Abstract
The traditional Chinese medicine Buyang Huanwu Decoction has been shown to improve the neu-rological function of patients with stroke. However, the precise mechanisms underlying its effect remain poorly understood. In this study, we established a rat model of cerebral ischemia by middle cerebral artery occlusion and intragastrically administered 5 g/kg Buyang Huanwu Decoction, once per day, for 1, 7, 14 and 28 days after cerebral ischemia. Immunohistochemical staining revealed a number of cells positive for the neural stem cell marker nestin in the cerebral cortex, the subven-tricular zone and the ipsilateral hippocampal dentate gyrus in rat models of cerebral ischemia. Buyang Huanwu Decoction significantly increased the number of cells positive for 5-bromodeoxyuridine (BrdU), a cell proliferation-related marker, microtubule-associated protein-2, a marker of neuronal differentiation, and growth-associated protein 43, a marker of synaptic plasticity in the ischemic rat cerebral regions. The number of positive cells peaked at 14 and 28 days after intragastric administration of Buyang Huanwu Decoction. These findings suggest that Buyang Huanwu Decoction can promote the proliferation and differentiation of neural stem cells and hance synaptic plasticity in ischemic rat brain tissue.
Keywords: neural regeneration, traditional Chinese medicine, Buyang Huanwu Decoction, cerebral ischemia, nestin, BrdU, microtubule-associated protein-2, growth-associated protein 43, neural stem cells, proliferation, differentiation, cerebral cortex, subventricular zone, dentate gyrus, grants-supported paper, neuroregeneration
Research Highlights
-
(1)
There have been few reports describing the mechanism by which Buyang Huanwu Decoction can promote neurogenesis in ischemic cerebral tissue. Most previous studies regarding the effects of Buyang Huanwu Decoction on cerebral ischemia have investigated the effects of Buyang Huanwu Decoction on angiogenesis and the recovery of injured cerebral tissue.
-
(2)
This study aimed to investigate the effects of Buyang Huanwu Decoction on the proliferation and neuronal differentiation of neural stem cells.
INTRODUCTION
Recent studies have shown that cerebral ischemia induces neurogenesis in neuroproliferative regions of the adult rodent brain, including the subventricular zone, the subgranular zone of the dentate gyrus, and the peri-infarct area[1,2,3,4]. In addition, endogenous neurogenesis cannot be regulated by drugs but can be controlled by intrinsic genetic mechanisms and growth factors[5,6,7]. Growth-associated protein 43 (GAP-43) is a nervous tissue-specific cytoplasmic protein that modulates synaptic plasticity[8]. During development, GAP-43 expression in the brain is high and declines in mature neurons during adulthood; during neurogenesis, GAP-43 expression is significantly upregulated in proliferating neuroblasts and differentiating neurons[9,10,11]. Therefore, GAP-43 has been designated as a ‘growth’ or ‘plasticity’ protein[12,13,14].
Buyang Huanwu Decoction (BYHWD) is a classic herbal formula of traditional Chinese medicine that has been shown to improve neurological function in stroke patients[12]. In experimental studies, Buyang Huanwu Decoction has been shown to reduce infarct volume[13], attenuate the number of apoptotic cells, and suppress the expression of the caspase-3 cleavage product caspase-3p20[14]. In addition, Buyang Huanwu Decoction has been used to culture neural stem cells derived from the embryonic hippocampus[15,16,17].
In this study, we investigated the effect of Buyang Huanwu Decoction on the proliferation and differentiation of neural stem cells, and on the expression of GAP-43 in ischemic brain.
RESULTS
Quantitative analysis of experimental animals
A total of 128 male rats were randomly and equally divided into four groups: a normal group, a sham surgery group, a middle cerebral artery occlusion (MCAO) group, and a MCAO + Buyang Huanwu Decoction group. One rat separately from each of the MCAO and MCAO + Buyang Huanwu Decoction groups died from hemorrhage of the middle cerebral artery. One rat separately from each of the MCAO and MCAO + Buyang Huanwu Decoction groups died of unknown causes 2 days after surgery. These four animals were excluded, and no rats were lost in the normal and sham operated groups. Rats in the MCAO + Buyang Huanwu Decoction group were intragastrically fed 5 g/kg Buyang Huanwu Decoction for 1, 7, 14 and 28 days (n = 8 rats/time point), once per day, and in the other groups, rats were given the same volume of distilled water at each time point (n = 6 rats/time point). All 96 rats were included in the final analysis.
