Abstract
Sericin from discarded silkworm cocoons of silk reeling has been used in different fields, such as cosmetology, skin care, nutrition, and oncology. The present study established a rat model of type 2 diabetes by consecutive intraperitoneal injections of low-dose (25 mg/kg) streptozotocin. After intragastrical perfusion of sericin for 35 days, blood glucose levels significantly declined, and the expression of neurofilament protein in the sciatic nerve and nerve growth factor in L4–6 spinal ganglion and anterior horn cells significantly increased. However, the expression of neuropeptide Y in spinal ganglion and anterior horn cells significantly decreased in model rats. These findings indicate that sericin protected the sciatic nerve and related nerve cells against injury in a rat type 2 diabetic model by upregulating the expression of neurofilament protein in the sciatic nerve and nerve growth factor in spinal ganglion and anterior horn cells, and downregulating the expression of neuropeptide Y in spinal ganglion and anterior horn cells.
Keywords: neural regeneration, traditional Chinese medicine, peripheral nerve injury, diabetes mellitus, sericin, sciatic nerve, spinal ganglion cells, anterior horn cells, nerve cells, neurofilament protein, nerve growth factor, neuropeptide Y, streptozotocin, photographs-containing paper, neuroregeneration
Research Highlights
Sericin upregulated the expression of neurofilament protein in the sciatic nerve, and nerve growth factor in spinal ganglion and anterior horn cells, but downregulated the expression of neuropeptide Y in spinal ganglion and anterior horn cells of diabetic rats to protect against type-2 diabetes-induced injuries in the sciatic nerve and related nerve cells.
INTRODUCTION
Diabetic peripheral neuropathy is a common chronic complication of diabetes mellitus, and its morbidity gradually increases with a prolonged course of disease[1,2]. Most Chinese diabetic patients control blood glucose levels by taking Western medicine, but liver and kidney impairments occur early, and some diabetics even lose their ability to participate in normal activities of daily living in a short time[3,4]. Thus, drugs with minimal toxicity and adverse effects are needed for diabetic patients. Sericin is a potential drug candidate that meets the above characteristics. Sericin is a kind of water-soluble protein in the silkworm cocoons, but it is mostly discarded during silk reeling. Recently, sericin has been used in such diverse areas as cosmetology, skin care, nutrition, and oncology[5,6,7]. The silkworm cocoon soaked in water has been used to reduce blood glucose. Previous studies from our group showed that sericin effectively reduced blood glucose, improved blood fat metabolic disorders, and prevented blood glucose elevation in diabetes mellitus[8]. Sericin can protect pancreatic islet cells against diabetic injury by inhibiting apoptosis of beta islet cells and downregulating neuropeptide Y protein expression in islet cells[9,10]. Moreover, sericin can regulate testicular growth hormone/insulin-like growth factor 1 axis disorder, improve spermatogenic function and protect reproductive injuries in diabetic rats by upregulating testicular proliferating cell nuclear antigen and c-fos expression[11,12,13]. As the main component of the neuron cytoskeleton, neurofilament protein mainly distributes in the cell body and processes of neurons, and plays an important role in maintaining the normal morphology and structure of neurons. Nerve growth factor was first found by Montalcini in the 1950s. It binds to neurotrophic tyrosine kinase receptor type 1 and p75 receptors on the nerve ending and retrogrades to the cell body to induce protein synthesis and axon growth, promote sphingomyelin hydrolysis and nerve fiber regeneration, and inhibit neuron apoptosis[14,15]. Neuropeptide Y is extensively distributed in mammals, including the nervous, digestive, cardiovascular, respiratory and urinary systems, and is an important modulator of the neuroendocrine system[16,17]. The present study focused on the effects of sericin on diabetic peripheral neuropathy by observing the changes of neurofilament protein, nerve growth factor and neuropeptide Y protein expression, which can reflect the function of nerve cells, in the rat model of streptozotocin-induced type 2 diabetes, and investigated the protective effects of sericin on sciatic nerve and related nerve cells in diabetic rats.
