Abstract
This study established a rat model of cerebral hemorrhage by injecting autologous anticoagulated blood. Rat models were intragastrically administered 5, 10, 20 g/kg Poxue Huayu and Tianjing Busui Decoction, supplemented with Hirudo, raw rhubarb, raw Pollen Typhae, gadfly, Fructrs Trichosanthis, Radix Notoginseng, Rhizoma Acori Talarinowii, and glue of tortoise plastron, once a day, for 14 consecutive days. Results demonstrated that brain water content significantly reduced in rats with cerebral hemorrhage, and intracerebral hematoma volume markedly reduced after treatment. Immunohistochemical staining revealed that brain-derived neurotrophic factor, tyrosine kinase B and vascular endothelial growth factor expression noticeably increased around the surrounding hematoma. Reverse transcription-PCR revealed that brain-derived neurotrophic factor and tyrosine kinase B mRNA expression significantly increased around the surrounding hematoma. Neurologic impairment obviously reduced. These results indicated that Poxue Huayu and Tianjing Busui Decoction exert therapeutic effects on cerebral hemorrhage by upregulating the expression of brain-derived neurotrophic factor.
Keywords: neural regeneration, traditional Chinese medicine, cerebral hemorrhage, brain-derived neurotrophic factor, tyrosine kinase B, vascular endothelial growth factor, Poxue Huayu and Tianjing Busui, grants-supported paper, neuroregeneration
Research Highlights
Hemorrhagic stroke was treated with Poxue Huayu and Tianjing Busui Decoction, supplemented with Hirudo, raw rhubarb, raw Pollen Typhae, gadfly, Fructrs Trichosanthis, Radix Notoginseng, Rhizoma Acori Talarinowii, and glue of tortoise plastron. Poxue Huayu and Tianjing Busui Decoction markedly elevated the expression of brain-derived neurotrophic factor, tyrosine kinase B and vascular endothelial growth factor in rats with cerebral hemorrhage. This study provided a new scientific therapeutic method for protecting neurons after cerebral hemorrhage.
INTRODUCTION
Traditional Chinese medicine for the recovery of neurological function in the convalescent period of stroke has some advantages such as reduced toxicity and side effects, and a precise curative effect[1]. On the basis of the pathogenesis of Suixu Dusun [2], we propose a therapeutic principle for Poxue Huayu and Tianjing Busui (i.e., breaking blood stasis, replenishing essence). In this study, the neuroprotective effect of Poxue Huayu and Tianjing Busui Decoction, containing Hirudo, raw rhubarb, raw Pollen Typhae, gadfly, Fructrs Trichosanthis, Radix Notoginseng, Rhizoma Acori Talarinowii, and glue of tortoise plastron, was investigated. Poxue Huayu and Tianjing Busui Decoction has previously been shown to effectively improve the clinical symptoms of patients with cerebral hemorrhage, and elevate the daily activity of patients[3]. Therefore, this study sought to observe the effects of Poxue Huayu and Tianjing Busui Decoction on brain-derived neurotrophic factor, tyrosine kinase B and vascular endothelial growth factor expression in brain tissues of rats with cerebral hemorrhage at the acute stage, and explore the increased secretion and expression of brain-derived neurotrophic factor, tyrosine kinase B and vascular endothelial growth factor in rat neurons after cerebral hemorrhage. In addition, the protective effect of Poxue Huayu and Tianjing Busui Decoction on neurons and its possible mechanism of action were investigated.
RESULTS
Quantitative analysis of experimental animals
Among the 150 Wistar rats, 120 rats were randomly selected to establish a model of cerebral hemorrhage by injecting anticoagulated autologous blood. Rat models were randomly assigned to five groups: model group, low-, moderate- and high-dose drug groups, and a positive control drug group. Rats in the low-, moderate- and high-dose drug groups, and positive control drug group were intragastrically administered 5, 10 and 20 g/kg Poxue Huayu and Tianjing Busui Decoction and 0.3 g/kg Xingnao Jianshen Capsule, respectively. Of the remaining 30 rats, 24 rats served as a sham surgery group, and 6 rats served as a normal control group. Rats that did not survive were supplemented with new rats during the experiment.