Buyang Huanwu Decoction promoted the proliferation of neural stem cells in ischemic brain tissue
At 1 day after intragastric administration, the number of nestin-positive cells was increased in the cerebral cortex, the subventricular zone and the dentate gyrus of the ipsilateral hippocampus in the MCAO and MCAO + Buyang Huanwu Decoction groups (Figure 1). In the MCAO and MCAO + Buyang Huanwu Decoction groups, the number of nestin-positive cells in the cerebral cortex reached its peak at 14 days (P < 0.05) and decreased at 28 days (P < 0.05). At 7, 14 and 28 days after intragastric administration, the number of nestin-positive cells in the cerebral cortex in the MCAO + Buyang Huanwu Decoction group was significantly higher than that in the MCAO group (P < 0.05; Figure 1). These findings suggest that Buyang Huanwu Decoction can promote the proliferation of neural stem cells.
Figure 1.
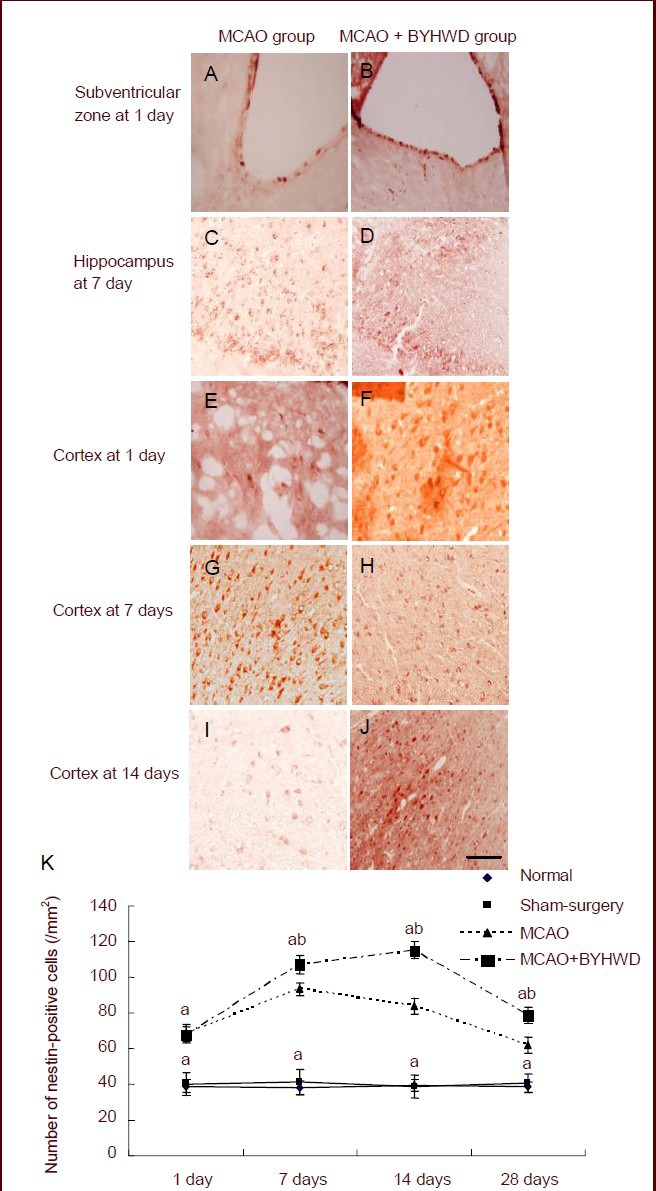
Effects of Buyang Huanwu Decoction (BYHWD) on the proliferation of neural stem cells in the infarct periphery after cerebral ischemia.
(A–J) Immunohistochemical staining of nestin-positive cells (red) in the middle cerebral artery occlusion (MCAO) group (cerebral cortex or subventricular zone at 1 day and hippocampus at 7 days) and MCAO + BYHWD group (cerebral cortex or subventricular zone at 1 day and hippocampus at 7 days) after cerebral ischemia (× 200; scale bars: 50 μm).
(K) Quantification of nestin-positive cells in the cerebral cortex. aP < 0.05, vs. normal group; bP < 0.05, vs. MCAO group (mean ± SD; n = 6 rats in each group, Student's t-test).
Buyang Huanwu Decoction promoted neuronal differentiation of neural stem cells in ischemic brain tissue
5-Bromodeoxyuridine (BrdU)/microtubule- associated protein-2 (MAP-2) double-labeled cells were not present in the normal and sham operated groups. At 1 day after intragastric administration, few BrdU/ MAP-2 double-labeled cells were observed in the subventricular zone and cerebral cortex of rats in the MCAO and MCAO + Buyang Huanwu Decoction groups. The number of double-labeled cells increased at 7 days, peaked at 14 days (P < 0.05) and decreased at 28 days (P < 0.05). There were a few BrdU/ MAP-2 double-labeled cells in the ipsilateral hippocampal dentate gyrus in both groups (Figure 2).