RESULTS
Quantitative analysis of experimental animals
A total of 36 male Sprague-Dawley were used; 12 were randomly selected as the control group, and were not treated. The remaining 24 rats were intraperitoneally injected with low-dose streptozotocin (25 mg/kg) for 3 consecutive days to establish type 2 diabetic models. All the rats became diabetic. Twelve rats were randomly selected as the diabetic model group, and did not receive any additional treatment. The sericin group comprised the remaining 12 diabetic rats, which were intragastrically perfused with sericin for 35 days. All 36 rats were included in the final analysis.
Sericin reduced blood glucose levels in diabetic rats
The glucose oxidase method showed that the fasting blood glucose level was 10.83 ± 2.03 mM in the control group; the fasting blood glucose level significantly increased in the model group (29.45 ± 4.82 mM; P < 0.01) compared with the control group, but significantly decreased after sericin treatment (13.20 ± 4.09 mM; P < 0.01) compared with the model group. None of the rats showed adverse effects after sericin treatment throughout the experiments.
Sericin promoted neurofilament protein expression in the sciatic nerve of diabetic rats
Immunohistochemistry showed that neurofilament protein positive products, stained as brown yellow particles, were distributed in the neurites of the sciatic nerve (Figure 1).
Figure 1.
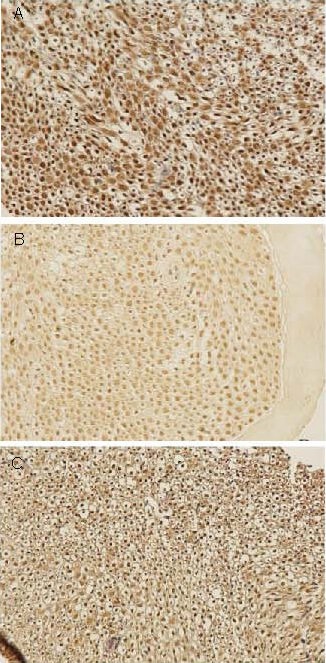
Effect of sericin on neurofilament proteinexpression in sciatic nerves of diabetic rats (immunohistochemistry, × 200).
Neurofilament protein positive products were presented as brown yellow particles.
(A) High levels of neurofilament protein expression were observed in the sciatic nerve of control rats.
(B) Significantly reduced levels of neurofilament protein expression were observed in the sciatic nerve of model rats.
(C) Levels of neurofilament protein expression were significantly increased following sericin treatment in diabetic rats.
The neurofilament protein expression level was significantly lower in the model group (0.206 7 ± 0.028 6) compared with the control group (0.294 1 ± 0.028 6; P < 0.01), but significantly increased in the sericin group (0.268 8 ± 0.055 0; P < 0.05) compared with the model group.
Sericin promoted nerve growth factor expression in L4–6 spinal ganglion and anterior horn cells
Immunohistochemistry showed nerve growth factor protein positive products in the cytoplasm and nuclei of L4–6 spinal ganglion and anterior horn cells in all groups, presented as brown yellow particles mainly in the cytoplasm (Figures 2, 3).
Figure 2.
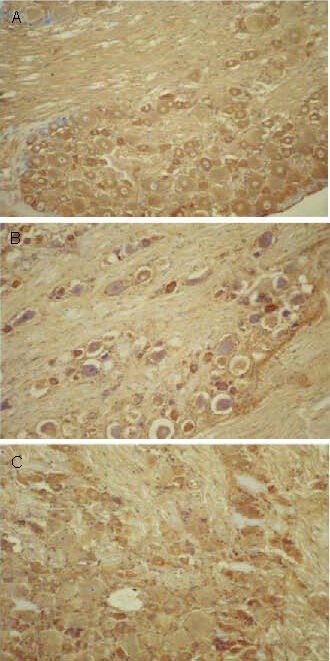
Effect of sericin on nerve growth factor expression in spinal ganglion cells of diabetic rats (immunohistochemistry, × 200).
Nerve growth factor positive products were presented as brown yellow particles.
(A) High levels of nerve growth factor expression were observed in spinal ganglion cells of control rats.
(B) Significantly reduced levels of nerve growth factor expression were observed in spinal ganglion cells of model rats.
(C) Levels of nerve growth factor expression in spinal ganglion cells were significantly increased following sericin treatment in diabetic rats.
Figure 3.
