Abstract
A rat model of middle cerebral artery permanent occlusion was established using the modified Longa method. Successfully established model animals were treated by blood-letting puncture at twelve Jing-Well points of the hand, and/or by injecting mannitol into the caudal vein twice daily. Brain tissue was collected at 24, 48 and 72 hours after modeling, and blood was collected through the retinal vein before Evans blue was injected, approximately 1 hour prior to harvesting of brain tissue. Results showed that Evans blue leakage into brain tissue and serum nitric oxide synthase activity were significantly increased in model rats. Treatment with blood-letting punctures at twelve Jing-Well points of the hand and/or injection of mannitol into the caudal vein reduced the amount of Evans blue leakage into the brain tissue and serum nitric oxide synthase activity to varying degrees. There was no significant difference between single treatment and combined treatment. Experimental findings indicate that blood-letting punctures at twelve Jing-Well points of the hand can decrease blood-brain barrier permeability and serum nitric oxide synthase activity in rats following middle cerebral artery occlusion, and its effect is similar to that of mannitol injection alone and Jing-Well points plus mannitol injection.
Keywords: neural regeneration, brain injury, Jing-Well points of hand, acupoint, blood-letting, mannitol, middle cerebral artery occlusion, cerebral ischemia, cerebral infarction, blood-brain barrier, nitric oxide synthase, cerebral edema, neuroprotection, grants-supported paper, neuroregeneration
Research Highlights
(1) Blood-letting punctures at twelve Jing-Well points of the hand for the treatment of ischemic cerebral edema were explored.
(2) Blood-letting puncture at twelve Jing-Well points of the hand could decrease blood-brain barrier permeability and serum nitric oxide synthase activity, thus attenuating cerebral edema in rats with middle cerebral artery occlusion.
(3) Blood-letting puncture at twelve Jing-Well points of the hand had a similar effect in preventing ischemia as that of mannitol injection alone and Jing-Well points plus mannitol injection.
INTRODUCTION
Stroke is the third leading cause of death in the western world[1]. It is also the second most common cause of death and the major cause of disability worldwide[2]. Ischemic stroke accounts for approximately 60–80% of stroke events. Brain edema is an important complication of ischemic stroke and is closely related to disability, mortality and recuperation of neural function after cerebral ischemia[3]. Currently western medicines, such as high permeability dehydrators and diuretics, are often used in clinical therapy. Although these drugs are reported to reduce the degree of edema, side effects may occur and include renal impairment and electrolyte disturbance[4,5,6]. Thus, effective and convenient treatments with fewer adverse effects are required.
Blood-letting puncture at twelve Jing-Well points of the hand is a classical emergent remedy for stroke in traditional Chinese medicine. This treatment has been popularized among the nation by the State Administration of Traditional Chinese Medicine in China. Our previous studies have demonstrated that blood-letting puncture at twelve Jing-Well points of the hand could not only improve cerebral blood flow[7,8], but also help restore conscious level in stroke patients[9,10]. Animal studies showed that blood-letting punctures at twelve Jing-Well points of the hand could reduce infarct size[11], prevent free radical damage[12], postpone development of hypoxia[13] and acidosis[14], and inhibit calcium overload[15,16]. Furthermore, blood-letting could also improve neurotoxicity[17,18,19] and healing ability of ischemic cells[20,21] in rat brains following middle cerebral artery occlusion. We have also demonstrated that blood-letting puncture at Jing-Well points can lessen the degree of cerebral edema in middle cerebral artery occluded rats[22,23], but the mechanism for this phenomenon still remains unclear.
The blood-brain barrier is a protective cellular layer which is formed by tight interendothelial cell connections, intact subtending basal laminas, and astrocyte end feet[24]. It plays an important part in normal central nervous system function, owing to its ability to regulate ion flux and supplementation of nutrients to the brain[25]. When ischemic stroke occurs, the morphology of the blood-brain barrier changes; for example, tight junctions open and endothelial cell membranes are damaged[26], thus increasing blood-brain barrier permeability. These changes allow normally excluded intravascular proteins and fluid to penetrate into the cerebral parenchymal extracellular space and thus may result in vasogenic edema.
Nitric oxide synthase is the key enzyme in nitric oxide biosynthesis, and a close relationship exists between the permeability of the blood-brain barrier and nitric oxide[27]. Studies have found that blood-letting puncture at twelve Jing-Well points of the hand can inhibit increased nitric oxide content and nitric oxide synthase activity in ischemic brain tissue from rats post middle cerebral artery occlusion. No dynamic observations were made in this study[28].
This study was designed to evaluate the effects of blood-letting puncture at Jing-Well points aimed at blood-brain barrier permeability and serum nitric oxide synthase activity. Furthermore, we investigated the mechanisms behind blood-letting puncture at Jing-Well points.