Figure 2.
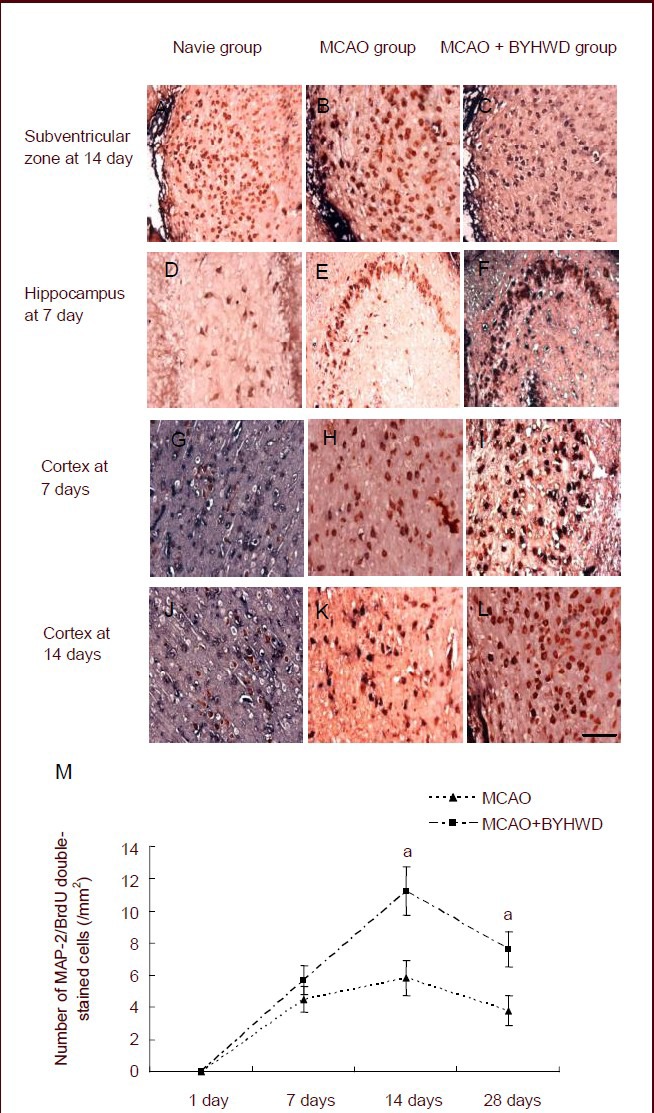
Effects of Buyang Huanwu Decoction (BYHWD) on the quantity of newly regenerated neurons in the cerebral cortex of rats with cerebral ischemia.
(A–L) Immunohistochemical staining of microtubule-associated protein-2 (MAP2)/5-bromodeoxyuridine (BrdU) double-stained cells in the subventricular zone (14 days), hippocampus (7 days) or in the cerebral cortex (7 and 14 days) after cerebral ischemia (× 400; scale bars: 25 μm).
(M) Quantification of MAP2/BrdU double-stained cells. aP < 0.05, vs. middle cerebral artery occlusion (MCAO) group (mean ± SD; n = 6 rats in each group, Student's t-test).
At 14 and 28 daysafter intragastric administration, the number of BrdU/MAP-2 double-labeled cells was significantly higher in the MCAO + Buyang Huanwu Decoction group than in the MCAO group (Figure 2; P < 0.05).
Buyang Huanwu Decoction regulated GAP-43 expression in ischemic brain
At 14 days after intragastric administration, the number of GAP-43-positive cells in the cerebral cortex and hippocampus in the MCAO and MCAO + Buyang Huanwu Decoction groups reached its peak and then decreased (P < 0.05; Figure 3A).
Figure 3.
