10.1016/j.neurol.2008.07.016. [DOI] [PubMed] [Google Scholar]
- 30.Roosink M, Renzenbrink GJ, Geurts AC, et al. Towards a mechanism-based view on post-stroke shoulder pain: theoretical considerations and clinical implications. Neuro Rehabilitation. 2012;30(2):153–165. doi: 10.3233/NRE-2012-0739. [DOI] [PubMed] [Google Scholar]
- 31.Vuagnat H, Chantraine A. Shoulder pain in hemiplegia revisited: contribution of functional electrical stimulation and other therapies. J Rehabil Med. 2003;35(2):49–56. doi: 10.1080/16501970306111. [DOI] [PubMed] [Google Scholar]
- 32.Yu XW, Zhang Z, Yang J, et al. Quantitative evaluations of ischemic stroke based on meta-analysis. Zhonghua Yi Xue Za Zhi. 2011;91(43):3066–3070. [PubMed] [Google Scholar]
- 33.Fil A, Armutlu K, Atay AO, et al. The effect of electrical stimulation in combination with Bobath techniques in the prevention of shoulder subluxation in acute stroke patients. Clin Rehabil. 2011;25(1):51–59. doi: 10.1177/0269215510375919. [DOI] [PubMed] [Google Scholar]
- 34.Chan MK, Tong RK, Chung KY. Bilateral upper limb training with functional electric stimulation in patients with chronic stroke. Neurorehabil Neural Repair. 2009;23(4):357–365. doi: 10.1177/1545968308326428. [DOI] [PubMed] [Google Scholar]
- 35.Yasar E, Vural D, Safaz I, et al. Which treatment approach is better for hemiplegic shoulder pain in stroke patients: intra-articular steroid or suprascapular nerve block?A randomized controlled trial. Clin Rehabil. 2011;25(1):60–68. doi: 10.1177/0269215510380827. [DOI] [PubMed] [Google Scholar]
- 36.Ada L, Foongchomcheay A. Efficacy of electrical stimulation in preventing or reducing subluxation of the shoulder after stroke: a meta-analysis. Aust J Physiother. 2002;48(4):257–267. doi: 10.1016/s0004-9514(14)60165-3. [DOI] [PubMed] [Google Scholar]
- 37.Chae J, Sheffler L, Knutson J. Neuromuscular electrical stimulation for motor restoration in hemiplegia. Top Stroke Rehabil. 2008;15(5):412–426. doi: 10.1310/tsr1505-412. [DOI] [PubMed] [Google Scholar]
- 38.Murie-Fernández M, Carmona Iragui M, Gnanakumar V, et al. Painful hemiplegic shoulder in stroke patients: causes and management. Neurologia. 2012;27(4):234–244. doi: 10.1016/j.nrl.2011.02.010. [DOI] [PubMed] [Google Scholar]
- 39.Ratmansky M, Defrin R, Soroker N. A randomized controlled study of segmental neuromyotherapy for post-stroke hemiplegic shoulder pain. J Rehabil Med. 2012;44(10):830–836. doi: 10.2340/16501977-1021. [DOI] [PubMed] [Google Scholar]
- 40.Zeilig G, Rivel M, Weingarden H, et al. Hemiplegic shoulder pain: evidence of a neuropathic origin. Pain. 2013;154(2):263–271. doi: 10.1016/j.pain.2012.10.026. [DOI] [PubMed] [Google Scholar]
- 41.The Chinese Neuroscience Society, The Chinese Neuro-surgery Society. Cerebrovascular Disease Diagnosis Points. Zhonghua Shenjingke Zazhi. 1996;29(6):379–380. [Google Scholar]
- 42.Huskisson EC. Measurement of pain. Lancet. 1974;2(7889):1127–1131. doi: 10.1016/s0140-6736(74)90884-8. [DOI] [PubMed] [Google Scholar]
- 43.Fugl-Meyer AR, Jääskö L, Leyman I, et al. The post-stroke hemiplegic patient. 1. a method for evaluation of physical performance. Scand J Rehabil Med. 1975;7(1):13–31. [PubMed] [Google Scholar]
- 44.Linacre JM, Heinemann AW, Wright BD, et al. The structure and stability of the Functional Independence Measure. Arch Phys Med Rehabil. 1994;75(2):127–132. [PubMed] [Google Scholar]