RESULTS
Quantitative analysis of experimental animals
A total of 140 Wistar rats were randomly divided into six groups: control (n = 5), sham operation (n = 15), model (n = 30; middle cerebral artery occlusion), mannitol (n = 30; middle cerebral artery occlusion + mannitol intravenous injection), Jing-Well points (n = 30; middle cerebral artery occlusion + blood-letting puncture at twelve Jing-Well points of the hand) or Jing-Well points plus mannitol (n = 30; middle cerebral artery occlusion + blood-letting puncture at twelve Jing-Well points of the hand plus mannitol injection) groups.
Brain samples were taken at 24, 48 and 72 hours after middle cerebral artery occlusion modeling (except in the control group), and the blood was collected via the retinal vein 1 hour before brains were harvested. Five rats were used at each time point in the sham operation group and control group. Ten rats were used in the model group and the other three treatment groups. All 140 rats were involved in the final analysis.
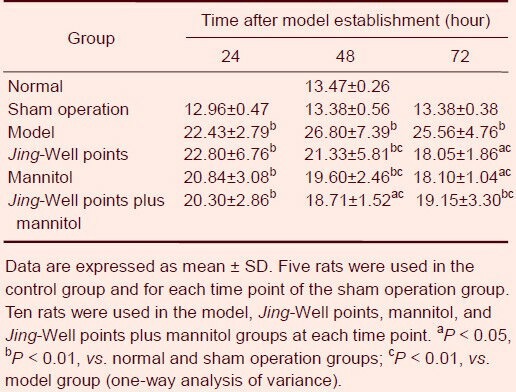
Effects of blood-letting puncture at twelve Jing-Well points of the hand on blood-brain barrier permeability in middle cerebral artery occluded rats
Evans blue leakage in the brain tissue of rats in the control and sham operation groups was almost the same, with little Evans blue exudation.
Increased Evans blue exudation was observed at 24 hours after middle cerebral artery occlusion, peaked at 48 hours, and then gradually decreased at 72 hours. Evans blue leakage in the model group was obviously greater than that of the control and sham operation groups (P < 0.01), and was significantly higher than that of the treatment groups at 48 and 72 hours (P < 0.01).
There was no statistically significant difference in effect among the three treatment groups at the same time point (P > 0.05; Table 1).
Table 1.
Effects of blood-letting puncture at twelve Jing-Well points of the hand on blood-brain barrier permeability (μg/g) in middle cerebral artery occluded rats
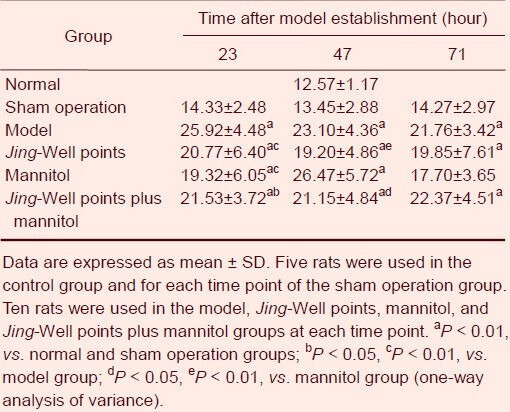
Effects of blood-letting puncture at twelve Jing-Well points of the hand on serum nitric oxide synthase activity in middle cerebral artery occluded rats
Blood was collected for the determination of serum nitric oxide synthase activity via the retinal vein 1 hour before brains were harvested.
Serum nitric oxide synthase activity of rats in the control and sham operation groups was not significantly different. Serum nitric oxide synthase activity in the model group was significantly higher than that of sham operated animals (P < 0.01) and gradually decreased with time.
Serum nitric oxide synthase activities in the Jing-Well point and mannitol groups were markedly lower than that of the model group at 23 hours after model establishment (P < 0.01). Serum nitric oxide synthase activity in the Jing-Well points plus mannitol group was also significantly lower than that of the model group (P < 0.05).
At 47 and 71 hours after establishing the model, no statistical differences between the treatment group and the model group were observed (P > 0.05). The Jing-Well points group was significantly different from the mannitol group at 47 hours after establishing the model (P < 0.01), as was the Jing-Well points plus mannitol group (P < 0.05; Table 2).
Table 2.