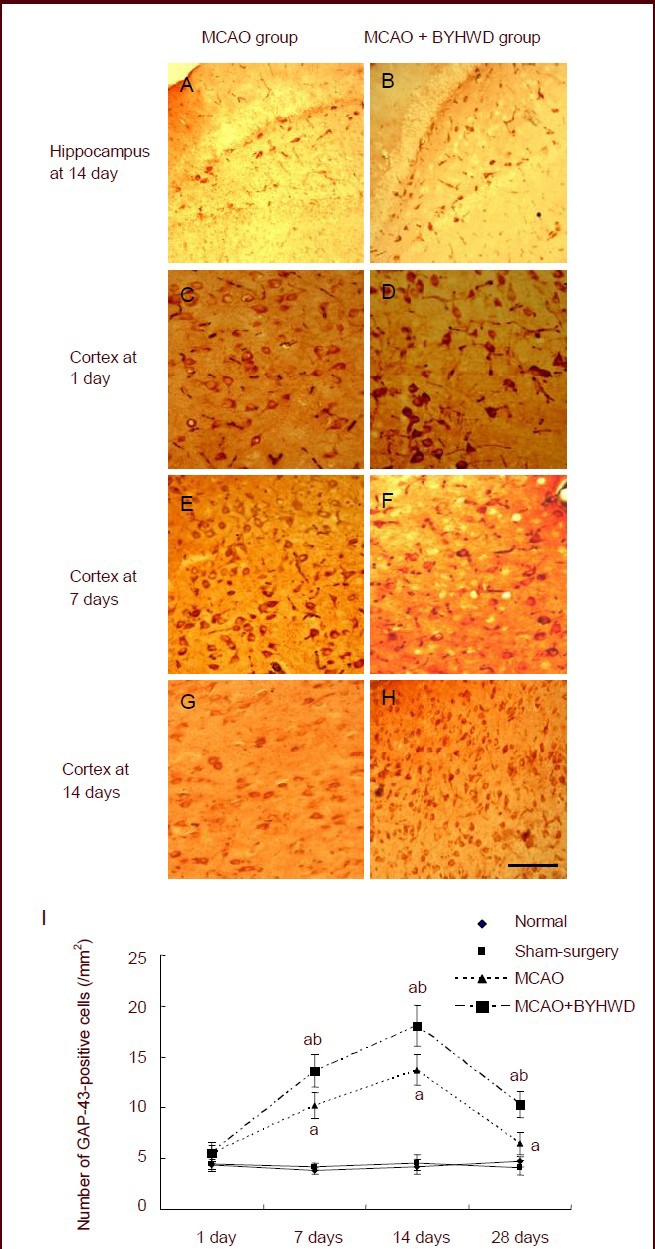
Effects of Buyang Huanwu Decoction (BYHWD) on expression of growth associated protein-43 (GAP-43) in the cerebral cortex of rats with cerebral ischemia.
(A–H) Immunohistochemical staining of GAP-43-positive cells in the cerebral cortex at 1, 7 and 14 days after cerebral ischemia in the middle cerebral artery occlusion (MCAO; C, E, G) and MCAO + BYHWD groups (D, F, H) and in the hippocampus at 14 days after cerebral ischemia in the MCAO (A) and MCAO + BYHWD groups (B) (counterstained with 3-amino-9-ethylcarbazole, × 400; scale bars: 25 μm).
(I) Quantification of GAP-43-positive cell expression in the cerebral cortex. aP < 0.05, vs. normal group; bP < 0.05, vs. MCAO group (mean ± SD; n = six rats in each group per time point, Student's t-test).
At 7, 14 and 28 days after intragastric administration, the number of GAP-43-positive cells in the MCAO + Buyang Huanwu Decoction group was significantly higher than that in the MCAO group (P < 0.05; Figure 3B).
DISCUSSION
In this study, we revealed two major findings. First, Buyang Huanwu Decoction improved the proliferation and neuronal differentiation of neural stem cells in the brain after MCAO. Second, Buyang Huanwu Decoction increased the expression of GAP-43.
After stroke, a reduction in cerebral blood flow and consequent hypoxia trigger a complex pathophysiologic response, which leads to cell death and neurological function deficits. Current therapies include neuroprotection and thrombolysis, which are aimed at reducing neuron death and saving damaged cells. Neither therapy is capable of regenerating new neurons and repairing injured tissues[18,19]. Therapies that stimulate neurogenesis and rebuild the neural system remain to be developed for the treatment of stroke.
Traditionally, the mammalian adult central nervous system is viewed as having a lack of regenerative capacity. However, recent studies have demonstrated that the adult brain has regenerative areas that reside in the subventricular zone and the hippocampal dentate gyrus[20,21,22,23]. Neurogenesis involves neural stem/progenitor cell proliferation, migration and neural differentiation[24]. Therefore, the effects of therapeutics at any stage of this complex process will have an impact on neurogenesis. Previous studies have detected few newly-generated neurons after cerebral ischemia[6,25]. More importantly, endogenous neurogenesis by neural stem/progenitor cells can be modulated by environmental factors and by administration of various pharmaceutical agents[5,6] and cytokines[26,27].
Buyang Huanwu Decoction is a well-known formula for stroke treatment in China, and it enhances the proliferation and differentiation of neural progenitor cells in vitro[15]. In addition, Buyang Huanwu Decoction has been shown to have an anti-inflammatory effect and increase vascular endothelial growth factor expression after cerebral ischemia[13,28]. Inflammation after stroke impairs neurogenesis and inhibits microglial activation[29], and vascular endothelial growth factor acts as a neurogenic factor, promoting neurogenesis in the brain after cerebral ischemia[30]. Therefore, we presume that Buyang Huanwu Decoction can enhance neurogenesis. In the present study, we found that Buyang Huanwu Decoction can enhance the expression of nestin and the numbers of newborn neurons, which indicates that Buyang Huanwu Decoction can affect neural regeneration.
