Abstract
Chronic stress models, established in adult Sprague-Dawley rats through a 14-day subcutaneous injection of 40 mg/kg corticosterone, once per day, were given a daily oral feeding of 50 mg/kg baicalin. The study was an attempt to observe the effect of baicalin on neurogenesis in chronically stressed rats. Results showed that subcutaneous injection of corticosterone significantly decreased the total number of doublecortin-positive neurons in the hippocampus. The reduced cell number caused by corticosterone was mainly due to the decrease of class II doublecortin-positive neurons, but the class I doublecortin-positive neurons were unaffected. Baicalin treatment increased the number of both class I and class II doublecortin-positive neurons. In addition, doublecortin-positive neurons showed less complexity in dendritic morphology after corticosterone injection, and this change was totally reversed by baicalin treatment. These findings suggest that baicalin exhibits a beneficial effect on adult neurogenesis.
Keywords: neural regeneration, traditional Chinese medicine, neurogenesis, neurodegenerative disease, baicalin, stress, hippocampus, neurons, doublecortin, dendrites, cognition, mood regulation, grants-supported paper, photographs-containing paper, neuroregeneration
Research Highlights
(1) The influence of baicalin on the differentiation and maturation of rat neural precursor cells into neurons was investigated, using in vivo experimental evidence to show that baicalin promotes neurogenesis.
(2) Using classification methods, the effect of baicalin on the number and morphology of class I and class II doublecortin-positive newborn neurons was observed.
(3) Baicalin promoted the proliferation and maturation of neural precursor cells, and exhibited neuroprotective activity in chronically stressed rats.
(4) Baicalin appears to have therapeutic actions and clinical applications for the improvement of cognitive function and emotional regulation.
INTRODUCTION
Adult neurogenesis is a dynamic process that occurs in the dentate gyrus and subventricular zone in mammals. This life-long process has been regarded to be closely related with the occurrence of psychiatric disorders and cognitive functions[1].
Adult neurogenesis in the dentate gyrus is vulnerable to various challenges, including inhibitory and stimulating challenges[2,3,4,5]. One inhibitory challenge is to induce chronic hypercortisolemia in experimental animals by the daily injection[6] or oral administration of corticosterone[7]. Numerous studies have shown that chronic treatment of high-dose corticosterone impairs adult neurogenesis in the dentate gyrus[8,9,10], and damaged neurogenesis is consequently associated with behavioral deficits, including depression-like behaviors[11,12], reduced learning and memory[13], and impaired sexual activities[14]. The significance of adult neurogenesis and effective ways to prevent corticosterone toxicity of adult neurogenesis need to be explored.
Baicalin, a commercially used Chinese herbal medicine, is a flavonoid isolated from the root of Scutellaria baicalensis Georgi[15]. As shown in Figure 1, purified baicalin, with a molecular weight of 447.092 Da (C21H18O11), is known to be capable of effectively passing through the blood-brain barrier and the gastrointestinal tract[16].
Figure 1.
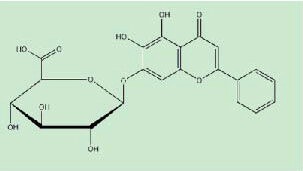
Structure of baicalin.
Its anti-inflammatory activities have been well recognized in different infective models, including pancreatitis[17,18,19], hepatitis[20], and autoimmune encephalomyelitis[21]. Furthermore, it has been reported that baicalin promotes the neuronal differentiation of several cell lines in vitro, including human umbilical cord blood mesenchymal stem cells[22], rat bone marrow stromal cells[23], and neural progenitor cells[24]. However, little is known if baicalin has any effects on adult neurogenesis in vivo. Adult neurogenesis consists of multiple steps: proliferation, differentiation, migration, maturation and synaptic integration[25]. Briefly, young neurons are generated from the progenitor cell pool in the proliferation stage and differentiation stage; a small number of young neurons migrate to their target region and acquire mature neuronal features, and eventually they integrate into the existing neuronal circuits. Doublecortin, a protein promoting microtubule polymerization, is present in migrating neuroblasts and young neurons[26,27], and is barely expressed in non-neurogenic regions in the mature central nervous system[28]. During adult neurogenesis, expression of doublecortin starts as neuroblasts are generated, peaks in the 2nd week, and is downregulated concomitantly with the appearance of the mature neuronal marker NeuN[29,30]. Therefore, doublecortin can be used as a long-term marker in adult neurogenesis. Immunohistochemical staining of doublecortin has been used as a powerful tool in basic research to study the positive[31,32,33], as well as negative modulators[34,35] of the number and morphology of newly formed neurons in adult neurogenesis.
The aim of this study was to investigate the protective effects of baicalin on adult neurogenesis in the dentate gyrus of chronically stressed rats, using the immunohistochemical staining of doublecortin.
RESULTS
Quantitative analysis of experimental animals
Twelve adult rats were randomly divided into four groups and subjected to the following treatments: (1) PBS + vehicle group (14-day oral feeding with PBS plus vehicle injection); (2) PBS + corticosterone group (14-day oral feeding with PBS plus 40 mg/kg corticosterone injection); (3) baicalin + vehicle group (14-day oral feeding with 50 mg/kg baicalin plus vehicle injection); (4) baicalin + corticosterone group (14-day oral feeding with 50 mg/kg baicalin plus 40 mg/kg corticosterone injection). There were three rats in each group. All rats were involved in the final analysis.
Baicalin significantly increased the number of doublecortin-positive cells in the hippocampus of corticosterone-treated chronically stressed rats
To study the effects of baicalin on adult neurogenesis, we checked the immunoreactivity of doublecortin in the hippocampus, a well-known protein expressed in newly formed neurons, which is associated with migration and differentiation of neuronal progenitor cells[36]. As shown in Figure 2A, most of the doublecortin-positive cell bodies were located at or just beneath the bottom of the granular cell layer, with short or long processes. First, the total population of doublecortin-positive neurons was quantified by Stereo Investigator software. Two-way analysis of variance revealed a significant effect of corticosterone (F(1, 11) = 174.828, P < 0.001) and an interactive effect of corticosterone and baicalin (F(1, 11) = 198.20, P < 0.001) on the total number of doublecortin-positive cells (Figures 2A, C). Corticosterone injection significantly reduced the total number of doublecortin-positive cells (P < 0.001). This effect was reversed by baicalin treatment (P < 0.001; Figure 2A, C).