Effects of blood-letting puncture at twelve Jing-Well points of the hands on serum nitric oxide synthase activity (U/mL) in middle cerebral artery occluded rats
DISCUSSION
Evans blue is a fluorescent dye that combines with serum albumin in the blood. Normally, serum albumin can not pass through the blood-brain barrier. When blood-brain barrier permeability is increased via a variety of factors such as ischemia, the albumin, combined with Evans blue, may get through the blood-brain barrier into the brain tissue. Therefore, blood-brain barrier permeability is reflected by the quantity of Evans blue that diffuses through the blood vessels. In this study, we showed that Evans blue leakage was significantly increased by ischemia, with the peak being reached at 48 hours after ischemia. This result was similar to those of previous studies[29,30]. Our data clearly demonstrated that blood-letting puncture at twelve Jing-Well points of the hand could significantly attenuate blood-brain barrier permeability at 48 and 72 hours after ischemia. Likewise, mannitol injection and blood-letting punctures induced the same effect. Why are blood-letting punctures at these 12 points effective against blood-brain barrier disruption? According to the theory of root and end, which is derived from the Medical Classic of the Yellow Emperor, since the Jing-Well points are the beginning of meridians, stimulation at these points can treat diseases located where these meridians pass, such as ailments of the head, chest or abdomen. Blood-letting punctures at Jing-Well points will eliminate toxic factors, remove stagnation, promote resuscitation, and activate Qi and blood circulation in the meridians to cure the diseases of the head.
Nitric oxide synthase is the key enzyme in nitric oxide biosynthesis. Kader et al[31] found a sharp, transient increase in the activity of nitric oxide synthase during the first hour of cerebral ischemia following middle cerebral artery occlusion in rats, which leads to a burst in nitric oxide production. Chi et al[32] reported that the nitric oxide synthase inhibitor (NG-nitro-L-arginine methyl ester) decreased the blood-brain barrier transfer coefficient of 14C-alpha-aminoisobutyric acid in rats with middle cerebral artery occlusion under isoflurane anesthesia. They considered that nitric oxide might participate in increasing transport of small hydrophilic molecules across the blood-brain barrier in focal ischemia. In this study, we have also shown that serum nitric oxide synthase activity was significantly increased by ischemia. Moreover, blood-letting punctures at twelve Jing-Well points of the hand significantly attenuated nitric oxide synthase activity within 23 hours. Hence, this observation may indicate one of the mechanisms by which blood-letting punctures at Jing-Well points of the hand can regulate blood-brain barrier permeability.
There are three isoforms of nitric oxide synthase. These include neuronal nitric oxide synthase, inducible nitric oxide synthase, and endothelial nitric oxide synthase. Both neuronal nitric oxide synthase and endothelial nitric oxide synthase are expressed constitutively and activated by calcium and calmodulin. Inducible nitric oxide synthase is a calcium-independent enzyme and can be induced by various cerebral diseases, including ischemia, trauma and infection[33]. It has been demonstrated that the peak of inducible nitric oxide synthase expression is between 24 and 48 hours after cerebral ischemia[34]. Our results have shown that serum nitric oxide synthase activities from the Jing-Well points group at 23 hours after establishing the model were markedly lower than those of the model group (P < 0.01). Therefore, blood-letting puncture at twelve Jing-Well points of the hand may regulate inducible nitric oxide synthase activity. Our previous studies have demonstrated that blood-letting puncture at twelve Jing-Well points of the hand can regulate calcium and calmodulin[15,16], therefore possibly also adjusting the activity of neuronal nitric oxide synthase and endothelial nitric oxide synthase. Serum nitric oxide synthase activity in the mannitol group gradually increased from 23 hours and peaked at 47 hours. If experimental error is excluded, this result may suggest that mannitol can decrease the activity of neuronal nitric oxide synthase and endothelial nitric oxide synthase, but may play a lesser role in decreasing the activity of inducible nitric oxide synthase.
The mechanism of blood-brain barrier disruption after cerebral ischemia is very complex. Both in vitro and in vivo studies[35,36,37] have shown that hypoxia and ischemia can damage the primary endothelial barrier and also the secondary barrier. This secondary barrier consists of the basal lamina and the integrin-mediated interactions of cells with the extracellular matrix of the blood-brain barrier. Calcium is critical to normal blood-brain barrier function[38]. In fact, hypoxia has been clearly shown to cause an increase in intracellular calcium in brain microvessel endothelial cell culture[39,40,41]. As confirmation, blockade of calcium flux[42] or blockade of calcium regulated signaling cascades prevented hypoxia-induced disruption of blood-brain barrier monolayer integrity. During ischemia, free radicals such as reactive oxygen species produced by cells of the immune system cannot be scavenged, which results in the overproduction of oxygen-derived free radicals[43]. Reactive oxygen species can cause considerable damage to membrane lipids in the central nervous system, also threatening the integrity of the blood-brain barrier[44,45]. Our previous studies have demonstrated that blood-letting punctures at Jing-Well points of the hand can delay the development of cerebral hypoxia[13], decrease intracellular calmodulin activity[15] and inhibit a decrease in extracellular calcium ion concentration[16] in ischemic tissue following middle cerebral artery occlusion. Huang et al[12] also found that blood-letting punctures at Jing-Well points of the hand could obviously decrease the content of malondialdehyde and increase the activity of superoxide dismutase in ischemic brain tissue from rats that underwent middle cerebral artery occlusion. These results could help explain the regulating effect of blood-letting punctures at Jing-Well points of the hand on blood-brain barrier permeability. There are some studies[46,47] suggesting that modulation of expression of tight junction proteins could probably be reflected in permeability changes of the blood-brain barrier or edema, and the extracellular and intracellular calcium ion concentration is closely related with these changes. Therefore, one of the mechanisms causing the effect of blood-letting punctures on blood-brain barrier permeability may be via the regulation of extracellular and intracellular calcium ion concentration to influence the expression of tight junction proteins. However, the specific mechanism by which this effect occurs still requires further investigation.
