Abstract
Based on previous studies that have shown flavonoids from the stems and leaves of Scutellaria baicalensis Georgi are neuroprotective agents in a naturally senile, D-galactose, aging in vivo model, as well as an in vitro model of oxidative/hypoxic injury, we established a cerebral ischemia/reperfusion model in rats by middle cerebral artery occlusion. The light/electron microscopic observations found significant neuropathological changes including neuron loss or swelling and rough endoplasmic reticulum injury. Moreover, the activities of lactate dehydrogenase, Na+-K+-ATPase, Ca2+-ATPase and superoxide dismutase were significantly lowered, and the levels of malonaldehyde increased. In addition, the memory of rats worsened. However, treatment with flavonoids from Scutellaria baicalensis Georgi (35, 70 and 140 mg/kg) for 13 days dramatically improved the above abnormal changes. These results suggest that the ability of flavonoids from Scutellaria baicalensis Georgi in attenuating cerebral functional and morphological consequences after cerebral ischemia/reperfusion may be beneficial for the treatment of ischemic brain disease.
Keywords: neural regeneration, traditional Chinese medicine, brain injury, Scutellaria baicalensis Georgi, cerebral ischemia/reperfusion, cognitive impairment, neuronal damage, lactate dehydrogenase, Na+-K+-ATPase, Ca2+-ATPase, superoxide dismutase, malonaldehyde, grants-supported paper, photographs-containing paper, neuroregeneration
Research Highlights
(1) Flavonoids from Scutellaria baicalensis Georgi, at a concentration range of 35–140 mg/kg, attenuated neuron injury in rats with cerebral ischemia/reperfusion.
(2) Flavonoids from Scutellaria baicalensis Georgi enhanced the activities of lactate dehydrogenase, Na+-K+-ATPase, Ca2+-ATPase and superoxide dismutase, and decreased levels of malonaldehyde.
(3) Flavonoids from Scutellaria baicalensis Georgi improved learning and memory behaviors in rats with cerebral ischemia/reperfusion.
INTRODUCTION
In general, treatment of cerebral ischemia requires the timely recovery of blood flow to the ischemic areas. Although reperfusion can save neurons injured in the infract area, it can also increase neuronal injury and advance neuron death[1]. This injury is commonly termed acute cerebral ischemia/reperfusion damage. Cerebral ischemia/reperfusion injury involves complicated pathophysiological processes and a cascade of reactions, such as ATP depletion, excitatory amino acid toxicity, excessive free radical production, neuronal apoptosis, intracellular calcium overload and inflammatory responses[2]. The accumulation of these detrimental neuronal changes in the brain contributes to neuronal damage and dysfunction, and advances learning and memory deficits[3]. Therefore, strategies that can attenuate the detrimental effects of middle cerebral artery occlusion may act as promising treatments for ischemic brain injury in a clinical setting.
Flavonoids from Scutellaria baicalensis Georgi have been affirmed as a promising agent in an in vivo palliative memory deficit model[4]. In our previous studies, flavonoids isolated from the stems and leaves of Scutellaria baicalensis Georgi were neuroprotective in a naturally senile, D-galactose, aging in vivo model and in in vitro oxidative/hypoxic models[5,6,7]. From these observations, we hypothesized that flavonoids from Scutellaria baicalensis Georgi may be effective against acute cerebral damage and represent a new treatment strategy. Middle cerebral artery occlusion-induced ischemia/reperfusion in rats is commonly used to study ischemic brain injury and the pharmacological effects of drugs. The model cannot completely replicate the status of patients with ischemic brain injury in a clinical setting, but it partially reproduces neuron and metabolite disorders, including learning/memory impairments[8]. Here, we aimed to investigate the beneficial effects of flavonoids from Scutellaria baicalensis Georgi on memory, neuron dysfunction and metabolite disruption following cerebral ischemia/reperfusion in rats.
RESULTS
Quantitative analysis of experimental animals
Of the 40 Sprague-Dawley rats, 32 were randomly selected to establish the cerebral ischemia/reperfusion model. The remaining eight rats, with exposed but not clamped blood vessels, were used as the sham-surgery group. The 32 model rats were randomly and equally assigned to model, and 35, 70 and 140 mg/kg flavonoids from Scutellaria baicalensis Georgi groups and intragastrically perfused with normal saline, 35, 70 and 140 mg/kg flavonoids from Scutellaria baicalensis Georgi, respectively. The sham group rats were given normal saline. All 40 rats were included in the final analysis.