Figure 2.
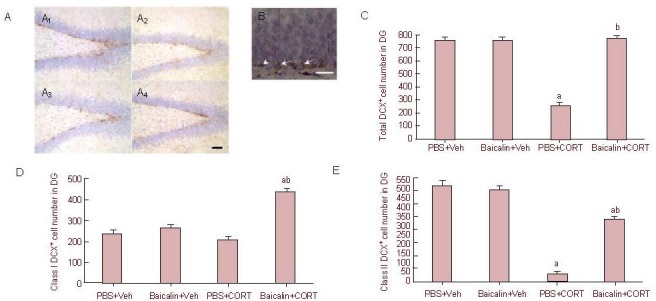
Effect of baicalin treatment on doublecortin (DCX) expression in the hippocampus, and quantitative analysis of DCX- positive cells (immunohistochemical staining).
(A) Immunoreactivity of DCX in the hippocampus. Corticosterone (CORT) treatment greatly decreased the expression of DCX in the dentate gyrus (DG), while baicalin treatment restored the expression of DCX (scale bar: 20 μm). (A1): PBS + vehicle (Veh) group; (A2): baicalin + Veh group; (A3): PBS + CORT group; (A4): baicalin + CORT group.
(B) Morphological characterization of class I (arrowhead) and class II (arrow) DCX-positive cells (scale bar: 50 μm).
(C–E) Quantitative analysis of cell number of total (C), class I (D) and class II (E) DCX-positive cells. Data are expressed as mean ± SEM, and there were three rats in each group. Six sections in each animal containing the dorsal hippocampus were picked and counted under a 40 × lens using Stereo Investigator software. aP < 0.01, vs. PBS + Veh group, bP < 0.01, vs. PBS + CORT group by two-way analysis of variance followed by Student-Newman-Keuls test.
To further address which subset of the doublecortin-positive cells was affected, i.e. the relatively younger or older cells, we counted class I and II doublecortin-positive cells (Figure 2B). As shown in Figure 2B, class I doublecortin-positive cells only had very short horizontal processes, without a long process extending through the granular cell layer. On the contrary, class II doublecortin-positive cells displayed a long vertical process branching through the granular cell layer. Almost no effect of corticosterone injection was found on the number of class I cells (P > 0.05; Figure 2D), and no effect of baicalin treatment alone was found on the number of class I cells (P > 0.05; Figure 2D). However, there was a significant interactive effect of corticosterone and baicalin treatment (P < 0.001) on class I doublecortin-positive cells. Baicalin treatment in corticosterone-treated rats displayed a higher number of class I doublecortin-positive cells than the rats in the PBS + vehicle group (control, P < 0.001; Figure 2D).
Two-way analysis of variance revealed that corticosterone significantly reduced the number of class II doublecortin-positive cells (F(1, 11) = 54.624, P < 0.001; Figure 2E). Baicalin treatment alone had no effect in control rats (P = 0.273). However, baicalin treatment significantly increased the number of class II doublecortin-positive cells in the corticosterone-treated chronically stressed rats, but the increased number was still lower than the control level. The above findings demonstrated that reduced neurogenesis in the hippocampus caused by corticosterone injection might be mainly due to the reduced number of class II doublecortin-positive cells, whereas class I doublecortin-positive cells were slightly affected, and baicalin treatment restored this reduction in neurogenesis by increasing the number of both class I and class II doublecortin-positive cells.
Baicalin restored the morphological changes of doublecortin-positive cells in the hippocampus of corticosterone-treated chronically stressed rats
Dendritic morphology is regarded as one of the important indicators in neuronal maturation[37,38]. In the present study, we adopted dendritic length and the number of intersections in Sholl analysis[39] to study the complexity of dendritic morphology in doublecortin-positive cells (Figure 3A). In brief, the outline of one single neuron was traced.
Figure 3.
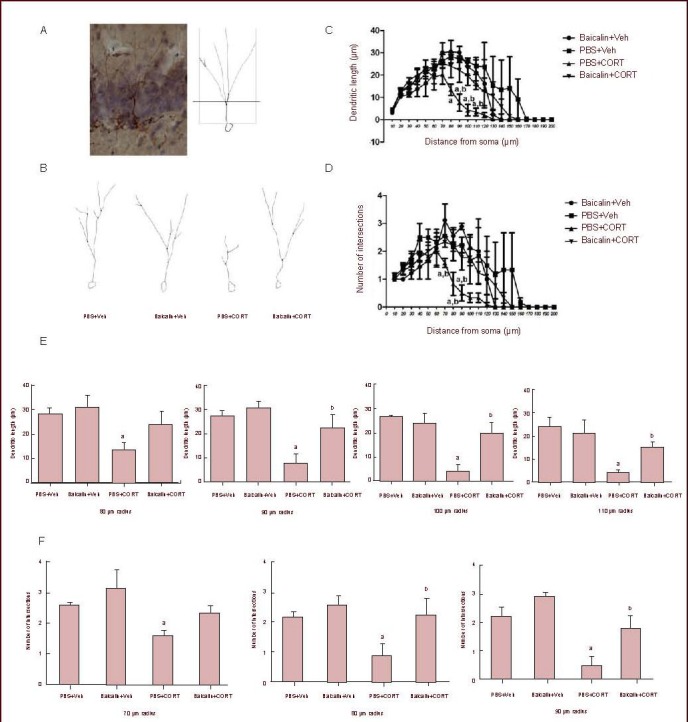
Effect of baicalin treatment on dendritic tree of doublecortin-positive cells in the dentate gyrus of corticosterone (CORT)-treated rats.
(A) Representative image (left) and traces by Filament tracer software of doublecortin-positive cells in the dentate gyrus.