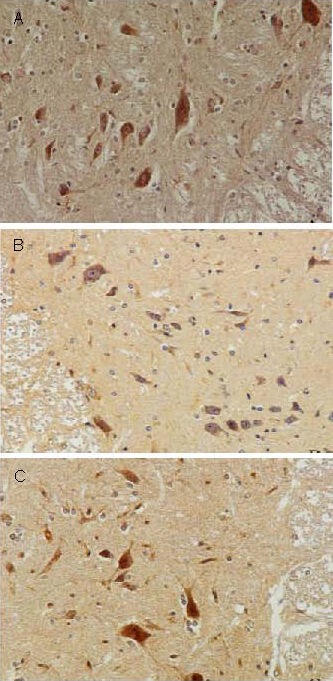
Effect of sericin on nerve growth factor expression in anterior horn cells of diabetic rats (immunohistochemistry, × 200).
Nerve growth factor positive products were presented as brown yellow particles.
(A) High levels of nerve growth factor expression were observed in anterior horn cells of control rats.
(B) Significantly reduced levels of nerve growth factor expression were observed in anterior horn cells of model rats.
(C) Levels of nerve growth factor expression in anterior horn cells were significantly increased following sericin treatment in diabetic rats.
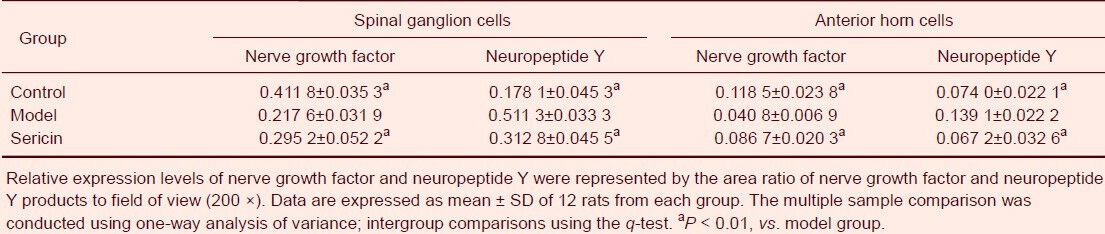
Nerve growth factor protein expression in spinal ganglion and anterior horn cells was significantly lower in the model group compared with the control group (P < 0.01), and was significantly higher in the sericin group compared with the model group (P < 0.01; Table 1).
Table 1.
Nerve growth factor and neuropeptide Y expression in spinal ganglion and anterior horn cells of rats
Sericin decreased neuropeptide Y expression in L4–6 spinal ganglion and anterior horn cells
Neuropeptide Y protein positive products distributed in the cytoplasm of L4–6 spinal ganglion and anterior horn cells of all groups, presented as brown yellow particles (Figures 4, 5).
Figure 4.
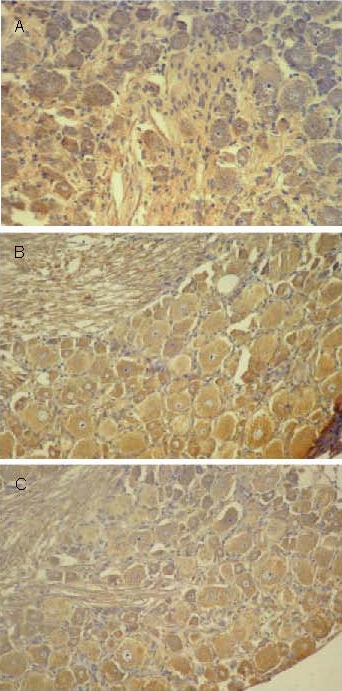
Effect of sericin on neuropeptide Y expression in spinal ganglion cells of diabetic rats (immunohistochemistry, × 200).
Neuropeptide Y positive products were presented as brown yellow particles.
(A) Low levels of neuropeptide Y expression were observed in spinal ganglion cells of control rats.
(B) Significantly increased levels of neuropeptide Y expression were observed in spinal ganglion cells of model rats.
(C) Levels of neuropeptide Y expression in spinal ganglion cells of diabetic rats were significantly decreased following sericin treatment.
Figure 5.