Re-establishing connections between newborn and neighboring cells after injury is important for neurological rehabilitation. GAP-43 is the most prominent molecule involved in neural regeneration[31]. The expression of GAP-43 is high in the brain during development and declines in most neurons when mature synapses are formed. However, after cerebral ischemia, expression of GAP-43 increases[32], and overexpression of GAP-43 has been shown to enhance neurite outgrowth in vitro and axonal sprouting in vivo[33,34]. Therefore, regulating GAP-43 can affect neurogenesis.
In summary, our present study revealed that there is limited neurogenesis after stroke. Buyang Huanwu Decoction enhanced neural stem cell proliferation and differentiation as well as the expression of GAP-43 in ischemic brain. However, further studies are required to understand its mechanism of action and neurological rehabilitation effect.
MATERIALS AND METHODS
Design
A randomized, controlled animal experiment.
Time and setting
Experiments were performed at Key Laboratory of Internal Medicine, Hunan University of Traditional Chinese Medicine, China from November 2009 to July 2011.
Materials
Animals
Adult male Wistar rats (n = 128), weighing 280–300 g, were obtained from Hunan Slac Jingda Laboratory Animal Company Ltd. (Changsha, China; license No. SCXK (Xiang) 2009-0004). Investigations using experimental animals were conducted in accordance with internationally accepted principles for laboratory animal use and care.
The experimental protocol was in accordance with Guidance Suggestions for the Care and Use of Laboratory Animals issued by Ministry of Science and Technology of the People's Republic of China[35].
Drugs
Buyang Huanwu Decoction was composed of Astragalus membranaceus (120 g), Angelica sinensis (10 g), Paeonia lactiflora (10 g), Ligusticum chuanxiong (10 g), Carthamus tinctorius (10 g), Semen Persicae (10 g) and Flos carthami (4.5 g). All of the herbal components (GAP grade) were purchased from the First Affiliated Hospital of Hunan University of Traditional Chinese Medicine, China. To ensure the consistency of the herbal formula, we made Buyang Huanwu drug extracts using standard methods described in the Chinese Pharmacopoeia (China Pharmacopoeia and Committee, 2000). Buyang Huanwu Decoction was obtained by boiling, concentrated to contain 2 g of crude material per liter, and stored at 4°C.
Methods
Preparation of rat models of local cerebral ischemia
Focal cerebral ischemia was induced by middle cerebral artery occlusion as described previously[36]. Briefly, rats were intraperitoneally (i.p.) anesthetized with 400 mg/kg chloral hydrate. During surgery, rat body temperature was maintained at 37.0°C by placing animals on a heated bed. The right carotid bifurcation was exposed, and the external carotid artery was ligated distal to the bifurcation. A silicone-coated 4-0 nylon monofilament suture was inserted through an incision of the external cerebral artery and gently advanced 17.0 ± 2.0 mm to occlude the middle cerebral artery. Sham-operated rats underwent the same surgical procedures as their ischemic counterparts but without suture-induced occlusion. The neurological behavior status was carefully evaluated at 24 hours after surgery according to Bederson's method[37]. A higher score indicated worse neurobehavioral dysfunction, vice versa. Rats that scored 1–3 were included in the subsequent experiments.
Buyang Huanwu Decoction treatments
Rats in the treatment group were orally gavaged with 5 g/kg Buyang Huanwu Decoction per day. The dosage was based on a clinical regimen for adult patients. The animals in other groups were given the same volume of distilled water.
BrdU labeling
To measure cell proliferation, animals received daily i.p. injections of BrdU (50 mg/kg per day; Sigma, St. Louis, MO, USA) in the first week after the occlusion and twice a week (5th, 7th days) thereafter. BrdU incorporation into DNA was used as an index of dividing cells and their progeny[38].
Tissue preparation
Animals were killed at 1, 7, 14 and 28 days after onset of middle cerebral artery occlusion under anesthesia and then perfused transcardially with saline solution, followed by 4% (w/v) paraformaldehyde. The brains were removed and fixed in 0.01 mol/L PBS containing 4% (w/v) cold paraformaldehyde for 24 hours. The fixed tissues were transferred to 30% (w/v) sucrose solution and kept at 4°C. Subsequently, the specimens were cut into 15 μm slices with a cryostat and mounted onto silane-coated slides. After they were dried at 37°C for 60 minutes, the sections were stored at –70°C for examination by microscopy (Olympus, Tokyo, Japan).