(B) PBS and baicalin-treated rats displayed similar dendritic trees; CORT-treated rats displayed a less complex morphology of dendritic tree, while baicalin treatment was able to restore the morphological damage caused by CORT.
(C, D) The dendritic length (C) and the number of intersections (D) were analyzed in different groups. Both the number of intersections and dendritic length represent the complexity of the dendrite.
(E, F) Quantitative analysis of dendritic length (E) and the number of intersections (F) in different concentric circles.
Data are expressed as mean ± SEM; there are three rats in each group. Three cells in the granular cell layer from each animal were analyzed, under a 40 × lens of a light microscope. aP < 0.05, vs. PBS + vehicle (Veh) group; bP < 0.05, vs. PBS + CORT group by two-way analysis of variance followed by Student-Newman-Keuls test.
The outlined dendritic tree was analyzed using a set of concentric circles (diameter 10–200 μm) centered at the centroid of the cell body. The intersections with the concentric circles and the dendritic length covered by every two concentric circles were counted, respectively. It was found that 40 mg/kg corticosterone injection dramatically reduced the dendritic length (PBS + corticosterone vs. PBS + vehicle: 80 μm P = 0.025, 90 μm P = 0.006, 100 μm P = 0.001, 110 μm P = 0.004; Figures 3C, E) and the number of intersections (PBS + corticosterone vs. PBS + vehicle: 70 μm P = 0.01, 80 μm P = 0.04, 90 μm P = 0.004; Figures 3D, F), two parameters that reflect the complexity of dendritic trees in neurons. However, after 50 mg/kg baicalin treatment, the dendritic length of doublecortin-positive cells (baicalin + corticosterone vs. PBS + corticosterone: 80 μm P = 0.014, 90 μm P = 0.024, 100 μm P = 0.009, 110 μm P = 0.05; Figures 3C, E), as well as the number of intersections in corticosterone-treated rats significantly increased (baicalin + corticosterone vs. PBS + corticosterone: 70 μm P = 0.138, 80 μm P = 0.035, 90 μm P = 0.018; Figures 3D, F). No obvious changes in dendritic morphology were observed after baicalin treatment alone (Figures 3B–F). These findings showed that baicalin treatment was able to maintain the dendritic complexity of newborn neurons subjected to 40 mg/kg corticosterone challenge.
DISCUSSION
In the present study, baicalin treatment enhanced neurogenesis by increasing the number of both class I and class II doublecortin-positive cells in the hippocampus of corticosterone-induced chronically stressed rats. Furthermore, baicalin treatment restored the morphological complexity of doublecortin-positive cells in the corticosterone-treated rats. This neuroprotective effect of baicalin agreed with previous findings[22,23,24,40,41]. Several studies have shown that baicalin has a powerful ability to promote neuronal differentiation in different lines of stem cells, including mouse C17.2 neural stem cells[40] and human mesenchymal stem cells[41]. However, one limitation in all the studies was that those experiments just provided in vitro evidence showing the beneficial effects of baicalin. To our knowledge, the present data provided the first line of in vivo evidence that baicalin can exhibit a protective effect on adult neural progenitor cells.
During adulthood in mammals, new neurons are continuously produced from a pool of neural progenitor cells located in two brain regions: the dentate gyrus and the subventricular zone. Then, a small amount of newly formed neurons manage to mature and integrate into the existing neuronal circuits. The whole process is called “adult neurogenesis”, consisting of several stages: proliferation, differentiation, migration, maturation, and synaptic integration. Adult neurogenesis in mammals is a highly conserved activity, existing from lower rodents[42,43] to primates[44,45,46]. Therefore, using specific markers such as Pax6 (a transcription factor during embryonic development)[47,48] and Tuj 1 (the neuron-specific class III-tubulin)[49,50] to visualize the different stages of neurons in adult neurogenesis is a useful tool to understand the details and mechanisms of adult neurogenesis. As a microtubule-associated protein[51], doublecortin can be used to differentially detect the proliferative progenitor cell stage from the post-mitotic phase with long dendrites[52,53]. That is to say, doublecortin immunoreactivity can be found both in proliferating progenitor cells and in immature newborn neurons. Furthermore, based on the location and apical dendrites[35,53], doublecortin-positive cells can be divided into two types in a simple way. Smaller cells (class I cells) without a dendrite that penetrate the granule cell layer are known to be progenitor cells, since 2/3 of the population of these types of cells are in the cell cycle. A later study[54] also showed younger neurons expressed lesser levels of doublecortin using immunofluorescence cell sorting. Therefore, 2/3 of class I cells labeled by doublecortin could be regarded as proliferating progenitor cells. Longer cells (class II cells) with dendrites extending into the molecular layer are considered as immature post-mitotic neurons (Figure 1B). Here, our data found that in corticosterone-treated rats, the number of class II cells was greatly decreased while leaving the class I cells nearly untouched. The data indicated that corticosterone injection impaired neurogenesis in the hippocampus, mainly by reducing the number of newly born neurons. However, after baicalin treatment, both the number of class I and class II doublecortin-positive cells were dramatically increased. The findings strongly suggest that baicalin might promote the survival of both the proliferating progenitor cells and post-mitotic neurons in adult neurogenesis, and consequently increase the number of both class I and class II doublecortin-positive cells in the hippocampus. A few previous studies found that baicalin treatment was able to promote the survival of adult neurons. Jung et al[55] performed a study showing that baicalin protected retinal ganglion cells in vitro from ischemic and oxidative insults. Other studies also found that baicalin treatment enhanced the survival of cortical neurons under deprivation of oxygen/glucose or glutamate toxicity[56,57]. Here, our data added to the knowledge that baicalin could enhance the survival of proliferating progenitor cells and newly formed neurons during the adult neurogenesis in the hippocampus.