Taken together, blood-letting punctures at twelve Jing-Well points of the hand can decrease blood-brain barrier permeability, serum nitric oxide synthase activity and cerebral edema in a middle cerebral artery occlusion model in rats. The effect of puncture was similar to that of mannitol injection and Jing-Well points plus mannitol. Compared with mannitol, blood-letting punctures at twelve Jing-Well points may have less side effects, and it is more easy to use and more affordable. This method is very suitable for pre-hospital care.
MATERIALS AND METHODS
Design
A randomized, controlled, animal experiment.
Time and setting
Experiments were performed at the Research Center Laboratory of Experimental Acupuncture, Tianjin University of Traditional Chinese Medicine, China, from September 2010 to July 2011.
Materials
Altogether, 140 male Wistar rats weighing 230–250 g, aged 3 months were selected from the Animal Center of Tianjin University of Traditional Chinese Medicine, China [license No. SCXK (Tianjin) 2009-0001]. Animals were allowed to acclimatize for 1 week prior to the experiment, and were housed under a 12-hour light-dark cycle subsisted with a standard laboratory diet and tap water ad libitum. Animal treatments were performed in a manner to minimize pain or discomfort in accordance with the Guidance Suggestions for the Care and Use of Laboratory Animals, formulated by the Ministry of Science and Technology of China[48].
Methods
Establishment of middle cerebral artery occlusion models
A modified Longa method was applied to prepare the model of middle cerebral artery embolism in rats[49]. Briefly, rats were anesthetized by abdominal injection with 10% chloral hydrate at a dose of 3.3 mL/kg and then fixed in a supine position. A midline incision was made at the neck to separate the common carotid artery, the external carotid artery and the internal carotid artery on the left side. Arteries were marked by ligatures on the external carotid artery and the proximal end of the common carotid artery. A 0.285-mm nylon thread was inserted from the distal end of the common carotid artery to the internal carotid artery, about 18.0 ± 0.5 mm in depth to the anterior cerebral artery. This thread gave rise to the blockage of the middle cerebral artery to induce focal cerebral ischemia (Figure 1). After animals recovered, deficits in neurological function were scored according to the method described by Longa[49]. Rats with a score of 1–3 were considered to have undergone successful cerebral ischemic injury. If rats did not score 1–3, they were not used for further experimentation. The nylon thread was not applied in the sham operation group.
Figure 1.
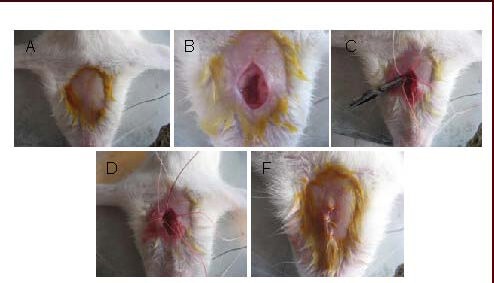
The process of establishing the middle cerebral artery occlusion model.
(A) Rats were anesthetized and then fixed in a supine position.
(B) A midline incision of the neck was made to separate the common carotid artery, the external carotid artery and the internal carotid artery on the left side.
(C) Arteries were marked by ligatures on the external carotid artery and the proximal end of the common carotid artery.
(D) A 0.285-mm nylon thread was inserted from the distal end of the bifurcation of the common carotid artery to the internal carotid artery, approximately 18.0 ± 0.5 mm in depth to the anterior cerebral artery.
(E) The wound was then sutured and disinfected.
Blood-letting puncture at twelve Jing-Well points of the hand
After model establishment, a 21 gauge blood lancet (Suzhou Sterilance Medical Equipment Co., Ltd., Suzhou, Jiangsu Province, China) was used to prick the acupoints, in sequence, for blood-letting at Shaoshang (LU11), Shangyang (LI1), Zhongchong (PC9), Guanchong (TE1), Shaochong (HT9) and Shaoze (SI1) at a depth of 1 mm[49] (Figure 2). The needle first penetrated vertically into the left foreleg and was not retained. This method was then repeated on the right side, at analogous Jing-Well points of the human hand[50]. Squeezing of the finger was done until bleeding stopped. Rats underwent surgery twice daily at 9:00–10:00 a.m. and 4:00–5:00 p.m. Rats from the control and sham operation groups were similarly grasped with equal strength minus the blood-letting puncture.