Flavonoids from Scutellaria baicalensis Georgi improved memory impairment in cerebral ischemia/reperfusion rats
To assess learning ability acquisition, the Morris water maze[9] was used to train rats for 5 consecutive days. During the 5-day learning acquisition trial, all rats took a progressively declined time to locate the hidden platform (latency). Figure 1A shows that the model group rats took longer to find the hidden platform than sham-surgery group rats [Group × Time F(4, 28) = 3.36, n = 8, P < 0.05]. However, when the model group rats were treated with flavonoids from Scutellaria baicalensis Georgi for 12 days, the prolonged latency was dramatically shortened. Figure 1A also shows a comparison of the effects of the three doses of flavonoids from Scutellaria baicalensis Georgi on latency [F(4, 28) = 12.8, P < 0.01] for 35 mg/kg, [F(4, 28) = 4.85, P < 0.01] for 70 mg/kg and [F(4, 28) = 3.62, P < 0.05] for 140 mg/kg with model rats. The time for swimming in the target quadrant (third quadrant), where the platform was located during the 5-day training trial, was used to estimate the memory retention in the probe trial. As compared with the sham-surgery group rats, the swimming time of model group rats decreased by 47.36% in the target quadrant, and the declined swimming time was attenuated by 21.93%, 41.35% and 81.86% with treatment of 35, 70 and 140 mg/kg flavonoids from Scutellaria baicalensis Georgi, respectively (Figure 1B).
Figure 1.
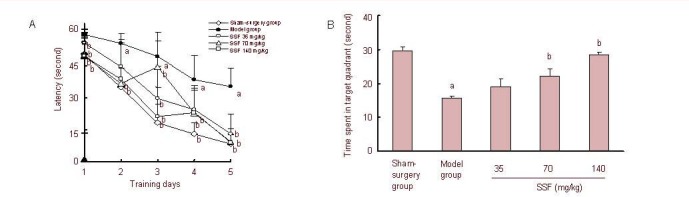
Effect of flavonoids from Scutellaria baicalensis Georgi (SSF) on Morris water maze performance following cerebral ischemia/reperfusion in rats.
Rats were daily and orally administered SSF (35, 70 and 140 mg/kg) for 12 days, from days 2 to 13 after operation. Each rat was subjected to two trials daily for 5 consecutive days from day 8 after operation. Data are expressed as the mean ± SEM. aP < 0.01, vs. sham-surgery group; bP < 0.01, vs. model group.
(A) Mean latency to find the hidden platform in the learning ability acquisition trial. Data were analyzed by two-way analysis of variance (group × days, 5 × 5) with repeated measures (n = 8).
(B) Time spent in target quadrant within 60 seconds in the probe trial (no platform). Each rat was immediately subjected to 60-second observations following the last learning ability acquisition trial on day 13 after operation. Groups differences were analyzed by one-way analysis of variance followed by the Duncan's multiple-range test (n = 8).
Flavonoids from Scutellaria baicalensis Georgi attenuated neuronal injury in the cerebral cortex of cerebral ischemia/reperfusion rats
No conspicuous histological changes were observed with the naked eye in all rats. Neuron morphology was viewed by light/electron microscopy. Neurons in the sham-surgery group presented as normal status, with normal appearing nuclei and cytoplasm, and no signs of injury. However, there were typical neuropathological changes in cerebral ischemia model rats, including notable neuron loss, nuclei pyknosis, neurofibrillary degeneration, neurogliocyte proliferation and neuronophagia.
In addition, neurons in the model group showed severe injury, with thickening and irregularities in the nuclear membrane, heterochromatin block formation, rough endoplasmic reticulum dilation, and broken or vacuolar mitochondrial ridges containing dense granules. Interestingly, when model group rats received any of the three doses of flavonoids from Scutellaria baicalensis Georgi for 13 days, neuronal injury was ameliorated (Figures 2 and 3).
Figure 2.
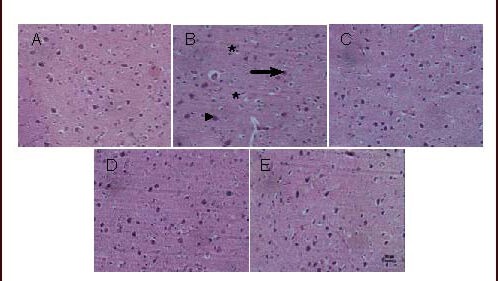
Representative photomicrographs of neurons in the cerebral cortex of cerebral ischemia/reperfusion rats treated with flavonoids from Scutellaria baicalensis Georgi (SSF) (hematoxylin-eosin staining). Scale bar: 20 μm.
All rats were sacrificed 60 minutes after final drug administration.
(A) Sham-surgery group; (B) model group: neuron loss, nuclear shrinkage (▴arrow), neurofibrillary degeneration (→ arrow), neurogliocyte proliferation and neuronophagia (*arrow); (C–E) group treated with SSF at doses of 35, 70 and 140 mg/kg, respectively. The three doses of SSF protected neurons in a dose-dependent manner.
Figure 3.
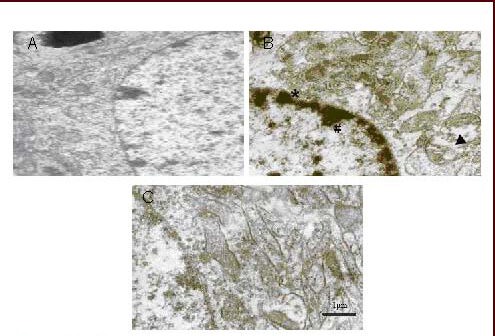
Electron micrographs of cerebral cortex neurons in cerebral ischemia/reperfusion rats treated with flavonoids from Scutellaria baicalensis Georgi (SSF).