In addition, the dendritic toxicity of corticosterone has long been recognized in mature neurons in the adult central nervous system[58,59]. Previous studies have shown that daily injection or oral administration of corticosterone greatly impairs apical dendritic morphology of the pyramidal cells in the CA3 region of the hippocampus. Corticosterone treatment decreased the length and branches of apical dendrites in CA3 pyramidal cells[60,61]. Similar changes were also found in CA1 pyramidal cells in the adult hippocampus[62,63]. The reduced dendritic length and branches in mature pyramidal cells caused by corticosterone were further thought to be related to the release of excitatory amino acids[64]. More recently, studies have also reported that corticosterone was able to affect spine stability and dendritic morphology in developing neurons[65,66,67]. In spite of mature neurons, research has proven that corticosterone treatment is also able to affect the dendritic dynamics in developing neurons. In young hippocampal organic cultures, high-dose application of corticosterone induced the atrophy of the apical dendritic tree in CA1 pyramidal cells in tissue slices[66]. Another example of corticosterone inhibiting dendritic development in young neurons was in prenatally stressed experimental animals[68,69,70,71]. Offspring animals with prenatal stress were shown to display elevated serum corticosterone and were found to have reduced spine density and dendritic complexity in the hippocampus[70], as well as in the prefrontal cortex[71].
In the present study, our data also found that the reduced dendritic complexity in doublecortin-positive neurons was induced by 2-week corticosterone injection. Considering the criteria in analyzing the doublecortin-positive cells with tertiary dendrites, the qualified doublecortin-positive neurons were more likely to be class II doublecortin-positive cells, which were newly formed neurons during the process of neurogenesis. In the current study, after baicalin treatment, the dendritic damage to class II doublecortin-positive cells caused by corticosterone injection was reversed. This finding indicated that corticosterone injection impaired the dendritic development of the newly formed neurons during the process of neurogenesis; while baicalin treatment protected the dendritic morphology of the doublecortin-positive cells in adult neurogenesis from corticosterone toxicity. Previous studies have shown that by using antagonists of corticosterone receptors (RU38468) or N-methyl-D-aspartic acid receptor knockdown mice, the inhibitory effects of corticosterone on dendritic growth were blocked in the CA1 and CA3 fields, respectively[66,72]. Additionally, it was well established by Garcia and colleagues that the glucocorticoid receptors were abundantly expressed in the newly formed neurons generated from the neural progenitor cells during neurogenesis[73]. An in vitro study has shown that, similar to MK801 (an antagonist of N-methyl-D-aspartic acid receptor), baicalin exhibited the ability to inhibit the activation of 5-lipoxygenase mediated by the N-methyl-D-aspartic acid receptor, and consequently reduced the damage by oxygen/glucose deprivation on the cultured cortical neurons[74]. Hence, one possible explanation here is that baicalin might antagonize the adverse effects of corticosterone on the dendritic development of young neurons by modulating the bioactivity of the N-methyl-D-aspartic acid receptors or the glucocorticoid receptors, or both.
Altogether, the present in vivo study showed that baicalin exhibited beneficial effects on proliferating progenitor cells and newborn neurons during adult neurogenesis in the dentate gyrus. Whether baicalin has the potential to treat psychological disorders or to improve cognitive functions requires further investigation, such as behavioral tests and cell signaling studies.
MATERIALS AND METHODS
Design
A randomized, controlled, animal experiment.
Time and setting
All the experiments were conducted from September 2011 to May 2012 in the Anesthesia Research Institute, Central South University, China.
Materials
Animals
Twelve 2-month-old male Sprague-Dawley rats, weighing 220–250 g, were obtained from the Department of Laboratory Animal at Central South University, China, with license number SCXK (Xiang) 2009-0004. The animals were maintained at a controlled temperature and 12-hour light/dark cycle with access to food and water ad libitum. All experimental procedures were in accordance with the Guidance Suggestions for the Care and Use of Laboratory Animals, formulated by the Ministry of Science and Technology of China[75].
Drug
Baicalin, 95% purity, was purchased from Sigma-Aldrich (Cat No. 572667, St. Louis, MO, USA).
Methods
Establishment of corticosterone-induced stress models and drug treatment
Corticosterone (Sigma-Aldrich) was suspended and sonicated in sesame oil as previously described[13]. The ready-to-use suspension was subcutaneously injected into PBS + corticosterone and baicalin + corticosterone groups at a dose of 40 mg/kg daily for 14 days to induce stress. At the same time, an oral feeding with 50 mg/kg baicalin dissolved in 0.01 M PBS was started, and was given to the baicalin + corticosterone group for 14 days. The PBS + corticosterone rats were orally given 0.01 M PBS at the same volume. The PBS + vehicle rats were orally given 0.01 M PBS plus vehicle injection (sesame oil) for 14 days; the baicalin + vehicle rats were orally dosed with 50 mg/kg baicalin dissolved in 0.01 M PBS plus vehicle injection for 14 days.
Sample collection and tissue processing
After treatment, all animals were anesthetized by over-dose of 2% sodium phenobarbital and transcardially prefixed with 4% paraformaldehyde. All the brains were post-fixed for 48 hours in 4% paraformaldehyde and immersed in 30% sucrose solution until each brain sank to the bottom of the solution. Serial coronary frozen sections (40 μm thickness) were cut with a freezing microtome and prepared for immunohistochemistry.
Immunohistochemistry for doublecortin expression
The brain sections were brought to room temperature and were blocked with a solution containing 0.01 M PBS, 0.1% Triton X-100 and 10% normal goat serum solution for 30 minutes, and subsequently incubated at 4°C overnight with polyclonal rabbit anti-doublecortin (1:500; Cell Signaling Technology, Boston, MA, USA). After thorough washes, sections were then incubated with goat anti-rabbit IgG (1:200; Vector Laboratories, Burlingame, CA, USA) and followed by avidin-biotin complex (1:200; Vector Laboratories) at room temperature for 60 minutes, respectively. Chromogen reactions were performed with diaminobenzidine (Sigma-Aldrich) and 0.1% H2O2 for 10 minutes. The sections were dehydrated in graded alcohol and cleared in toluene, and then coverslipped. The stained sections were observed using a CHBS light microscope (Olympus, New York Microscope Company Inc., NY, USA).