Figure 2.
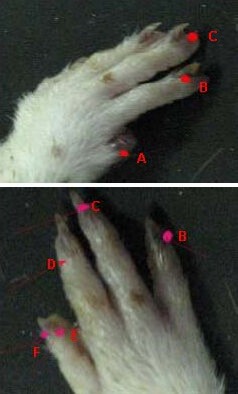
The acupoints of the left foreleg used for blood-letting in rats.
A: Shaoshang (LU11), B: Shangyang (LI1), C: Zhongchong (PC9), D: Guanchong (TE1), E: Shaochong(HT9), F: Shaoze(SI1).
Mannitol injection
After model establishment, 0.5 g/kg of 20% mannitol (Tianan Pharmaceutical Co., Ltd., Tianjin, China) was injected into the caudal vein of rats twice a day. In the Jing-Well points plus mannitol group, blood-letting puncture and mannitol injection were performed simultaneously.
Determination of blood-brain barrier permeability with Evans blue
At 23, 47, and 71 hours after model establishment, 4 mL/kg of 2% Evans blue (Sigma, St. Louis, MO, USA) in physiological saline was injected into the caudal vein of rats. One hour later, the rats were anesthetized with 10% chloral hydrate and were perfused with physiological saline. Dyed brain samples (0.1 g) were weighed and homogenized in 3 mL formamide (Sigma), incubated for 24 hours at 54°C, and centrifuged at 3 000 r/min for 15 minutes. The supernatants were analyzed at 620 nm using a 722-type spectrophotometer (Shanghai Third Analytical Instrument Factory, Shanghai, China).
Evans blue was made into a series of concentrations (0.05, 0.025, 0.012 5, 0.006 3, 0.003 1, 0.001 6, 0.000 7 μg/mL), and then absorbance was determined. A standard curve was plotted and linear regression revealed the equation y = 7.846x + 0.002 7, r = 0.999. These findings showed that Evans blue content was closely related to Evans blue absorbance.
Brain tissue Evans blue content (μg/g) was calculated using the equation: A × formamide quantity (mL)/brain tissue wet weight (g), where A is obtained by substituting the Evans blue absorbance into the linear regression equation.
Determination of nitric oxide synthase activity using spectrophotometry
Blood (2–3 mL) was collected from the retinal vein of rats before Evans blue was injected, and was centrifuged at 3 000 r/min for 15 minutes. Serum was analyzed at 530 nm using the 722-type spectrophotometer. Determination of nitric oxide synthase activity, using a kit, was strictly based on the instructions provided by the Nanjing Jiancheng Bioengineering Institute (Nanjing, Jiangsu Province, China).
Total nitric oxide synthase activity (U/mL) was determined using the following equation: (absorbance value of detected tube – absorbance value of blank tube)/molar extinction coefficient × (volume of reaction solution/sampling amount)/(1/chromatic light diameter × reaction time)/1 000 ≈ 141.57 × (absorbance value of detected tube – absorbance value of blank tube).
Statistical analysis
All results were expressed as mean ± SD for each group. Statistical analyses were performed with SPSS 11.0 software (SPSS, Chicago, IL, USA). One-way analysis of variance was used to compare mean differences in Evans blue leakage and nitric oxide synthase activity among groups. A P value less than 0.05 was considered to be a significant difference.
Acknowledgments:
We would like to show our deepest gratitude to Yue Zhang from the Experimental Acupuncture Research Center of Tianjin University of Traditional Chinese Medicine for providing valuable guidance in the translation of this thesis, and to thank all the teachers and students in the Research Center of Experimental Acupuncture-Moxibustion Science of Tianjin University of Traditional Chinese Medicine for their encouragement and support.
Footnotes
Xuan Lu, Master.
Funding: This study was sponsored by the Open Research Fund of Zhejiang First-foremost Key Subject—Acupuncture & Moxibustion, No. ZTK2010A07.
Conflicts of interest: None declared.
Ethical approval: The experimental protocol was approved by the Animal Ethics Committee of Tianjin University of Traditional Chinese Medicine in China.