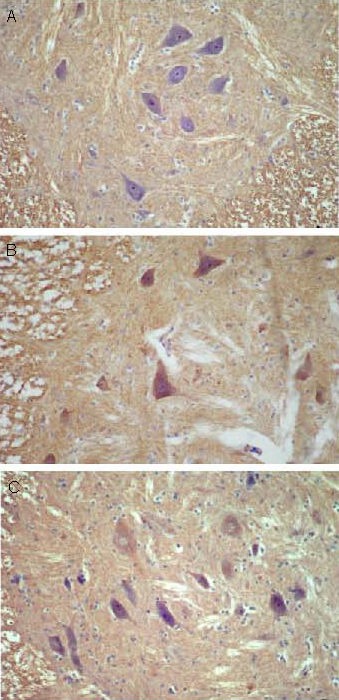
Effect of sericin on neuropeptide Y expression in anterior horn cells of diabetic rats (immunohistochemistry, × 200).
Neuropeptide Y positive products were presented as brown yellow particles.
(A) Low levels of neuropeptide Y expression were observed in anterior horn cells of control rats.
(B) Significantly increased levels of neuropeptide Y expression were observed in anterior horn cells of model rats.
(C) Levels of neuropeptide Y expression in anterior horn cells of diabetic rats were significantly decreased following sericin treatment.
Neuropeptide Y protein expression in spinal ganglion and anterior horn cells was significantly greater in the model group compared with the control group (P < 0.01; Table 1), and was significantly less in the sericin group compared with model group (P < 0.01; Table 1).
DISCUSSION
Effects of sericin on neurofilament protein expression in the sciatic nerves of diabetic rats
Neurofilament protein plays a role in axoplasmic transport, and serves as the major marker identifying function of synapses[18]. In addition, neurofilament protein is associated with DNA transcription and translation[18].
Studies showed evident gene alterations in skeletal proteins occur in diabetes mellitus[19,20]. Neurofilament protein expression is reduced in the nervous system of diabetics, with accompanying decreases in the quantity and density of neurofilament protein-positive fibers and microtubules[21,22]. The reduced neurofilament protein expression results from a disturbance in neuronal substance synthesis and transport, leading to abnormal activities in the nervous system[23,24]. In the present study, neurofilament protein expression in the sciatic nerve was significantly elevated in the sericin group compared with the model group, indicating that sericin can increase neurofilament protein expression in the sciatic nerve, improve neuronal axoplasmic transport, and maintain the normal morphology and structures of neurons in diabetes mellitus. The sericin-increased neurofilament protein expression allows neurofilament protein to coordinate molecular information transfer and substance exchange among neurons, recover functions of the sciatic nerve, and protect the sciatic nerve.
Effects of sericin on nerve growth factor expression in the spinal ganglion and anterior horn cells of diabetic rats
Studies showed that nerve growth factor synthesis is reduced in diabetes mellitus because of a lack of insulin and hyperglycemia. In diabetes mellitus models, the affinity of nerve growth factor for the neurotrophic tyrosine kinase receptor type 1 receptor is decreased and nerve growth factor reverse axoplasmic transport is injured, leading to reduced nerve growth factor expression in various tissues[25,26]. Results from the present study showed that nerve growth factor expression in spinal ganglion and anterior horn cells significantly increased in the sericin group compared with the model group, demonstrating that sericin can protect peripheral nerves against diabetic-induced injury.
Effects of sericin on neuropeptide Y expression in the spinal ganglion and anterior horn cells of diabetic rats
Neuropeptide Y expression is the highest in the mammalian nervous system, specifically in the spinal cord, hippocampus, basal nuclei, hypothalamus and cortex[27]. In the nervous system, neuropeptide Y can excite synapses and is closely correlated with sympathetic preganglionic neurons, indicating that neuropeptide Y may participate in neuromodulator or neuroendocrine release, and can inhibit neurotransmitter release from various neurons[28,29]. Neuropeptide Y also resides in the heart and surrounding blood vessels[30,31]. It constricts blood vessels and vascular smooth muscle, enhances the sensitivity of blood vessels to adrenaline and 5-hydroxytryptamine, and suppresses the dilatation of blood vessels to adenosine and acetylcholine, significantly reducing blood flow to corresponding organs[32,33,34].
In conclusion, neuropeptide Y expression in the spinal ganglion and anterior horn cells was significantly increased in the model group compared with the controls, which may result in nervous system injury because of nerve tissue ischemia and hypoxia induced by reduced neurotransmitter release and vasoconstriction. However, compared with the model group, neuropeptide Y expression significantly declined following sericin treatment, indicating that sericin can reduce neuropeptide Y expression, attenuate the inhibitory effects of neuropeptide Y on neurotransmitter release and vasoconstriction to ameliorate nerve tissue ischemia and hypoxia, restore nerve physiologic function, and protect peripheral nerves against diabetes mellitus-induced injury.