SSF were administered daily and orally for 13 days. All rats were sacrificed 60 minutes after final drug administration.
(A) Sham-surgery group; (B) model group: the neurons showed severe injury, including nuclear membrane thickening and irregularities (* arrow), heterochromatin block formation and side-movement (# arrow); rough endoplasmic reticulum dilation; broken mitochondrial ridges and vacuoles (▴arrow); (C) 140 mg/kg group treated with SSF; SSF showed protective effects on injured neurons.
Flavonoids from Scutellaria baicalensis Georgi enhances lactate dehydrogenase, Na+-K+-ATPase and Ca2+-ATPase activities in the hippocampus and cerebral cortex of cerebral ischemia/reperfusion rats
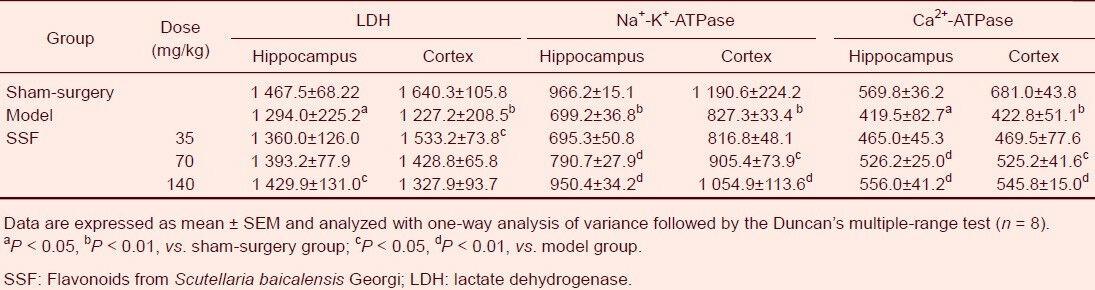
Compared with sham-surgery group rats, enzyme activities in the hippocampus and cerebral cortex of model group rats decreased by 11.82% and 25.18%, respectively, for lactate dehydrogenase; 27.63% and 30.52%, respectively, for Na+-K+-ATPase; and 26.37% and 37.91%, respectively, for Ca2+-ATPase (P < 0.01). However, these disturbances were markedly reversed following administration of 35, 70 and 140 mg/kg flavonoids from Scutellaria baicalensis Georgi for 13 days (Table 1).
Table 1.
Effects of SSF on the activities of LDH (U/L), Na+-K+-ATPase (μmolPi/g/h) and Ca2+-ATPase (μmolPi/g/h) in cerebral ischemia/reperfusion rats
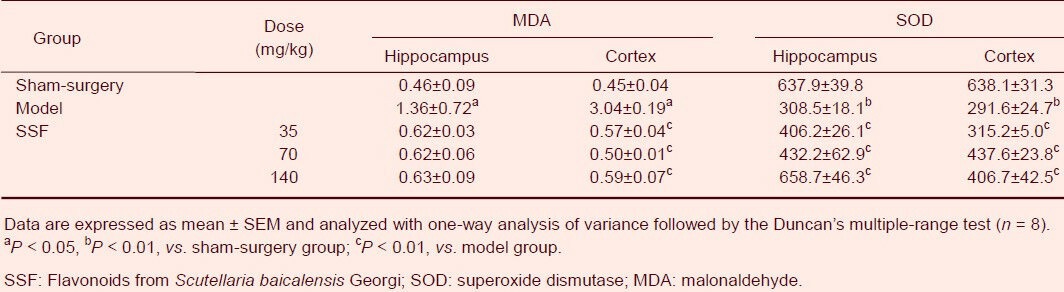
Flavonoids from Scutellaria baicalensis Georgi inhibited malonaldehyde expression and enhanced superoxide dismutase activity in the hippocampus and cerebral cortex of rats
Malonaldehyde levels and superoxide dismutase activity in the hippocampus and cerebral cortex of the ischemia/reperfusion group also exhibited marked disruption. Malonaldehyde levels in the hippocampus and cerebral cortex were 2.96 (P < 0.05) and 6.76 (P < 0.05) fold higher, respectively, than in sham-surgery group rats, and superoxide dismutase activity decreased by 51.64% (P < 0.01) in the hippocampus and 54.29% (P < 0.01) in the cerebral cortex, when compared with sham-surgery group rats. However, malonaldehyde levels and superoxide dismutase activity in the two brain regions of the model group were ameliorated to different extents with 35, 70 and 140 mg/kg flavonoids from Scutellaria baicalensis Georgi treatment for 13 days (Table 2).
Table 2.