(Edited by Wei JZ, Tian YF/Yang Y/Wang L)
REFERENCES
- [1].Lo EH, Dalkara T, Moskowitz MA. Mechanisms, challenges and opportunities in stroke. Nat Rev Neurosci. 2003;4(5):399–415. doi: 10.1038/nrn1106. [DOI] [PubMed] [Google Scholar]
- [2].Donnan GA, Fisher M, Macleod M, et al. Stroke. Lancet. 2008;371(9624):1612–1623. doi: 10.1016/S0140-6736(08)60694-7. [DOI] [PubMed] [Google Scholar]
- [3].Manley GT, Fujimura M, Ma T, et al. Aquaporin-4 deletion in mice reduces brain edema after acute water intoxication and ischemic stroke. Nat Med. 2000;6(2):159–163. doi: 10.1038/72256. [DOI] [PubMed] [Google Scholar]
- [4].Kofke WA. Mannitol: potential for rebound intracranial hypertension? J Neurosurg Anesthesiol. 1993;5(1):1–3. doi: 10.1097/00008506-199301000-00001. [DOI] [PubMed] [Google Scholar]
- [5].Khanna S, Davis D, Peterson B, et al. Use of hypertonic saline in the treatment of severe refractory posttraumatic intracranial hypertension in pediatric traumatic brain injury. Crit Care Med. 2000;28(4):1144–1151. doi: 10.1097/00003246-200004000-00038. [DOI] [PubMed] [Google Scholar]
- [6].Node Y, Nakazawa S. Clinical study of mannitol and glycerol on raised intracranial pressure and on their rebound phenomenon. Adv Neurol. 1990;52:359–363. [PubMed] [Google Scholar]
- [7].Guo Y, Zhang YJ, Wang XY, et al. Effects of blood-letting puncture at twelve Jing-Well points of hand on the intracranial haemodynamics of stroke patients. Zhenjiu Linchuang Zazhi. 1995;11(6):21–23. [Google Scholar]
- [8].Li Q, Wang X, Ren HZ, et al. Effects of blood-letting puncture at twelve Jing-Well points of hand on the cerebral blood flow. Zhongguo Zhongyiyao Xinxi Zazhi. 2000;7(3):51. [Google Scholar]
- [9].Ding J, Guo Y. Effects of pricking blood at twelve Jing points of hand on state of consciousness in the patient of early stroke. Zhongguo Zhenjiu. 2004;24(10):673–676. [Google Scholar]
- [10].Guo Y, Wang XY, Xu TP, et al. Clinical observation of the influence of puncture and blood letting at twelve Hand Jing Point on consciousness and heart rate in patients with wind stroke. Tianjin Zhongyiyao. 2003;20(2):35–37. [Google Scholar]
- [11].Zhang XH, Sun SX, Xu BJ. The basic research on the effect of blood-letting puncture at Jing-Well points on the infarction volume after focal cerebral ischemia in rats. Zhenjiu Linchuang Zazhi. 2004;20(12):47–48. [Google Scholar]
- [12].Huang BL, Yu LZ, Liu SX, et al. The effects of blood-letting puncture in twelve-well points of the hand on content of MDA and activity of SOD after focal cerebral ischemia in rats. Xianning Xueyuan Xuebao. 2005;19(1):4–6. [Google Scholar]
- [13].Ma YF, Guo Y, Zhang YJ, et al. Dynamic observation of the influence of blood-letting puncture of hand twelve well points on partial pressure of oxygen in ischemic brain tissue in rats with experimental cerebral ischemia. Shanghai Zhenjiu Zazhi. 2000;19(1):40–42. [Google Scholar]
- [14].He SQ, Guo Y, Ma YF, et al. Experimental research on the effects of blood-letting puncture at twelve Jing-Well points of hand on hydrogen ion concentration of ischemia area in rats with experimental cerebral ischemia. Zhenjiu Linchuang Zazhi. 2002;18(2):43–45. [Google Scholar]
- [15].Ma YF, Guo Y, Wang XY, et al. The experimental observation on affect the CaM content of the cerebral ischemia region intracellular of MCAO model rat by blood-letting puncture in twelve-well points. Zhenci Yanjiu. 1999;24(2):105–107. [Google Scholar]
- [16].Guo Y, Hu LM, Zhang YJ, et al. Dynamic observation of the influence of blood-letting puncture of hand twelve well points on extracellular calcium ion concentration in rats with experimental cerebral ischemia. Zhenjiu Linchuang Zazhi. 1999;15(6):48–50. [Google Scholar]
- [17].Ren XJ, Tu Y, Guo Y, et al. Dynamic observation of the effects of bloodletting of the 12 hand Jing-Points on the level of excitatory amino acid in the brain of the rats with cerebral ischemia. Beijing Zhongyiyao Daxue Xuebao. 2001;24(6):48–51. [Google Scholar]