MATERIALS AND METHODS
Design
A randomized, controlled, animal study.
Time and setting
The experiments were performed at the Institute of Basic Medicine, Chengde Medical University, China from June 2010 to September 2011.
Materials
Experimental animals
A total of 36 healthy male Sprague-Dawley rats of clean grade, weighing 200–250 g, were provided by the Experimental Animal Center of Hebei Medical University (license No. 712024). They were housed at 20 ± 2°C with a humidity of 40–70%. Animal procedures were conducted in accordance with the Guidance Suggestions for the Care and Use of Laboratory Animals, formulated by the Ministry of Science and Technology of China[35].
Drugs
Sericin was made from color silkworm cocoons (Sericultural Research Institute, Chengde Medical University) by soaking, water decoction, filtration and condensation.
Methods
Establishment of type 2 diabetic models
The rats were intraperitoneally injected with 2% streptozotocin (25 mg/kg; Sigma, St. Louis, MO, USA; dissolved in citric acid-sodium citrate buffer, pH 4.4) for 3 consecutive days to establish type 2 diabetic models. Rats were included if the fasting blood glucose was ≥ 16.7 mM after 7 days[36,37]. The control group rats were normally fed and intraperitoneally injected with the same volume of citric acid-sodium citrate buffer.
Sericin treatment
Following model establishment, sericin group rats were intragastrically perfused with sericin (2.4 g/kg per day), once a day for 35 consecutive days[38,39]. The control and model groups were intragastrically perfused with the same volume of normal saline for 35 days.
Blood glucose detection
All rats were deprived of food for 12 hours, and the rats were anesthetized by intraperitoneal injection with 4% chloral hydrate. 3 mL of blood was harvested from the posterior orbital venous plexus, centrifuged at 3 000 r/min for 20 minutes, and serum was stored at –20°C. Blood glucose levels were detected using the glucose oxidase method (Blood Glucose Detection Kit, No. 20071030; Baoding Great Wall Clinical Reagents Co., Ltd., Baoding, China) in a Boehringer Mannheim/Hitachi 717 automatic clinical biochemical analyzer (Hitachi, Tokyo, Japan)[8].
Immunohistochemistry detection for neurofilament protein, nerve growth factor and neuropeptide Y expression
The rats were sacrificed after the blood sampling, and the sciatic nerve (4 cm above the knee joint), spinal cord at L4–6 levels and corresponding spinal ganglia were harvested, fixed in Bouin's solution, paraffin embedded, and sectioned into 5-μm-thick blocks. The expression level of neurofilament protein in sciatic nerve cells, and of nerve growth factor and neuropeptide Y in spinal ganglion and anterior horn cells was determined using streptavidin-peroxidase immunohistochemistry[12,40]. The sections were incubated with mouse anti-neurofilament protein polyclonal antibody (1:50; Santa Cruz Biotechnology, Santa Cruz, CA, USA), rabbit anti-nerve growth factor polyclonal antibody (1:50; Santa Cruz Biotechnology) and rabbit anti-neuropeptide Y polyclonal antibody (1:50; Wuhan Boster, Wuhan, China) overnight at 4°C, followed by biotin-labeled goat anti-rabbit/mouse IgG (undiluted; Beijing Zhongshan Goldenbridge Biotechnology, Beijing, China) at 37°C for 30 minutes. The sections were visualized by diaminobenzidine, and the reaction was terminated by tap water. The nuclei were counterstained with hematoxylin. Negative controls were treated with PBS instead of the primary antibody. A positive neurofilament protein reaction was documented by brown yellow particles in the neurites of sciatic nerves; positive reactions of nerve growth factor and neuropeptide Y were represented by brown yellow particles in the nuclei or cytoplasm of spinal ganglion and anterior horn cells. Six rats from each group and six sections from each rat were randomly selected. Three fields of view were randomly selected from each section and quantitatively analyzed by 200 × light microscope (Olympus, Tokyo, Japan) using the MiVnt image analysis system (Echung Electronics, Shandong, China). Relative expression levels were represented by the mean value of the area ratio of positive products to field of view[41].