Effects of SSF on MDA content (nmol/mg) and SOD activity (μg/min/g) in cerebral ischemia/reperfusion rats
DISCUSSION
Cerebral ischemia is a neurological disorder that results in neuronal injury and metabolic disturbances that are accompanied by cognitive deficits[10]. Many studies have shown that energy depletion, oxidative stress, inflammation, intracellular calcium overload and other neuronal responses are considered responsible elements for cognitive deficits following cerebral ischemia[11]. In the laboratory, the middle cerebral artery occlusion model is used to replicate the cognitive deficits and cerebral pathophysiological changes observed following ischemic injury. Several papers have reported that repetitive cerebral ischemia/reperfusion in rats may produce more severe damage than a single insult, which closely resembles the pathophysiological status of ischemic cerebral disease in the clinical setting[12,13].
In the present study, we found that middle cerebral artery occlusion rats showed a profound impairment in learning and memory performance. The results were in agreement with previous reports that showed cerebral ischemic rats took longer to climb onto the hidden platform and swam for a shorter time in the target quadrant when compared to sham-surgery group rats[14]. We also observed cerebral tissue damage in middle cerebral artery occlusion rats, particularly neuron loss or swelling, neuronophagia, mitochondria and rough endoplasmic reticulum injury, and heterochromatin blocks. The results confirmed that brain state contributes to cerebral function including learning, memory and cognition. However, middle cerebral artery occlusion rats treated with flavonoids from Scutellaria baicalensis Georgi 35, 70 and 140 mg/kg for 12 days dramatically improved water maze performance when compared with middle cerebral artery occlusion rats.
In addition, oral administration of flavonoids from Scutellaria baicalensis Georgi also attenuated cerebral injury and these paralleled with the improvement on cognitive deficits in middle cerebral artery occlusion rats. Thus, our data indicates the possible beneficial value of flavonoids from Scutellaria baicalensis Georgi on cognitive deficits following middle cerebral artery occlusion, which is most likely due to its neuroprotective properties. These data also suggest that neuron injury delayed by flavonoids from Scutellaria baicalensis Georgi may inhibit memory impairment induced by cerebral ischemia.
There is mounting evidence that decreased cerebral blood flow is one of the critical insults during cerebral ischemia. Energy supplied to the brain is rapidly exhausted within 10 minutes because of low ATP and glycogen levels reserved for the brain. Under normal conditions, the brain is dependent on a constant energy supply from ATP through oxidative phosphorylation. The energy released from ATP by Na+-K+-ATPase supports brain function. However, when the brain undergoes ischemic injury, the brain uses ATP generated from anaerobic metabolism. Brain pH markedly decreases following excessive lactate production during anaerobic metabolism and an inability to clear H+. When brain pH decreases, the Na+/H+ channel and H+ clearance are inhibited, resulting in a further increase in H+. In addition, the high levels of H+ can also combine with the imidazole group of lactate dehydrogenase, resulting in lactate dehydrogenase activity suppression, which results in reduced affinity of lactate dehydrogenase for lactic acid and lactic acid accumulation, further aggravating acidosis. This disruption to H+ clearance damages the mitochondrial respiratory chain and leads to a series of metabolic changes, including ATP synthesis impairment, ATPase activity inhibition, intracellular Na+ enhancement, and an increase in calcium influx. These alterations finally lead to neuronal swelling and necrosis[15,16].
Na+-K+-ATPase and Ca2+-ATPase are located on the cell membrane and can hydrolyze ATP to build a reversed gradient to transfer ions. This process is the physical basis of nerve conduction. Therefore, Na+-K+-ATPase activity is considered a good indicator of cell status. When the brain undergoes cerebral ischemia, ATP is rapidly exhausted and Na+ pump activity, which is dependent on energy, decreases. Therefore, Na+ cannot be transferred and is retained intracellularly. This intracellular accumulation of Na+ allows water to enter the cell and subsequent cerebral edema. In addition, the reduced Na+-K+-ATPase activity induces K+ enhancement of the synaptosome and the depolarization of the presynaptic membrane, which further affects synaptic excitability conduction and neurotransmitter release. Simultaneously, Na+-K+-ATPase can also regulate intracellular Ca2+ concentrations, which regulate neurotransmitter release and excitability transmission. Intracellular Ca2+ regulation is dependent on various Ca2+ channels, the Na+/Ca2+ exchanger and Ca2+-ATPase. In particular, Ca2+-ATPase activity is considered an important factor for Ca2+ uptake, storage and release. When the brain is exposed to an insult, the function of Ca2+-ATPase appears to be impaired and results in intracellular Ca2+ overload. In addition, the high intracellular Ca2+ can trigger Ca2+-regulated pathways to induce free radical production, lipid peroxidation, mitochondrial injury and ion channel function impairment[17]. In our study, we found that lactate dehydrogenase, Na+-K+-ATPase and Ca2+-ATPase activities significantly decreased in the brain following middle cerebral artery occlusion in rats. However, these decreases were reversed following administration of 35, 70 and 140 mg/kg flavonoids from Scutellaria baicalensis Georgi for 13 days. In addition, flavonoids from Scutellaria baicalensis Georgi treatment reduced neuronal damage and cognitive deficits following middle cerebral artery occlusion. Thus, our results indicate that flavonoids from Scutellaria baicalensis Georgi may be beneficial for the treatment of cognitive deficits caused by cerebral ischemia/reperfusion.