Analysis of the number of doublecortin-positive cells
To determine the number of doublecortin-positive cells in the hippocampus, six sections (at 440 μm intervals) were selected in each animal and processed for immunohistochemical staining. The doublecortin-positive cells in the dentate gyrus in the dorsal part of hippocampus were counted using Stereo Investigator software (Micro-Bright Field Biotechnology, New York, NY, USA) by a skilled and blinded experimenter. The software randomly offered 30–50 visual fields under 40 × lens and the qualified cells were counted. The selected doublecortin-positive cells were further divided into class I and class II doublecortin-positive cells, as previously described[35,53]. Briefly, the most mature class II doublecortin-positive cells displayed a primary dendrite that was orientated perpendicular to the subgranular zone. The class I doublecortin-positive cells were located in the subgranular zone, without a dendrite, or only a short dendrite.
Dendritic complexity of doublecortin-positive neurons
Dendritic outline of doublecortin-positive cells including dendritic length and number of intersections (branch points) in the granule cells layer of the hippocampal dentate gyrus were traced using Filament tracer software (Bitplane Inc., South Windsor, CT, USA) and then analyzed by Imaris track software (Bitplane Inc.). A qualified neuron for analysis displayed a comparatively independent dendritic tree with at least tertiary branches. Tracings were analyzed by Sholl analysis in concentric circles (diameter 10–200 μm) with their center in the cell body[39]. In brief, the dendritic length counted as the length of the tracing between every two concentric circles. The intersection number was the number of tracings crossing every circle. All tracings were done by a skilled experimenter blind to the groups and treatments. A higher value in dendritic length or the number of intersections represented a neuron with more complex dendritic branches.
Statistical analysis
All data were presented as mean ± SEM. Comparisons of multiple groups were done by two-way analysis of variance-dependent experimental designs and followed by Student-Newman-Keuls test for intergroup comparisons with SPSS 16.0 statistical software (SPSS, Chicago, IL, USA). Significance was set at a probability level of P < 0.05.
Footnotes
Xinghua Jiang, Studying for doctorate, Attending physician.
Funding: This work was supported by the National Natural Science Foundation of China, No. 81070994 and the Scientific Research Foundation for the Returned Overseas Chinese Scholars, Ministry of Education of the People's Republic of China, No. 2009/8.
Conflicts of interest: None declared.
Ethical approval: All protocols were approved by the Animal Ethics Committee of Central South University in China.
(Edited by Yao ZX, Li JT/Yang Y/Wang L)
REFERENCES
- [1].Kim WR, Christian K, Ming GL, et al. Time-dependent involvement of adult-born dentate granule cells in behavior. Behav Brain Res. 2012;227(2):470–479. doi: 10.1016/j.bbr.2011.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Tomášová L, Smajda B, Sevc J. Effects of prenatal irradiation on behaviour and hippocampal neurogenesis in adult rats. Acta Physiol Hung. 2012;99(2):126–132. doi: 10.1556/APhysiol.99.2012.2.5. [DOI] [PubMed] [Google Scholar]
- [3].van Praag H, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat Neurosci. 1999;2(3):266–270. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- [4].Ziv Y, Ron N, Butovsky O, et al. Immune cells contribute to the maintenance of neurogenesis and spatial learning abilities in adulthood. Nat Neurosci. 2006;9(2):268–275. doi: 10.1038/nn1629. [DOI] [PubMed] [Google Scholar]
- [5].Santarelli L, Saxe M, Gross C, et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301(5634):805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- [6].Huang Z, Zhong XM, Li ZY, et al. Curcumin reverses corticosterone-induced depressive-like behavior and decrease in brain BDNF levels in rats. Neurosci Lett. 2011;493(3):145–148. doi: 10.1016/j.neulet.2011.02.030. [DOI] [PubMed] [Google Scholar]
- [7].Nacher J, Gomez-Climent MA, McEwen B. Chronic non-invasive glucocorticoid administration decreases polysialylated neural cell adhesion molecule expression in the adult rat dentate gyrus. Neurosci Lett. 2004;370(1):40–44. doi: 10.1016/j.neulet.2004.07.062. [DOI] [PubMed] [Google Scholar]
- [8].Brummelte S, Galea LA. Chronic high corticosterone reduces neurogenesis in the dentate gyrus of adult male and female rats. Neuroscience. 2010;168(3):680–690. doi: 10.1016/j.neuroscience.2010.04.023. [DOI] [PubMed] [Google Scholar]
- [9].David DJ, Samuels BA, Rainer Q, et al. Neurogenesis-dependent and -independent effects of fluoxetine in an animal model of anxiety/depression. Neuron. 2009;62(4):479–493. doi: 10.1016/j.neuron.2009.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Gilhooley MJ, Pinnock SB, Herbert J. Rhythmic expression of per1 in the dentate gyrus is suppressed by corticosterone: implications for neurogenesis. Neurosci Lett. 2011;489(3):177–181. doi: 10.1016/j.neulet.2010.12.011. [DOI] [PubMed] [Google Scholar]
- [11].Hellsten J, Wennström M, Mohapel P, et al. Electroconvulsive seizures increase hippocampal neurogenesis after chronic corticosterone treatment. Eur J Neurosci. 2002;16(2):283–290. doi: 10.1046/j.1460-9568.2002.02093.x. [DOI] [PubMed] [Google Scholar]