Immunohistochemical detection
To monitor neural stem cells, we immunostained cells for nestin, which is an intermediate filament protein, transiently and abundantly expressed early in embryogenesis[39], e.g., in neuroepithelium. Five brain tissue sections from rats killed at each time point were incubated in horse serum blocking solution for 10 minutes and then in primary antibody (mouse anti-nestin monoclonal antibody, 1:500; Chemicon, Millipore, Billerica, MA, USA) for 2 hours at room temperature. The slides were washed with 0.01 mol/L PBS containing 0.1% (v/v) Triton X-100 and incubated with a biotinylated secondary broad goat anti-mouse IgG antibody (Zymed Laboratories, Inc., San Francisco, CA, USA). After additional incubation with streptavidin peroxidase, the slides were stained with 3-amino-9-ethylcarbazole (Zymed Laboratories). Newborn neurons were identified using double immunohistochemistry, as described in the protocol of the Histostain-DS kit (Zymed Laboratories).
Briefly, endogenous peroxidase activity was quenched by 30-minute incubation in freshly prepared 3% (v/v) H2O2-methanol solution. The sections were pretreated with 2 mol/L HCl for 10 minutes at room temperature to denature DNA and then blocked for 10 minutes at room temperature with horse serum blocking solution. A mouse anti-BrdU antibody (1:100; ThermoFisher Scientific, Sunnyvale, CA, USA) diluted in PBS was added and allowed to incubate for 1 hour at 37°C. Anti-BrdU was initially detected by streptavidin-alkaline phosphatase and stained with 5-bromo-4-chloro-3-indolyl phosphate/nitroblue tetrazolium salt. The same staining procedure was repeated by incubating brain sections with the neuron-specific marker mouse anti-MAP-2 antibody (1:400; Millipore). Immunolabeling was detected with the biotinylated secondary broad IgG (1:400; Zymed Laboratories) followed by streptavidin-peroxidase and stained with AEC. The expression of mouse anti-GAP-43 (1:200; Zymed Laboratories) was monitored using immunohistochemistry as described above.
Nestin-positive cells had intense red cytoplasmic staining. Three coronal brain sections were randomly selected. The number of nestin, BrdU/MAP-2 and GAP-43 positive cells per mm2 of area was calculated through the use of Olympus MicroImage 4.0 software (Olympus).
Statistical analysis
Measurement data are expressed as mean ± SD. Statistical analysis was performed using the Student's t-test, with SPSS 11.0 software (SPSS, Chicago, IL, USA). P < 0.05 was considered statistically significant.
Footnotes
Funding: This study was supported by grants from the National Nature Science Foundation of China, No. 30873355, 81072939, 81273989, 81202694; the Foundation of Educational Commission of Hunan Province in China, No. 11C0954.
Conflicts of interest: None declared.
Ethical approval: The experimental protocol was approved by the Ethics Committee, Hunan University of Traditional Chinese Medicine in China.
(Reviewed by McGowan D, Raye Z, Ma HH, Wang RG)
(Edited by Wang J, Li CH, Song LP, Liu WJ, Zhao M)
REFERENCES
- 1.Curtis MA, Kam M, Nannmark U, et al. Human neuroblasts migrate to the olfactory bulb via a lateral ventricular extension. Science. 2007;315(5816):1243–1249. doi: 10.1126/science.1136281. [DOI] [PubMed] [Google Scholar]
- 2.Duan X, Kang E, Liu CY, et al. Development of neural stem cell in the adult brain. Curr Opin Neurobiol. 2008;18(1):108–115. doi: 10.1016/j.conb.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thored P, Arvidsson A, Cacci E, et al. Persistent production of neurons from adult brain stem cells during recovery after stroke. Stem Cells. 2006;24(3):739–747. doi: 10.1634/stemcells.2005-0281. [DOI] [PubMed] [Google Scholar]
- 4.Jin K, Minami M, Lan JQ, et al. Neurogenesis in dentate subgranular zone and rostral subventricular zone after focal cerebral ischemia in the rat. Proc Natl Acad Sci U S A. 2001;98(8):4710–4715. doi: 10.1073/pnas.081011098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ormerod BK, Galea LA. Reproductive status influences cell proliferationand cell survival in the dentate gyrus of adult female meadowvoles: a possible regulatory role for estradiol. Neuroscience. 2001;102(2):369–379. doi: 10.1016/s0306-4522(00)00474-7. [DOI] [PubMed] [Google Scholar]