There is a consensus of opinion that free radical system disorders are involved in ischemia/reperfusion-induced cerebral injury. Excessive free radicals can result in lipid peroxidation, and in some cases, cell membrane structure damage, protein degradation, nucleic acid trunk chain rupture, and cell dysfunction[18]. Abnormal changes to malonaldehyde levels and superoxide dismutase activity in the brain have been reported as the main cause of free radical disorders, and have thus been used as indicators of brain injury[18]. Furthermore, mitochondrial injury and enzyme deactivation, including lactate dehydrogenase, Na+-K+-ATPase and Ca2+-ATPase, are also causes of free radical disorder. In our study, we found that levels of malonaldehyde markedly increased and that activity of superoxide dismutase significantly decreased in the hippocampus and cerebral cortex of middle cerebral artery occlusion rats, which coincided with previous reports that free radical system disorders contribute to cerebral damage in rats subjected to cerebral ischemia/reperfusion injury[19,20]. However, these disturbances in malonaldehyde content and superoxide dismutase activity were attenuated following treatment with 35, 70 and 140 mg/kg flavonoids from Scutellaria baicalensis Georgi for 13 days. These results were in parallel with the neuroprotective effects of flavonoids from Scutellaria baicalensis Georgi and the improvement to learning and memory in middle cerebral artery occlusion rats.
The present study confirmed the beneficial effects of flavonoids from Scutellaria baicalensis Georgi on the cognitive deficits, neuronal pathological changes, free radical production, and energy and Ca2+ metabolism disorders following middle cerebral artery occlusion. These properties of flavonoids from Scutellaria baicalensis Georgi are primarily derived from its polyphenol structure. In addition, our experimental evidence also suggests that the flavonoids not only act as antioxidants, but also modulate enzyme metabolism and signaling pathways involved in reactive oxygen species formation[21]. Therefore, flavonoids from Scutellaria baicalensis Georgi, a group of flavonoid compounds, are able to block free radical-induced cytotoxicity, which may be helpful in the treatment of ischemic cerebrovascular diseases.
MATERIALS AND METHODS
Design
A randomized, controlled, animal study.
Time and setting
The experiment was performed at the Department of Pharmacology of Basic Medical School, Wuhan University and Institute of Traditional Chinese Medicine, Chengde Medical College, China from June to December 2011.
Materials
Animals
Forty adult, male Sprague-Dawley rats, aged 3–3.5 months, weighing 350–400 g, were purchased from the Laboratory Animal Center, Hebei Medical University (Clean grade, Certification No. 04057), China. The rats were housed in groups (four or five per cage) in a room controlled at 23 ± 1°C and maintained in an alternating 12-hour light/dark cycle. Food and water were freely available. All animal procedures were performed in accordance with the Regulations of Experimental Animal Administration, issued by the State Committee of Science and Technology of China[22].
Drugs
Flavonoids, isolated from the stems and leaves of Scutellaria baicalensis Georgi (Huangqin), were prepared by the Phytochemistry Laboratory, Institute of Traditional Chinese Medicine, Chengde Medical College, and the Huangqin herb was planted by our laboratory. With regard to preparation, the dried aerial part of Scutellaria baicalensis (1 kg) was boiled with 80% (v/v) alcohol for 1 hour and then filtered. The filtrate was concentrated to the appropriate volume at 60°C under vacuum distillation. The concentrated solution was adjusted to pH 2 with 1 M HCl and the suspension was filtered after still standing for 24 hours at room temperature[23]. The residue was identified as flavonoids from Scutellaria baicalensis Georgi, in which the purity of flavonoids from Scutellaria baicalensis Georgi was not less than 80% and scutellarein was determined to be the major ingredient by high performance liquid chromatography analysis. flavonoids from Scutellaria baicalensis Georgi were dissolved in distilled water prior to administration, and the pH of the solution was adjusted to 7.2–7.4 with saturated sodium bicarbonate.