- [12].Crupi R, Mazzon E, Marino A, et al. Melatonin treatment mimics the antidepressant action in chronic corticosterone-treated mice. J Pineal Res. 2010;49(2):123–129. doi: 10.1111/j.1600-079X.2010.00775.x. [DOI] [PubMed] [Google Scholar]
- [13].Yau SY, Lau BW, Tong JB, et al. Hippocampal neurogenesis and dendritic plasticity support running-improved spatial learning and depression-like behaviour in stressed rats. PLoS One. 2011;6(9):e24263. doi: 10.1371/journal.pone.0024263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Lau BW, Lee JC, Li Y, et al. Polysaccharides from wolfberry prevents corticosterone-induced inhibition of sexual behavior and increases neurogenesis. PLoS One. 2012;7(4):e33374. doi: 10.1371/journal.pone.0033374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Lin B. Polyphenols and neuroprotection against ischemia and neurodegeneration. Mini Rev Med Chem. 2011;11(14):1222–1238. doi: 10.2174/13895575111091222. [DOI] [PubMed] [Google Scholar]
- [16].Tarragó T, Kichik N, Claasen B, et al. Baicalin, a prodrug able to reach the CNS, is a prolyl oligopeptidase inhibitor. Bioorg Med Chem. 2008;16(15):7516–7524. doi: 10.1016/j.bmc.2008.04.067. [DOI] [PubMed] [Google Scholar]
- [17].Tu XK, Yang WZ, Shi SS, et al. Baicalin inhibits TLR2/4 signaling pathway in rat brain following permanent cerebral ischemia. Inflammation. 2011;34(5):463–470. doi: 10.1007/s10753-010-9254-8. [DOI] [PubMed] [Google Scholar]
- [18].Zhang X, Feng G, Weng W, et al. Protective effects of baicalin and octreotide on intestinal mucosa of rats with severe acute pancreatitis. Turk J Gastroenterol. 2009;20(2):108–115. [PubMed] [Google Scholar]
- [19].Zhang X, Tian H, Wu C, et al. Effect of baicalin on inflammatory mediator levels and microcirculation disturbance in rats with severe acute pancreatitis. Pancreas. 2009;38(7):732–738. doi: 10.1097/MPA.0b013e3181ad9735. [DOI] [PubMed] [Google Scholar]
- [20].Liu LL, Gong LK, Wang H, et al. Baicalin protects mouse from Concanavalin A-induced liver injury through inhibition of cytokine production and hepatocyte apoptosis. Liver Int. 2007;27(4):582–591. doi: 10.1111/j.1478-3231.2007.01450.x. [DOI] [PubMed] [Google Scholar]
- [21].Zeng Y, Song C, Ding X, et al. Baicalin reduces the severity of experimental autoimmune encephalomyelitis. Braz J Med Biol Res. 2007;40(7):1003–1010. doi: 10.1590/s0100-879x2006005000115. [DOI] [PubMed] [Google Scholar]
- [22].Yan XH, Huang RB. Differentiation of human umbilical cord blood mesenchymal stem cells toward neurons induced by baicalin in vitro. Zhonghua Er Ke Za Zhi. 2006;44(3):214–219. [PubMed] [Google Scholar]
- [23].Jia Y, Yang Y, Zhou Y, et al. Differentiation of rat bone marrow stromal cells into neuron induced by baicalin. Zhonghua Yi Xue Za Zhi. 2002;82(19):1337–1341. [PubMed] [Google Scholar]
- [24].Li Y, Zhuang P, Shen B, et al. Baicalin promotes neuronal differentiation of neural stem/progenitor cells through modulating p-stat3 and bHLH family protein expression. Brain Res. 2012;1429:36–42. doi: 10.1016/j.brainres.2011.10.030. [DOI] [PubMed] [Google Scholar]
- [25].von Bohlen Und Halbach O. Immunohistological markers for staging neurogenesis in adult hippocampus. Cell Tissue Res. 2007;329(3):409–420. doi: 10.1007/s00441-007-0432-4. [DOI] [PubMed] [Google Scholar]
- [26].Francis F, Koulakoff A, Boucher D, et al. Doublecortin is a developmentally regulated, microtubule-associated protein expressed in migrating and differentiating neurons. Neuron. 1999;23(2):247–256. doi: 10.1016/s0896-6273(00)80777-1. [DOI] [PubMed] [Google Scholar]
- [27].Gleeson JG, Allen KM, Fox JW, et al. Doublecortin, a brain-specific gene mutated in human X-linked lissencephaly and double cortex syndrome, encodes a putative signaling protein. Cell. 1998;92(1):63–72. doi: 10.1016/s0092-8674(00)80899-5. [DOI] [PubMed] [Google Scholar]
- [28].Couillard-Despres S, Winner B, Schaubeck S, et al. Doublecortin expression levels in adult brain reflect neurogenesis. Eur J Neurosci. 2005;21(1):1–14. doi: 10.1111/j.1460-9568.2004.03813.x. [DOI] [PubMed] [Google Scholar]
- [29].Rao MS, Shetty AK. Efficacy of doublecortin as a marker to analyse the absolute number and dendritic growth of newly generated neurons in the adult dentate gyrus. Eur J Neurosci. 2004;19(2):234–246. doi: 10.1111/j.0953-816x.2003.03123.x. [DOI] [PubMed] [Google Scholar]
- [30].Cooper-Kuhn CM, Kuhn HG. Is it all DNA repair? Methodological considerations for detecting neurogenesis in the adult brain. Brain Res Dev Brain Res. 2002;134(1-2):13–21. doi: 10.1016/s0165-3806(01)00243-7. [DOI] [PubMed] [Google Scholar]
- [31].Wang JW, David DJ, Monckton JE, et al. Chronic fluoxetine stimulates maturation and synaptic plasticity of adult-born hippocampal granule cells. J Neurosci. 2008;28(6):1374–1384. doi: 10.1523/JNEUROSCI.3632-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Komitova M, Mattsson B, Johansson BB, et al. Enriched environment increases neural stem/progenitor cell proliferation and neurogenesis in the subventricular zone of stroke-lesioned adult rats. Stroke. 2005;36(6):1278–1282. doi: 10.1161/01.STR.0000166197.94147.59. [DOI] [PubMed] [Google Scholar]