Statistical analysis
Data were analyzed using SPSS version 15.0 (SPSS, Chicago, IL, USA) and were expressed as mean ± SD. Multiple sample comparison was made by one-way analysis of variance, and intergroup differences were compared by q-test.
Acknowledgments:
We thank the Institute of Basic Medicine, Chengde Medical University for technical support.
Footnotes
Chengjun Song, Master, Associate professor.
Conflicts of interest: None declared.
Ethical approval: This study received permission from the Animal Ethics Committee of Hebei Province, China.
(Edited by Xu F, Wu ZB/Su LL/Song LP)
REFERENCES
- [1].Harati Y. Diabetic peripheral neuropathies. Methodist Debakey Cardiovasc J. 2010;6(2):15–19. doi: 10.14797/mdcj-6-2-15. [DOI] [PubMed] [Google Scholar]
- [2].Gebel E. New hope for ending complications. Working together to protect your body from diabetes damage. (59-60).Diabetes Forecast. 2012;65(4):56. [PubMed] [Google Scholar]
- [3].Shiyovich A, Sztarkier I, Nesher L. Toxic hepatitis induced by Gymnema sylvestre, a natural remedy for type 2 diabetes mellitus. Am J Med Sci. 2010;340(6):514–517. doi: 10.1097/MAJ.0b013e3181f41168. [DOI] [PubMed] [Google Scholar]
- [4].Russell-Jones D. The safety and tolerability of GLP-1 receptor agonists in the treatment of type-2 diabetes. Int J Clin Pract. 2010;64(10):1402–1414. doi: 10.1111/j.1742-1241.2010.02465.x. [DOI] [PubMed] [Google Scholar]
- [5].Kaewkorn W, Limpeanchob N, Tiyaboonchai W, et al. Effects of silk sericin on the proliferation and apoptosis of colon cancer cells. Biol Res. 2012;45(1):45–50. doi: 10.4067/S0716-97602012000100006. [DOI] [PubMed] [Google Scholar]
- [6].Nayak S, Talukdar S, Kundu SC. Potential of 2D crosslinked sericin membranes with improved biostability for skin tissue engineering. Cell Tissue Res. 2012;347(3):783–794. doi: 10.1007/s00441-011-1269-4. [DOI] [PubMed] [Google Scholar]
- [7].Zhaorigetu S, Yanaka N, Sasaki M, et al. Inhibitory effects of silk protein, sericin on UVB-induced acute damage and tumor promotion by reducing oxidative stress in the skin of hairless mouse. J Photochem Photobiol B. 2003;71(1-3):11–17. doi: 10.1016/s1011-1344(03)00092-7. [DOI] [PubMed] [Google Scholar]
- [8].Fu XM, Zhong MR, Fu WL, et al. Effects of sericine on blood glucose and blood lipid in type 2 diabetes rats. Zhongguo Laonian Xue Zazhi. 2011;31(1):103–105. [Google Scholar]
- [9].Fu XM, Ma HW, Fu WL, et al. Protective effects of sericin on pancreatic islet cells of type II diabetic rat. Jiepou Xue Zazhi. 2010;33(2):161–164. [Google Scholar]
- [10].Liu XY, Fu XM, Gao Y, et al. Protective effects of sericin on islet cells apoptosis of type 2 diabetes rats. Zhongguo Laonian Xue Zazhi. 2012;32(12):2525–2527. [Google Scholar]
- [11].Fu WL, He YQ, Ma HW, et al. Effects of sericine on proliferation and related factors of germ cells in testis of type 2 diabetic rats. Zhongguo Yike Daxue Xuebao. 2010;39(5):332–335. [Google Scholar]
- [12].Fu WL, Fu XM, Zhong MR, et al. Effects of sericine on growth hormone/insulin-like growth factor-1 axis of testis in type 2 diabetes mellitus rats. Jiepou Xuebao. 2011;42(1):104–109. [Google Scholar]
- [13].Fu WL, Zhong MR, He YQ, et al. Effects of sericine pretreatment on IGF-1 expression in testes of diabetes mellitus rat model. Zhongguo Zuzhi Huaxue yu Xibao Huaxue Zazhi. 2010;19(4):361–364. [Google Scholar]