Poxue Huayu and Tianjing Busui Decoction improved symptoms and neurological function of the nervous system in rats with cerebral hemorrhage
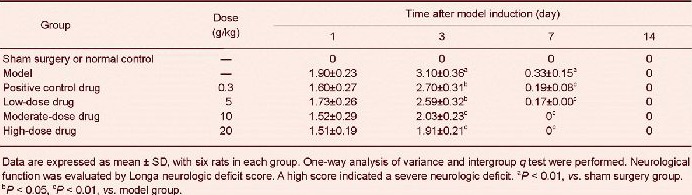
Limbs of rats in the normal control and sham surgery groups could move freely. Rats with cerebral hemorrhage experienced hemiplegia after right brain injury, and cycling to the right during walking. Moreover, neurological deficit scores increased (P < 0.01). The above-described symptoms noticeably improved in rats with cerebral hemorrhage after treatment with various doses of Poxue Huayu and Tianjing Busui Decoction or Xingnao Jianshen Capsule (P < 0.05, P < 0.01). At 7 days, nervous system symptoms recovered to normal in rats from the moderate- and high-dose drug groups (Table 1).
Table 1.
Effects of Poxue Huayu and Tianjing Busui Decoction on neurological function in rats with cerebral hemorrhage
Poxue Huayu and Tianjing Busui Decoction improved pathological morphology of tissue surrounding the hematoma in rats with cerebral hemorrhage
Hematoxylin-eosin staining results revealed that no necrotic cells were detected in the cortex and medulla of rat brain tissue in the normal control group. Brain structure remained intact and neural cells were arranged in an orderly manner and of uniform size. In the sham surgery group, neural cells were clearly arranged in rat brain tissue. A few necrotic cells were visible surrounding the pin hole, which was a physical injury induced by a needle. In the model group, low-, moderate- and high-dose drug groups, abundant necrotic neural cells and swollen glial cells were detected surrounding the hematoma, showing pyknosis and dark staining.
Macroscopically, swelling and necrosis of vascular endothelial cells, neural cells and glial cells were observed, and edema was visible surrounding the focus. At 14 days after model establishment, the hematoma and necrosis disappeared in the low-, moderate- and high-dose drug groups and the positive control drug group. Many neural cells were intact surrounding the hematoma, and the number of necrotic cells surrounding the focus markedly reduced (Figure 1).
Figure 1.
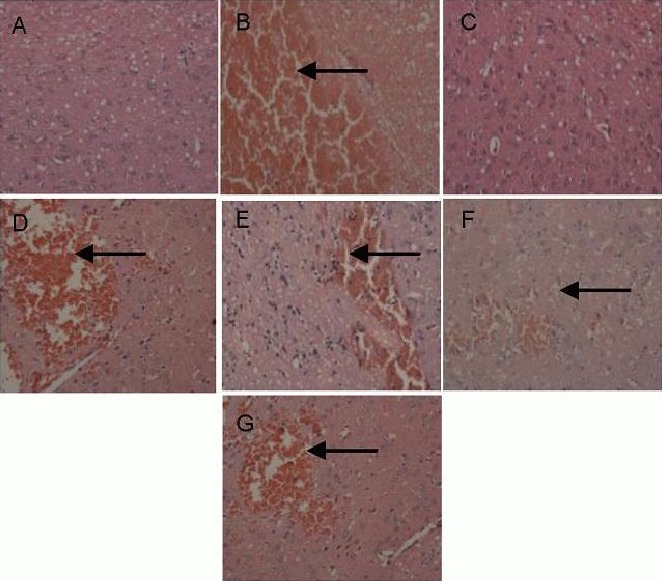
Effects of Poxue Huayu and Tianjing Busui Decoction on pathological morphology of rat brain tissue at 14 days after cerebral hemorrhage (hematoxylin-eosin staining, × 200).