- [18].Ren XJ, Tu Y, Guo Y, et al. Effects of the bloodletting of the 12 hand Jing-Points on the level of nitric oxide in the brain of the rats with cerebral ischemia. Beijing Zhongyiyao Daxue Xuebao. 2001;24(4):51–53. [Google Scholar]
- [19].Huang BL, Yu LZ, Cheng J. Intervention of blood-letting puncture on 12-well points of hand on activity of nitric oxide synthase after focal cerebral ischemia in rats. Zhongguo Zuzhi Gongcheng Yanjiu yu Linchuang Kangfu. 2006;10(7):174–176. [Google Scholar]
- [20].Wang XY, Li JS, Liu GW, et al. Effect of collateral puncture-bloodletting at Jing point on expression of HSP70 protein of cerebral cortex in rats with middle cerebral artery occlusion. Tianjin Zhongyiyao. 2005;22(6):477–479. [Google Scholar]
- [21].Wang XY, Li JS, Guo Y, et al. The influence of blood-letting puncture of twelve Jing-Well points on corticocerebral C-fos protein expression in rat MCAO model. Shanghai Zhenjiu Zazhi. 2004;23(12):39–41. [Google Scholar]
- [22].Gao L. Tianjin: Tianjin University of Traditional Chinese Medicine; 2009. The influences of blood-letting puncture at Jing-points and Chinese herb Job's tears (Yiyiren) on the survival rate and brain edema in experimental cerebral ischemic rats. [Google Scholar]
- [23].Tian LX. Tianjin: Tianjin University of Traditional Chinese Medicine; 2010. Study on the brain-protecting effect of bloodletting puncture at Jing(Well)-points and semen coicis (Yiyiren)—the influences on survival rate, survival time and brain edema in cerebral ischemic rats. [Google Scholar]
- [24].del Zoppo GJ, Mabuchi T. Cerebral microvessel responses to focal ischemia. J Cereb Blood Flow Metab. 2003;23(8):879–894. doi: 10.1097/01.WCB.0000078322.96027.78. [DOI] [PubMed] [Google Scholar]
- [25].Brown RC, Mark KS, Egleton RD, et al. Protection against hypoxia-induced increase in blood-brain barrier permeability: role of tight junction proteins and NF kappaB. J Cell Sci. 2003;116(Pt 4):693–700. doi: 10.1242/jcs.00264. [DOI] [PubMed] [Google Scholar]
- [26].Klatzo I. Pathophysiological aspects of brain edema. Acta Neuropathol. 1987;72(3):236–239. doi: 10.1007/BF00691095. [DOI] [PubMed] [Google Scholar]
- [27].Mayhan WG. Role of nitric oxide in histamine-induced increases in permeability of the blood-brain barrier. Brain Res. 1996;743(1-2):70–76. doi: 10.1016/s0006-8993(96)01021-9. [DOI] [PubMed] [Google Scholar]
- [28].Huang BL, Chen J. The effects of blood-letting puncture in “Twelve-Well Points of the Hand” on content of NO and activity of NOS after focal cerebral ischemia in rats. Xianning Xueyuan Xuebao: Yixue Ban. 2004;18(5):312–314. [Google Scholar]
- [29].Rosenberg GA, Estrada EY, Dencoff JE. Matrix metalloproteinases and TIMPs are associated with blood-brain barrier opening after reperfusion in rat brain. Stroke. 1998;29(10):2189–2195. doi: 10.1161/01.str.29.10.2189. [DOI] [PubMed] [Google Scholar]
- [30].Yang ZZ, Chen H, Yan MY, et al. Experimental study of alteration of blood-brain barrier leakage after acute cerebral ischemia in rats. Zhonghua Zhongyiyao Xuekan. 2009;27(3):26–28. [Google Scholar]
- [31].Kader A, Frazzini VI, Solomon RA, et al. Nitric oxide production during focal cerebral ischemia in rats. Stroke. 1993;24(11):1709–1716. doi: 10.1161/01.str.24.11.1709. [DOI] [PubMed] [Google Scholar]
- [32].Chi OZ, Wei HM, Sinha AK, et al. Effects of inhibition of nitric oxide synthase on blood-brain barrier transport in focal cerebral ischemia. Pharmacology. 1994;48(6):367–373. doi: 10.1159/000139202. [DOI] [PubMed] [Google Scholar]
- [33].Andrew PJ, Mayer B. Enzymatic function of nitric oxide synthases. Cardiovasc Res. 1999;43(3):521–531. doi: 10.1016/s0008-6363(99)00115-7. [DOI] [PubMed] [Google Scholar]
- [34].Sehara Y, Hayashi T, Deguchi K, et al. Distribution of inducible nitric oxide synthase and cell proliferation in rat brain after transient middle cerebral artery occlusion. Brain Res. 2006;1093(1):190–197. doi: 10.1016/j.brainres.2006.03.092. [DOI] [PubMed] [Google Scholar]