- [14].Diolaiti D, Bernardoni R, Trazzi S, et al. Functional cooperation between TrkA and p75(NTR) accelerates neuronal differentiation by increased transcription of GAP-43 and p21(CIP/WAF) genes via ERK1/2 and AP-1 activities. Exp Cell Res. 2007;313(14):2980–2992. doi: 10.1016/j.yexcr.2007.06.002. [DOI] [PubMed] [Google Scholar]
- [15].Renton JP, Xu N, Clark JJ, et al. Interaction of neurotrophin signaling with Bcl-2 localized to the mitochondria and endoplasmic reticulum on spiral ganglion neuron survival and neurite growth. J Neurosci Res. 2010;88(10):2239–2251. doi: 10.1002/jnr.22381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Nässel DR, Wegener C. A comparative review of short and long neuropeptide F signaling in invertebrates: Any similarities to vertebrate neuropeptide Y signaling? Peptides. 2011;32(6):1335–1355. doi: 10.1016/j.peptides.2011.03.013. [DOI] [PubMed] [Google Scholar]
- [17].Nguyen AD, Herzog H, Sainsbury A. Neuropeptide Y and peptide YY: important regulators of energy metabolism. Curr Opin Endocrinol Diabetes Obes. 2011;18(1):56–60. doi: 10.1097/MED.0b013e3283422f0a. [DOI] [PubMed] [Google Scholar]
- [18].Ackerley S, James PA, Kalli A, et al. A mutation in the small heat-shock protein HSPB1 leading to distal hereditary motor neuronopathy disrupts neurofilament assembly and the axonal transport of specific cellular cargoes. Hum Mol Genet. 2006;15(2):347–354. doi: 10.1093/hmg/ddi452. [DOI] [PubMed] [Google Scholar]
- [19].Xu G, Pierson CR, Murakawa Y, et al. Altered tubulin and neurofilament expression and impaired axonal growth in diabetic nerve regeneration. J Neuropathol Exp Neurol. 2002;61(2):164–175. doi: 10.1093/jnen/61.2.164. [DOI] [PubMed] [Google Scholar]
- [20].von Wilmowsky C, Stockmann P, Harsch I, et al. Diabetes mellitus negatively affects peri-implant bone formation in the diabetic domestic pig. J Clin Periodontol. 2011;38(8):771–779. doi: 10.1111/j.1600-051X.2011.01746.x. [DOI] [PubMed] [Google Scholar]
- [21].Pierson CR, Zhang W, Sima AA. Proinsulin C-peptide replacement in type 1 diabetic BB/Wor-rats prevents deficits in nerve fiber regeneration. J Neuropathol Exp Neurol. 2003;62(7):765–779. doi: 10.1093/jnen/62.7.765. [DOI] [PubMed] [Google Scholar]
- [22].Scott JN, Clark AW, Zochodne DW. Neurofilament and tubulin gene expression in progressive experimental diabetes: failure of synthesis and export by sensory neurons. Brain. 1999;122(Pt 11):2109–2118. doi: 10.1093/brain/122.11.2109. [DOI] [PubMed] [Google Scholar]
- [23].Li Y, Jung P, Brown A. Axonal transport of neurofilaments: a single population of intermittently moving polymers. J Neurosci. 2012;32(2):746–758. doi: 10.1523/JNEUROSCI.4926-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Shea TB, Lee S. Neurofilament phosphorylation regulates axonal transport by an indirect mechanism: a merging of opposing hypotheses. Cytoskeleton (Hoboken) 2011;68(11):589–595. doi: 10.1002/cm.20535. [DOI] [PubMed] [Google Scholar]
- [25].Kanbayashi H, Itoh H, Kashiwaya T, et al. Spatial distribution of nociceptive neuropeptide and nerve growth factor depletion in experimental diabetic peripheral nervous system. J Int Med Res. 2002;30(5):512–519. doi: 10.1177/147323000203000507. [DOI] [PubMed] [Google Scholar]
- [26].Gezginci-Oktayoglu S, Sacan O, Yanardag R, et al. Exendin-4 improves hepatocyte injury by decreasing proliferation through blocking NGF/TrkA in diabetic mice. Peptides. 2011;32(2):223–231. doi: 10.1016/j.peptides.2010.10.025. [DOI] [PubMed] [Google Scholar]