Methods
Establishment of middle cerebral artery occlusion models
The rats were anesthetized with 240 mg/kg chloral hydrate by intraperitoneal injection. The right middle cerebral artery was occluded according to an animal model of focal cerebral ischemia as previously described[24]. The rats were placed in a supine position and the skin was incised along the midline of the neck. The right common carotid artery, external carotid artery and internal carotid artery were exposed and separated. The common carotid artery and internal carotid artery were occluded using a bulldog clamp. The proximal and distal tips of the external carotid artery were ligated with 0# thread, and were cut at the bevel portal at the middle of the two ligated sites of the external carotid artery. The distal end of the external carotid artery was lifted so that it was in line with the internal carotid artery. A nylon thread (50 mm length and 0.26 mm diameter) was inserted approximately 20 mm from the bevel portal of the external carotid artery distal tip and through the internal carotid artery to the bifurcation of the common carotid artery. At this position, the tip of the nylon thread was inside the middle cerebral artery branches of the Willis circle of the brain, where blood flow was blocked by the nylon thread. The middle cerebral artery occlusion in rats lasted 20 minutes and then the nylon thread was retracted 10 mm to allow for blood reperfusion for 10 minutes. The same procedure was repeated three times. After the final ischemia and reperfusion, the external carotid artery stump was ligated and the surgical incision was sutured. The rats that received the same surgery without ischemia/reperfusion served as the sham-surgery group. The surgical rats were placed on a heating pad until they recovered from anesthesia.
Intragastric administration of flavonoids from Scutellaria baicalensis Georgi
Rats from the three treatment groups received flavonoids from Scutellaria baicalensis Georgi at doses of 35, 70 and 140 mg/kg daily by intragastric administration. The model and sham-surgery groups were subjected to the same volume of distilled water for 13 days from 24 hours after the surgery (2 mL per 100 g body weight). The dosage was based on a previous report[5].
Morris water maze test
Learning ability and memory retention was assessed using the Morris water maze[9]. Assessment of learning began on day 8 and the probe trial was carried out on day 12 after drug administration. During the learning performance test and the probe trial, the drug or its vehicle (distilled water) was administered 60 minutes before the trial. The water maze was a stainless steel circular tank of diameter 120 cm and depth 50 cm and was supplied by the Institute of Materia Medica, Chinese Academy of Medical Science & Peking Union Medical College. A circular platform, diameter 10 cm and 30 cm high, was placed in a quadrant of the tank. When the water maze test was carried out, the tank was added with water to a depth of 31.5 cm and the platform was set 1.5 cm under the water surface. The tank water was maintained at 23 ± 1°C and made white/opaque with non-fat milk powder. All spatial marks around the maze were kept invariable during the water maze test. The tank was subdivided into four equal quadrants by imaginary lines. The hidden platform was placed in the third quadrant. Learning ability was assessed over 5 consecutive days with two trials each day. The time taken to find the hidden platform (latency) was recorded and an average of the two trials was used to determine learning ability. On the first training day, each rat was left to swim for 120 seconds in the water maze to become familiar with the water conditions. On the second day, the rats were left to search for the hidden platform for 60 seconds. In cases where rats did not find the platform, the rats were placed on the hidden platform within 60 seconds. Regardless of whether rats discovered the hidden platform or not, the rats were maintained on the platform for 20 seconds and then removed from the tank. Rats were allowed to recuperate for 10 seconds between the two trials. The probe trial was performed immediately after the last learning ability test. The rats were allowed to swim for 60 seconds, in which the platform was taken away from the water. The time spent in the target quadrant (third quadrant), where the platform had been available during the learning ability test, was recorded as memory retention. Swimming activity was monitored using a video camera linked to computer-based graphics analytic software (Institute of Materia Medica, Chinese Academy of Medical Science & Peking Union Medical College).
Sample preparation of the hippocampus and cerebral cortex
Under ether anesthesia, all rats were sacrificed 60 minutes after the final administration of flavonoids from Scutellaria baicalensis Georgi or distilled water on day 14 of the operation. The hippocampus and cerebral cortex were separated according to a previously described method[25], and were placed on the ice for determining the neural morphology and biochemistry.
Measurement of neuronal morphology in the hippocampus and cerebral cortex
The brain was rapidly placed on ice, and the hippocampus and cerebral cortex were gently dissected. The separated cerebral cortex of the right hemisphere in four rats from each group was fixed with 4% (v/v) formalin and embedded in paraffin. Coronal sections were cut at 6 μm and stained with hematoxylin and eosin as previously described[8]. Stained cells were observed and pictures at 400 × magnification were captured with an Olympus VANOX microscope (Olympus, Tokyo, Japan). In addition, another cerebral cortex was fixed with 2.5% (v/v) glutaraldehyde and 1% (v/v) osmic acid, and then cut at 50 nm using an ultra-microtome. The slices were put on a copper grid with 200-mesh, and stained with uranyl acetate and lead nitrate–sodium citrate according to a previously described method[25]. The neuron ultra-status was viewed using a JELO100 CX-II-transmission electron microscope (JEOL, Japan) and images were captured at a magnification of 6 700–20 000 ×.