- [33].Valero J, España J, Parra-Damas A, et al. Short-term environmental enrichment rescues adult neurogenesis and memory deficits in APP(Sw,Ind) transgenic mice. PLoS One. 2011;6(2):e16832. doi: 10.1371/journal.pone.0016832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Van Bokhoven P, Oomen CA, Hoogendijk WJ, et al. Reduction in hippocampal neurogenesis after social defeat is long-lasting and responsive to late antidepressant treatment. Eur J Neurosci. 2011;33(10):1833–1840. doi: 10.1111/j.1460-9568.2011.07668.x. [DOI] [PubMed] [Google Scholar]
- [35].Oomen CA, Soeters H, Audureau N, et al. Severe early life stress hampers spatial learning and neurogenesis, but improves hippocampal synaptic plasticity and emotional learning under high-stress conditions in adulthood. J Neurosci. 2010;30(19):6635–6645. doi: 10.1523/JNEUROSCI.0247-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Brown JP, Couillard-Després S, Cooper-Kuhn CM, et al. Transient expression of doublecortin during adult neurogenesis. J Comp Neurol. 2003;467(1):1–10. doi: 10.1002/cne.10874. [DOI] [PubMed] [Google Scholar]
- [37].Livneh Y, Mizrahi A. Long-term changes in the morphology and synaptic distributions of adult-born neurons. J Comp Neurol. 2011;519(11):2212–2224. doi: 10.1002/cne.22625. [DOI] [PubMed] [Google Scholar]
- [38].van der Velden L, van Hooft JA, Chameau P. Altered dendritic complexity affects firing properties of cortical layer 2/3 pyramidal neurons in mice lacking the 5-HT3A receptor. J Neurophysiol. 2012;108(5):1521–1528. doi: 10.1152/jn.00829.2011. [DOI] [PubMed] [Google Scholar]
- [39].Sholl DA. Dendritic organization in the neurons of the visual and motor cortices of the cat. J Anat. 1953;87(4):387–406. [PMC free article] [PubMed] [Google Scholar]
- [40].Li M, Tsang KS, Choi ST, et al. Neuronal differentiation of C17.2 neural stem cells induced by a natural flavonoid, baicalin. Chembiochem. 2011;12(3):449–456. doi: 10.1002/cbic.201000570. [DOI] [PubMed] [Google Scholar]
- [41].Yang XS, Luo XM, Xiao B, et al. An experimental research on differentiation of mesenchymal stem cells derived from children with spinal muscular atrophy into neuron-like cells. Zhongguo Dang Dai Er Ke Za Zhi. 2007;9(5):453–456. [PubMed] [Google Scholar]
- [42].Kempermann G, Kuhn HG, Gage FH. More hippocampal neurons in adult mice living in an enriched environment. Nature. 1997;386(6624):493–495. doi: 10.1038/386493a0. [DOI] [PubMed] [Google Scholar]
- [43].van Praag H, Schinder AF, Christie BR, et al. Functional neurogenesis in the adult hippocampus. Nature. 2002;415(6875):1030–1034. doi: 10.1038/4151030a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Eriksson PS, Perfilieva E, Björk-Eriksson T, et al. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4(11):1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- [45].Gould E, Reeves AJ, Fallah M, et al. Hippocampal neurogenesis in adult Old World primates. Proc Natl Acad Sci U S A. 1999;96(9):5263–5267. doi: 10.1073/pnas.96.9.5263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Kornack DR, Rakic P. Continuation of neurogenesis in the hippocampus of the adult macaque monkey. Proc Natl Acad Sci U S A. 1999;96(10):5768–5773. doi: 10.1073/pnas.96.10.5768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Nacher J, Varea E, Blasco-Ibañez JM, et al. Expression of the transcription factor Pax 6 in the adult rat dentate gyrus. J Neurosci Res. 2005;81(6):753–761. doi: 10.1002/jnr.20596. [DOI] [PubMed] [Google Scholar]
- [48].Englund C, Fink A, Lau C, et al. Pax6, Tbr2, and Tbr1 are expressed sequentially by radial glia, intermediate progenitor cells, and postmitotic neurons in developing neocortex. J Neurosci. 2005;25(1):247–251. doi: 10.1523/JNEUROSCI.2899-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Gould E, Vail N, Wagers M, et al. Adult-generated hippocampal and neocortical neurons in macaques have a transient existence. Proc Natl Acad Sci U S A. 2001;98(19):10910–10917. doi: 10.1073/pnas.181354698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Parent JM, Yu TW, Leibowitz RT, et al. Dentate granule cell neurogenesis is increased by seizures and contributes to aberrant network reorganization in the adult rat hippocampus. J Neurosci. 1997;17(10):3727–3738. doi: 10.1523/JNEUROSCI.17-10-03727.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Karl C, Couillard-Despres S, Prang P, et al. Neuronal precursor-specific activity of a human doublecortin regulatory sequence. J Neurochem. 2005;92(2):264–282. doi: 10.1111/j.1471-4159.2004.02879.x. [DOI] [PubMed] [Google Scholar]
- [52].Filippov V, Kronenberg G, Pivneva T, et al. Subpopulation of nestin-expressing progenitor cells in the adult murine hippocampus shows electrophysiological and morphological characteristics of astrocytes. Mol Cell Neurosci. 2003;23(3):373–382. doi: 10.1016/s1044-7431(03)00060-5. [DOI] [PubMed] [Google Scholar]
- [53].Plümpe T, Ehninger D, Steiner B, et al. Variability of doublecortin-associated dendrite maturation in adult hippocampal neurogenesis is independent of the regulation of precursor cell proliferation. BMC Neurosci. 2006;7:77. doi: 10.1186/1471-2202-7-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Walker TL, Yasuda T, Adams DJ, et al. The doublecortin-expressing population in the developing and adult brain contains multipotential precursors in addition to neuronal-lineage cells. J Neurosci. 2007;27(14):3734–3742. doi: 10.1523/JNEUROSCI.5060-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Jung SH, Kang KD, Ji D, et al. The flavonoid baicalin counteracts ischemic and oxidative insults to retinal cells and lipid peroxidation to brain membranes. Neurochem Int. 2008;53(6-8):325–337. doi: 10.1016/j.neuint.2008.09.004. [DOI] [PubMed] [Google Scholar]