- [27].Castro A, Manso MJ, Anadón R. Distribution of neuropeptide Y immunoreactivity in the central and peripheral nervous systems of amphioxus (Branchiostoma lanceolatum Pallas) J Comp Neurol. 2003;461(3):350–361. doi: 10.1002/cne.10694. [DOI] [PubMed] [Google Scholar]
- [28].Zhaohui Z, Jingzhu Z, Guipeng D, et al. Role of neuropeptide Y in regulating hypothalamus-pituitary-gonad axis in the rats treated with electro-acupuncture. Neuropeptides. 2012;46(3):133–139. doi: 10.1016/j.npep.2012.03.002. [DOI] [PubMed] [Google Scholar]
- [29].Férézou I, Hill EL, Cauli B, et al. Extensive overlap of mu-opioid and nicotinic sensitivity in cortical interneurons. Cereb Cortex. 2007;17(8):1948–1957. doi: 10.1093/cercor/bhl104. [DOI] [PubMed] [Google Scholar]
- [30].Gonsalvez DG, Kerman IA, McAllen RM, et al. Chemical coding for cardiovascular sympathetic preganglionic neurons in rats. J Neurosci. 2010;30(35):11781–11791. doi: 10.1523/JNEUROSCI.0796-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Onuoha GN, Nicholls DP, Alpar EK, et al. Regulatory peptides in the heart and major vessels of man and mammals. Neuropeptides. 1999;33(2):165–172. doi: 10.1054/npep.1999.0017. [DOI] [PubMed] [Google Scholar]
- [32].Baldassano S, Wang GD, Mulè F, et al. Glucagon-like peptide-1 modulates neurally evoked mucosal chloride secretion in guinea pig small intestine in vitro. Am J Physiol Gastrointest Liver Physiol. 2012;302(3):G352–358. doi: 10.1152/ajpgi.00333.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Jacques D, Sader S, El-Bizri N, et al. Neuropeptide Y induced increase of cytosolic and nuclear Ca2+ in heart and vascular smooth muscle cells. Can J Physiol Pharmacol. 2000;78(2):162–172. [PubMed] [Google Scholar]
- [34].Jacques D, Abdel-Samad D. Neuropeptide Y (NPY) and NPY receptors in the cardiovascular system: implication in the regulation of intracellular calcium. Can J Physiol Pharmacol. 2007;85(1):43–53. doi: 10.1139/Y06-106. [DOI] [PubMed] [Google Scholar]
- [35].The Ministry of Science and Technology of the People's Republic of China. Guidance Suggestions for the Care and Use of Laboratory Animals 2006-09-30 [Google Scholar]
- [36].Xiang X, Wang Z, Zhu Y, et al. Dosage of streptozocin in inducing rat model of type 2 diabetes mellitus. Wei Sheng Yan Jiu. 2010;39(2):138–142. [PubMed] [Google Scholar]
- [37].Li X, Cui X, Sun X, et al. Mangiferin prevents diabetic nephropathy progression in streptozotocin-induced diabetic rats. Phytother Res. 2010;24(6):893–899. doi: 10.1002/ptr.3045. [DOI] [PubMed] [Google Scholar]
- [38].Zhan YL, Huang CF, Chen GL. Hypoglycemic activity of the decoction of cocoon shell on alloxan-diabetic model mice. Canye Kexue. 2003;29(4):446–448. [Google Scholar]
- [39].Chen ZH, He YQ, Fu WL, et al. Effects of sericin on heme oxygenase-1 expression in the hippocampus and cerebral cortex of type 2 diabetes mellitus rats. Neural Regen Res. 2011;6(6):423–427. [Google Scholar]
- [40].Kikuno N, Kawamoto K, Hirata H, et al. Nerve growth factor combined with vascular endothelial growth factor enhances regeneration of bladder acellular matrix graft in spinal cord injury-induced neurogenic rat bladder. BJU Int. 2009;103(10):1424–1428. doi: 10.1111/j.1464-410X.2008.08129.x. [DOI] [PubMed] [Google Scholar]
- [41].Chen ZH, He YQ, Song CJ, et al. Sericin can reduce hippocampal neuronal apoptosis by activating the Akt signal transduction pathway in a rat model of diabetes mellitus. Neural Regen Res. 2012;7(3):197–201. doi: 10.3969/j.issn.1673-5374.2012.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]