- [35].Witt KA, Mark KS, Hom S, et al. Effects of hypoxia-reoxygenation on rat blood-brain barrier permeability and tight junctional protein expression. Am J Physiol Heart Circ Physiol. 2003;285(6):H2820–2831. doi: 10.1152/ajpheart.00589.2003. [DOI] [PubMed] [Google Scholar]
- [36].Wagner S, Tagaya M, Koziol JA, et al. Rapid disruption of an astrocyte interaction with the extracellular matrix mediated by integrin alpha 6 beta 4 during focal cerebral ischemia/reperfusion. Stroke. 1997;28(4):858–865. doi: 10.1161/01.str.28.4.858. [DOI] [PubMed] [Google Scholar]
- [37].Hamann GF, Liebetrau M, Martens H, et al. Microvascular basal lamina injury after experimental focal cerebral ischemia and reperfusion in the rat. J Cereb Blood Flow Metab. 2002;22(5):526–533. doi: 10.1097/00004647-200205000-00004. [DOI] [PubMed] [Google Scholar]
- [38].Brown RC, Davis TP. Calcium modulation of adherens and tight junction function: a potential mechanism for blood-brain barrier disruption after stroke. Stroke. 2002;33(6):1706–1711. doi: 10.1161/01.str.0000016405.06729.83. [DOI] [PubMed] [Google Scholar]
- [39].Ikeda K, Nagashima T, Wu S, et al. The role of calcium ion in anoxia/reoxygenation damage of cultured brain capillary endothelial cells. Acta Neurochir Suppl. 1997;70:4–7. doi: 10.1007/978-3-7091-6837-0_2. [DOI] [PubMed] [Google Scholar]
- [40].Kimura C, Oike M, Ito Y. Hypoxia-induced alterations in Ca(2+) mobilization in brain microvascular endothelial cells. Am J Physiol Heart Circ Physiol. 2000;279(5):H2310–2318. doi: 10.1152/ajpheart.2000.279.5.H2310. [DOI] [PubMed] [Google Scholar]
- [41].Park JH, Okayama N, Gute D, et al. Hypoxia/aglycemia increases endothelial permeability: role of second messengers and cytoskeleton. Am J Physiol. 1999;277(6 Pt 1):C1066–1074. doi: 10.1152/ajpcell.1999.277.6.C1066. [DOI] [PubMed] [Google Scholar]
- [42].Abbruscato TJ, Davis TP. Combination of hypoxia/aglycemia compromises in vitro blood-brain barrier integrity. J Pharmacol Exp Ther. 1999;289(2):668–675. [PubMed] [Google Scholar]
- [43].Chan PH, Schmidley JW, Fishman RA, et al. Brain injury, edema, and vascular permeability changes induced by oxygen-derived free radicals. Neurology. 1984;34(3):315–320. doi: 10.1212/wnl.34.3.315. [DOI] [PubMed] [Google Scholar]
- [44].Griot C, Vandevelde M, Richard A, et al. Selective degeneration of oligodendrocytes mediated by reactive oxygen species. Free Radic Res Commun. 1990;11(4-5):181–193. doi: 10.3109/10715769009088915. [DOI] [PubMed] [Google Scholar]
- [45].Kim YS, Kim SU. Oligodendroglial cell death induced by oxygen radicals and its protection by catalase. J Neurosci Res. 1991;29(1):100–106. doi: 10.1002/jnr.490290111. [DOI] [PubMed] [Google Scholar]
- [46].Lamas M, González-Mariscal L, Gutiérrez R. Presence of claudins mRNA in the brain. Selective modulation of expression by kindling epilepsy. Brain Res Mol Brain Res. 2002;104(2):250–254. doi: 10.1016/s0169-328x(02)00328-5. [DOI] [PubMed] [Google Scholar]
- [47].Cruzalegui FH, Bading H. Calcium-regulated protein kinase cascades and their transcription factor targets. Cell Mol Life Sci. 2000;57(3):402–410. doi: 10.1007/PL00000702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].The Ministry of Science and Technology of the People's Republic of China. Guidance Suggestions for the Care and Use of Laboratory Animals 2006-09-30 [Google Scholar]
- [49].Longa EZ, Weinstein PR, Carlson S, et al. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20(1):84–91. doi: 10.1161/01.str.20.1.84. [DOI] [PubMed] [Google Scholar]
- [50].Gao L, Chen ZL, Tian LX, et al. Effects of bloodletting puncture at Jing-Well points in distal ends of finger and toe on survival rate and brain edema in cerebral ischemic rats. J Tradit Chin Med. 2012;32(3):471–476. doi: 10.1016/s0254-6272(13)60057-6. [DOI] [PubMed] [Google Scholar]