- [56].Liu LY, Wei EQ, Zhao YM, et al. Protective effects of baicalin on oxygen/glucose deprivation- and NMDA-induced injuries in rat hippocampal slices. J Pharm Pharmacol. 2005;57(8):1019–1026. doi: 10.1211/0022357056622. [DOI] [PubMed] [Google Scholar]
- [57].Lee HH, Yang LL, Wang CC, et al. Differential effects of natural polyphenols on neuronal survival in primary cultured central neurons against glutamate- and glucose deprivation-induced neuronal death. Brain Res. 2003;986(1-2):103–113. doi: 10.1016/s0006-8993(03)03197-4. [DOI] [PubMed] [Google Scholar]
- [58].Morales-Medina JC, Sanchez F, Flores G, et al. Morphological reorganization after repeated corticosterone administration in the hippocampus, nucleus accumbens and amygdala in the rat. J Chem Neuroanat. 2009;38(4):266–272. doi: 10.1016/j.jchemneu.2009.05.009. [DOI] [PubMed] [Google Scholar]
- [59].Magariños AM, McEwen BS. Stress-induced atrophy of apical dendrites of hippocampal CA3c neurons: comparison of stressors. Neuroscience. 1995;69(1):83–88. doi: 10.1016/0306-4522(95)00256-i. [DOI] [PubMed] [Google Scholar]
- [60].Magariños AM, Orchinik M, McEwen BS. Morphological changes in the hippocampal CA3 region induced by non-invasive glucocorticoid administration: a paradox. Brain Res. 1998;809(2):314–318. doi: 10.1016/s0006-8993(98)00882-8. [DOI] [PubMed] [Google Scholar]
- [61].Watanabe Y, Gould E, Cameron HA, et al. Phenytoin prevents stress- and corticosterone-induced atrophy of CA3 pyramidal neurons. Hippocampus. 1992;2(4):431–435. doi: 10.1002/hipo.450020410. [DOI] [PubMed] [Google Scholar]
- [62].Alfarez DN, Karst H, Velzing EH, et al. Opposite effects of glucocorticoid receptor activation on hippocampal CA1 dendritic complexity in chronically stressed and handled animals. Hippocampus. 2008;18(1):20–28. doi: 10.1002/hipo.20360. [DOI] [PubMed] [Google Scholar]
- [63].Sousa N, Lukoyanov NV, Madeira MD, et al. Reorganization of the morphology of hippocampal neurites and synapses after stress-induced damage correlates with behavioral improvement. Neuroscience. 2000;97(2):253–266. doi: 10.1016/s0306-4522(00)00050-6. [DOI] [PubMed] [Google Scholar]
- [64].Magariños AM, McEwen BS. Stress-induced atrophy of apical dendrites of hippocampal CA3c neurons: involvement of glucocorticoid secretion and excitatory amino acid receptors. Neuroscience. 1995;69(1):89–98. doi: 10.1016/0306-4522(95)00259-l. [DOI] [PubMed] [Google Scholar]
- [65].Liston C, Gan WB. Glucocorticoids are critical regulators of dendritic spine development and plasticity in vivo. Proc Natl Acad Sci U S A. 2011;108(38):16074–16079. doi: 10.1073/pnas.1110444108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Alfarez DN, De Simoni A, Velzing EH, et al. Corticosterone reduces dendritic complexity in developing hippocampal CA1 neurons. Hippocampus. 2009;19(9):828–836. doi: 10.1002/hipo.20566. [DOI] [PubMed] [Google Scholar]
- [67].Ishiwata H, Shiga T, Okado N. Selective serotonin reuptake inhibitor treatment of early postnatal mice reverses their prenatal stress-induced brain dysfunction. Neuroscience. 2005;133(4):893–901. doi: 10.1016/j.neuroscience.2005.03.048. [DOI] [PubMed] [Google Scholar]
- [68].Murmu MS, Salomon S, Biala Y, et al. Changes of spine density and dendritic complexity in the prefrontal cortex in offspring of mothers exposed to stress during pregnancy. Eur J Neurosci. 2006;24(5):1477–1487. doi: 10.1111/j.1460-9568.2006.05024.x. [DOI] [PubMed] [Google Scholar]
- [69].Martínez-Téllez RI, Hernández-Torres E, Gamboa C, et al. Prenatal stress alters spine density and dendritic length of nucleus accumbens and hippocampus neurons in rat offspring. Synapse. 2009;63(9):794–804. doi: 10.1002/syn.20664. [DOI] [PubMed] [Google Scholar]
- [70].Jia N, Yang K, Sun Q, et al. Prenatal stress causes dendritic atrophy of pyramidal neurons in hippocampal CA3 region by glutamate in offspring rats. Dev Neurobiol. 2010;70(2):114–125. doi: 10.1002/dneu.20766. [DOI] [PubMed] [Google Scholar]
- [71].Mychasiuk R, Gibb R, Kolb B. Prenatal stress alters dendritic morphology and synaptic connectivity in the prefrontal cortex and hippocampus of developing offspring. Synapse. 2012;66(4):308–314. doi: 10.1002/syn.21512. [DOI] [PubMed] [Google Scholar]
- [72].Christian KM, Miracle AD, Wellman CL, et al. Chronic stress-induced hippocampal dendritic retraction requires CA3 NMDA receptors. Neuroscience. 2011;174:26–36. doi: 10.1016/j.neuroscience.2010.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Garcia A, Steiner B, Kronenberg G, et al. Age-dependent expression of glucocorticoid- and mineralocorticoid receptors on neural precursor cell populations in the adult murine hippocampus. Aging Cell. 2004;3(6):363–371. doi: 10.1111/j.1474-9728.2004.00130.x. [DOI] [PubMed] [Google Scholar]
- [74].Ge QF, Hu X, Ma ZQ, et al. Baicalin attenuates oxygen-glucose deprivation-induced injury via inhibiting NMDA receptor-mediated 5-lipoxygenase activation in rat cortical neurons. Pharmacol Res. 2007;55(2):148–157. doi: 10.1016/j.phrs.2006.11.007. [DOI] [PubMed] [Google Scholar]
- [75].The Ministry of Science and Technology of the People's Republic of China. Guidance Suggestions for the Care and Use of Laboratory Animals 2006-09-30 [Google Scholar]