Detection of lactate dehydrogenase, Na+-K+-ATPase and Ca2+-ATPase activity in the hippocampus and cerebral cortex
The hippocampus and cerebral cortex were homogenized with cold saline to obtain 2% homogenates for measuring lactate dehydrogenase, Na+-K+-ATPase and Ca2+-ATPase activity. Lactate dehydrogenase activity was determined according to the manufacturers’ protocol (Beikong Bio-technology & Science Inc, Beijing, China) and using an ultraviolet spectrophotometer[26] (Shanghai Analytic Factory, Shanghai, China). ATPase activity was determined according to the manufactures protocol (Nanjing Jiancheng Institute of Biological Engineering, Nanjing, China). ATPase activity was determined by measuring the formation of phosphoric acid from ATP[27]. The phosphoric acid combines with ammonium molybdate to produce phosphato-molybdic acid salt. The latter was reduced into molybdenum blue, and the maximum absorptive wavelength of molybdenum blue was measured at 660 nm. ATPase activity was defined as the change in absorbance of molybdenum blue.
Detection of malonaldehyde production and superoxide dismutase activity in the hippocampus and cerebral cortex
The hippocampus and cerebral cortex were separated and homogenized with cold saline to obtain 10 % (w/v) and 1% (w/v) homogenates for assaying malonaldehyde production and superoxide dismutase activity, respectively. The principle of determining malonaldehyde was based on the methods of thiobarbituric acid[28]. The malonaldehyde production was determined by a change in absorbance. The measurement of superoxide dismutase activity was based on the xanthine-xanthine oxidase method[29]. Superoxide dismutase activity was determined by measuring the absorbance at 550 nm. Both assays were performed in an ice-cold environment according to the manufacturer's instructions (Nanjing Jiancheng Institute of Biological Engineering).
Statistical analysis
The Statpark software package (Oracle Corporation, Redwood Shores, CA, USA) was used to analyze the experimental results. Data are expressed as mean ± SEM and the latency from the Morris water maze training trial was evaluated by two-way analysis of variance with repeated measures for between group comparisons. One-way analysis of variance followed by the Duncan's multiple-range test was used to analyze group differences for the probe trial and biochemistry data. The level of significance was set at P < 0.05..
Footnotes
Yazhen Shang, Studying for doctorate, Professor, Master's supervisor.
Funding: The study was supported by the State Administration of Traditional Chinese Medicine of China, No. 02-03-ZP18; Hebei Provincial Education Department, No. 20015; and Hebei Provincial Hundred Outstanding Innovated Talents, First Batch.
Conflicts of interest: None declared.
Ethical approval: The study received full approval by the Animals Ethics Committee of Chengde Medical College, China.
(Edited by Yang N, Zhang YJ/Su LL/Wang L)
REFERENCES
- [1].Pennisi G, Ferri R, Cantone M, et al. A review of transcranial magnetic stimulation in vascular dementia. Dement Geriatr Cogn Disord. 2011;31(1):71–80. doi: 10.1159/000322798. [DOI] [PubMed] [Google Scholar]
- [2].Vaibhav K, Shrivastava P, Javed H, et al. Piperine suppresses cerebral ischemia-reperfusion-induced inflammation through the repression of COX-2, NOS-2, and NF-κB in middle cerebral artery occlusion rat model. Mol Cell Biochem. 2012;367(1-2):73–84. doi: 10.1007/s11010-012-1321-z. [DOI] [PubMed] [Google Scholar]
- [3].Car H, Zendzian-Piotrowska M, Fiedorowicz A, et al. The role of ceramides in selected brain pathologies: ischemia/hypoxia, Alzheimer disease. Postepy Hig Med Dosw (Online) 2012;66:295–303. doi: 10.5604/17322693.999024. [DOI] [PubMed] [Google Scholar]
- [4].Shang YZ, Miao H, Cheng JJ, et al. Effects of SSF on memory deficits in aluminum toxic mice. Zhongguo Yaoli Xue Tongbao. 2005;21(3):361–365. [Google Scholar]
- [5].Song HR, Cheng JJ, Miao H, et al. Scutellaria flavonoid supplementation reverses ageing-related cognitive impairment and neuronal changes in aged rats. Brain Inj. 2009;23(2):146–153. doi: 10.1080/02699050802649670. [DOI] [PubMed] [Google Scholar]
- [6].Shang YZ, Gong MY, Zhou XX, et al. Improving effects of SSF on memory deficits and pathological changes of neural and immunological systems in senescent mice. Acta Pharmacol Sin. 2001;22(12):1078–1083. [PubMed] [Google Scholar]
- [7].Shang YZ, Qin BW, Cheng JJ, et al. Effect of Scutellaria flavonoids on KCN-induced damages in rat pheochromocytoma PC12 cells. Indian J Med Res. 2008;127(6):610–615. [PubMed] [Google Scholar]