- 6.Yoshimura S, Takagi Y, Harada J, et al. FGF-2 regulation of neurogenesis in adult hippocampus after brain injury. Proc Natl Acad Sci U S A. 2001;98(10):5874–5879. doi: 10.1073/pnas.101034998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jin K, Zhu Y, Sun Y, et al. Vascular endothelial growth factor (VEGF) stimulates neurogenesis in vitro and in vivo. Proc Natl Acad Sci U S A. 2002;99(18):11946–11950. doi: 10.1073/pnas.182296499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Benowitz LI, Routtenberg A. GAP-43: an intrinsic determinant of neuronal development and plasticity. Trends Neurosci. 1997;20(2):84–91. doi: 10.1016/s0166-2236(96)10072-2. [DOI] [PubMed] [Google Scholar]
- 9.Denny J. Molecular Mechanisms, biological actions, and neuropharmacology of the growth-associated protein gap-43. Curr Neuropharmacol. 2006;4(4):293–304. doi: 10.2174/157015906778520782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shen Y, Mani S, Meiri KF. Failure to express GAP-43 leads to disruption of a multipotent precursor and inhibits astrocyte differentiation. Mol Cell Neurosci. 2004;26(3):390–405. doi: 10.1016/j.mcn.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 11.Zhao J, Yao Y, Xu C, et al. Expression of the neural specific protein, GAP-43, dramatically lengthens the cell cycle in fibroblasts. Int J Dev Neurosci. 2009;27(6):531–537. doi: 10.1016/j.ijdevneu.2009.06.013. [DOI] [PubMed] [Google Scholar]
- 12.Feng L, Sun XF. Effect of Buyang Huanwu decoction on rehabilitation of stroke patients. Zhongguo Kangfu Lilun yu Shijian. 2005;11(6):465–466. [Google Scholar]
- 13.Cai G, Liu B, Liu W, et al. Buyang Huanwu Decoction can improve recovery of neurological function, reduce infarction volume, stimulate neural proliferation and modulate VEGF and Flk1 expressions in transient focal cerebral ischaemic rat brains. J Ethnopharmacol. 2007;113(2):292–299. doi: 10.1016/j.jep.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 14.Li XM, Bai XC, Qin LN, et al. Neuroprotective effects of Buyang Huanwu Decoction on neuronal injury in hippocampus after transient forebrain ischemia in rats. Neurosci Lett. 2003;346(1-2):29–32. doi: 10.1016/s0304-3940(03)00522-6. [DOI] [PubMed] [Google Scholar]
- 15.Sun J, Bi Y, Guo L, Qi X, et al. Buyang Huanwu Decoction promotes growth and differentiation of neural stem cells: using a serum pharmacological method. J Ethnopharmacol. 2007;113(2):199–203. doi: 10.1016/j.jep.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 16.Sun JH, Gao YM, Yang L, et al. Effects of Buyang Huanwu Decoction on neurite outgrowth and differentiation of neuroepithelial stem cells. Chin J Physiol. 2007;50(4):151–156. [PubMed] [Google Scholar]
- 17.Li Y, Chopp M. Temporal profile of nestin expression after focal cerebral ischemia in adult rat. Brain Res. 1999;838(1-2):1–10. doi: 10.1016/s0006-8993(99)01502-4. [DOI] [PubMed] [Google Scholar]
- 18.Horner PJ, Gage FH. Regenerating the damaged central nervous system. Nature. 2000;407(6807):963–970. doi: 10.1038/35039559. [DOI] [PubMed] [Google Scholar]
- 19.Martinez-Vila E, Irimia P. Challenges of neuroprotection and neurorestoration in ischemic stroke treatment. Cerebrovasc Dis. 2005;20(Suppl 2):148–158. doi: 10.1159/000089369. [DOI] [PubMed] [Google Scholar]
- 20.Iwai M, Hayashi T, Zhang WR, et al. Induction of highly polysialylated neural cell adhension molecule (PSA- NCAM) in postischemic gerbil hippocampus mainly dissociated with neural stem cell proliferation. Brain Res. 2001;902(2):288–293. doi: 10.1016/s0006-8993(01)02399-x. [DOI] [PubMed] [Google Scholar]
- 21.Yagita Y, Kitagawa K, Ohtsuki T, et al. Neurogenesis by progenitor cells in the ischemic adult rat hippocampus. Stroke. 2001;32(8):1890–1986. doi: 10.1161/01.str.32.8.1890. [DOI] [PubMed] [Google Scholar]
- 22.Arias-Carrion O, Verdugo-Diaz L, Feria-Velasco A, et al. Neurogenesis in the subventricular zone following transcranial magnetic field stimulation and nigrostriatal lesions. J Neurosci Res. 2004;78(1):16–28. doi: 10.1002/jnr.20235. [DOI] [PubMed] [Google Scholar]