- [8].Pu F, Motohashi K, Kaneko T, et al. Neuroprotective effects of Kangen-karyu on spatial memory impairment in an 8-arm radial maze and neuronal death in the hippocampal CA1 region induced by repeated cerebral ischemia in rats. J Pharmacol Sci. 2009;109(3):424–430. doi: 10.1254/jphs.08245fp. [DOI] [PubMed] [Google Scholar]
- [9].Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods. 1984;11(1):47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- [10].Yu J, Liu C, Zhang X, et al. Acupuncture improved cognitive impairment caused by multi-infarct dementia in rats. Physiol Behav. 2005;86(4):434–441. doi: 10.1016/j.physbeh.2005.07.015. [DOI] [PubMed] [Google Scholar]
- [11].Takagi N. Pathology and strategies for the treatment of ischemic brain injury. Yakugaku Zasshi. 2009;129(10):1215–1219. doi: 10.1248/yakushi.129.1215. [DOI] [PubMed] [Google Scholar]
- [12].Nakano S, Kato H, Kogure K. Neuronal damage in the rat hippocampus in a new model of repeated reversible transient cerebral ischemia. Brain Res. 1989;490(1):178–180. doi: 10.1016/0006-8993(89)90448-4. [DOI] [PubMed] [Google Scholar]
- [13].Wishart T, McCrea S, Ijaz S, et al. Comparisons of repetitive-and single-insult ischaemia: effects on regional brain damage and behaviour. Neuroreport. 1994;5(12):1541–1454. doi: 10.1097/00001756-199407000-00033. [DOI] [PubMed] [Google Scholar]
- [14].Wattanathorn J, Jittiwat J, Tongun T, et al. Zingiber officinale mitigates brain damage and improves memory impairment in focal cerebral ischemic rat. Evid Based Complement Alternat Med 2011. 2011 doi: 10.1155/2011/429505. 429505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Ames A., 3rd CNS energy metabolism as related to function. Brain Res Brain Res Rev. 2000;34(1-2):42–68. doi: 10.1016/s0165-0173(00)00038-2. [DOI] [PubMed] [Google Scholar]
- [16].Obrenovitch TP, Garofalo O, Harris RJ, et al. Brain tissue concentrations of ATP, phosphocreatine, lactate, and tissue pH in relation to reduced cerebral blood flow following experimental acute middle cerebral artery occlusion. J Cereb Blood Flow Metab. 1988;8(6):866–874. doi: 10.1038/jcbfm.1988.144. [DOI] [PubMed] [Google Scholar]
- [17].Wang Y, Zhu HY, Guo Y, et al. The influence of puerarin on the brain edema and the activity of Na+-K+-ATPase, Ca2+ -ATPase in neuron of ischemia-reperfusion rats. Zhongguo Zhongyi Jizheng. 2007;16(1):70–71. [Google Scholar]
- [18].Allen CL, Bayraktutan U. Oxidative stress and its role in the pathogenesis of ischaemic stroke. Int J Stroke. 2009;4(6):461–470. doi: 10.1111/j.1747-4949.2009.00387.x. [DOI] [PubMed] [Google Scholar]
- [19].Mansoorali KP, Prakash T, Kotresha D, et al. Cerebroprotective effect of Eclipta alba against global model of cerebral ischemia induced oxidative stress in rats. Phytomedicine. 2012;19(12):1108–1116. doi: 10.1016/j.phymed.2012.07.004. [DOI] [PubMed] [Google Scholar]
- [20].Wang D, Yuan X, Liu T, et al. Neuroprotective activity of lavender oil on transient focal cerebral ischemia in mice. Molecules. 2012;17(8):9803–9817. doi: 10.3390/molecules17089803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Gutierrez-Merino C, Lopez-Sanchez C, Lagoa R, et al. Neuroprotective actions of flavonoids. Curr Med Chem. 2011;18(8):1195–1212. doi: 10.2174/092986711795029735. [DOI] [PubMed] [Google Scholar]
- [22].The Ministry of Science and Technology of the People's Republic of China. Guidance Suggestions for the Care and Use of Laboratory Animals 2006-09-30 [Google Scholar]
- [23].Shang Y, Cheng J, Qi J, et al. Scutellaria flavonoid reduced memory dysfunction and neuronal injury caused by permanent global ischemia in rats. Pharmacol Biochem Behav. 2005;82(1):67–73. doi: 10.1016/j.pbb.2005.06.018. [DOI] [PubMed] [Google Scholar]
- [24].He W, Liu YM, Chen H, et al. Effect of breviscapine on brain edema and neutrophilinfiltration induced by focal cerebral ischemia and reperfusion in rats. Zhongguo Yaoli Xue yu Duli Xue Zazhi. 2004;18(3):161–165. [Google Scholar]
- [25].Zhu CG. Beijing: People's Medical Publishing House; 2002. Neuroanatomy. [Google Scholar]
- [26].Rosenberg JC, Rush BF. An enzymatic-spectrophotometric determination of pyruvic and lactic acid in blood. Methodologic aspects. Clin Chem. 1966;12(5):299–307. [PubMed] [Google Scholar]
- [27].He YZ, Li M, Feng GL, et al. Inhibition of pyrethroid insecticides on nerve Na+-K+-ATPase in house flies (musca domestica) Kunchong Xuebao. 1999;42(1):19–24. [Google Scholar]
- [28].Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95(2):351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- [29].McCord JM, Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein) J Biol Chem. 1969;244(22):6049–6055. [PubMed] [Google Scholar]