- 23.Warner-Schmidt JL, Duman RS. Hippocampal neurogenesis: opposing effects of stress and antidepressant treatment. Hippocampus. 2006;16(3):239–249. doi: 10.1002/hipo.20156. [DOI] [PubMed] [Google Scholar]
- 24.Iwai M, Sato K, Omori N, et al. Three steps of neural stem cells develoment in gerbil dentate gyrus after transient ischemia. J Cereb Blood Flow Metab. 2002;22(4):411–419. doi: 10.1097/00004647-200204000-00005. [DOI] [PubMed] [Google Scholar]
- 25.Ninomiya M, Yamashita T, Araki N, et al. Enhanced neurogenesis in the ischemic striatum following EGF-induced expansion of transit-amplifying cells in the subventricular zone. Neurosci Lett. 2006;403(1-2):63–67. doi: 10.1016/j.neulet.2006.04.039. [DOI] [PubMed] [Google Scholar]
- 26.Koo JW, Russo SJ, Ferguson D, et al. Nuclear factor-κB is a critical mediator of stress-impaired neurogenesis and depressive behavior. Proc Natl Acad Sci U S A. 2010;107(6):2669–2674. doi: 10.1073/pnas.0910658107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seguin JA, Brennan J, Mangano E, et al. Proinflammatory cytokines differentially influence adult hippocampal cell proliferation depending upon the route and chronicity of administration. Neuropsychiatr Dis Treat. 2009;5:5–14. [PMC free article] [PubMed] [Google Scholar]
- 28.Liu Y, Lin R, Shi X, et al. The roles of buyang huanwu decoction in anti-inflammation, antioxidation and regulation of lipid metabolism in rats with myocardial ischemia. Evid Based Complement Alternat Med 2011. 2011 doi: 10.1093/ecam/neq028. 561396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ekdahl CT, Claasen JH, Bonde S, et al. Inflammation is detrimental for neurogenesis in adult brain. Proc Natl Acad Sci U S A. 2003;100(23):13632–13637. doi: 10.1073/pnas.2234031100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang YQ, Cui HR, Yang SZ, et al. VEGF enhance cortical newborn neurons and their neurite development in adult rat brain after cerebral ischemia. Neurochem Int. 2009;55(7):629–636. doi: 10.1016/j.neuint.2009.06.007. [DOI] [PubMed] [Google Scholar]
- 31.Skene JH. Axonal growth-associated proteins. Annu Rev Neurosci. 1989;12:127–156. doi: 10.1146/annurev.ne.12.030189.001015. [DOI] [PubMed] [Google Scholar]
- 32.Stroemer RP, Kent TA, Hulsebosch CE. Neocortical neural sprouting, synaptogenesis, and behavioral recovery after neocortical infarction in rats. Stroke. 1995;26(11):2135–2144. doi: 10.1161/01.str.26.11.2135. [DOI] [PubMed] [Google Scholar]
- 33.Aigner L, Arber S, Kapfhammer JP, et al. Overexpression of the neural growth-associated protein GAP-43 induces nerve sprouting in the adult nervous system of transgenic mice. Cell. 1995;83(2):269–278. doi: 10.1016/0092-8674(95)90168-x. [DOI] [PubMed] [Google Scholar]
- 34.Buffo A, Holtmaat AJ, Savio T, et al. Targeted overexpression of the neurite growth-associated protein B-50/GAP- 43 in cerebellar Purkinje cells induces sprouting after axotomy but not axon regeneration into growth-permissive transplants. J Neurosci. 1997;17(22):8778–8791. doi: 10.1523/JNEUROSCI.17-22-08778.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.The Ministry of Science and Technology of the People's Republic of China. Guidance Suggestions for the Care and Use of Laboratory Animals. 2006 Sep 30; [Google Scholar]
- 36.Longa EZ, Weinstein PR, Carlson S, et al. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20(1):84–91. doi: 10.1161/01.str.20.1.84. [DOI] [PubMed] [Google Scholar]
- 37.Bederson JB, Pitts LH, Tsuji M, et al. Rat middle cerebral artery occlusion: evaluation of the model and development of a neurologic examination. Stroke. 1986;17(3):472–476. doi: 10.1161/01.str.17.3.472. [DOI] [PubMed] [Google Scholar]
- 38.Choi YS, Lee MY, Sung KW, et al. Regional differences in enhanced neurogenesis in the dentate gyrus of adult rats after transient forebrain ischemia. Mol Cells. 2003;16(2):232–238. [PubMed] [Google Scholar]
- 39.Dahlstrand J, Zimmerman LB, McKay RD, et al. Characterization of the human nestin gene reveals a close evolutionary relationship to neurofilaments. J Cell Sci. 1992;103(Pt 2):589–597. doi: 10.1242/jcs.103.2.589. [DOI] [PubMed] [Google Scholar]