(A, C) Normal control group and sham surgery group: intact brain tissue, orderly arranged neural cells.
(B) Model group: abundant necrotic neural cells and swollen glial cells.
(D–G) Low-, moderate- and high-dose drug groups and positive control drug group: injury to brain tissue was significantly reduced.
Arrows show swollen glial cells.
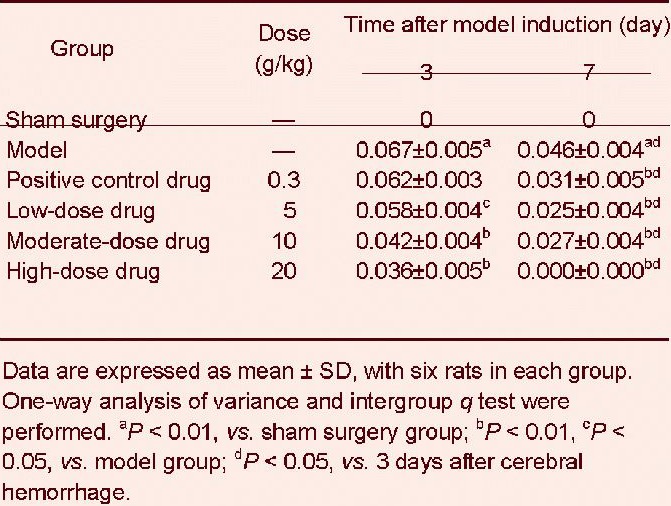
Poxue Huayu and Tianjing Busui Decoction reduced intracerebral hematoma volumes in rats with cerebral hemorrhage
At 3 days after cerebral hemorrhage, the intracerebral hematoma volume was larger in the model group than that in the sham surgery group (P < 0.01). The intracerebral hematoma volume was significantly reduced at 3 days after treatment with various doses of Poxue Huayu and Tianjing Busui Decoction (P < 0.05, P < 0.01). However, the intracerebral hematoma volume did not significantly change after treatment with Xingnao Jianshen Capsule (P > 0.05; Table 2).
Table 2.
Effects of Poxue Huayu and Tianjing Busui Decoction on intracerebral hematoma volume in rats with cerebral hemorrhage
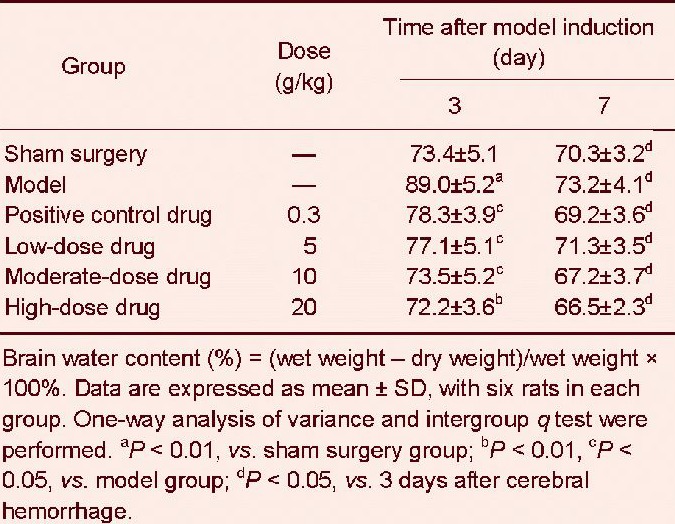
Poxue Huayu and Tianjing Busui Decoction reduced brain water content in rats with cerebral hemorrhage
Dry-wet method results revealed that at 3 days after cerebral hemorrhage, brain water content in the injured brain region in the model group was significantly greater than that in the sham surgery group (P < 0.01). Brain water content was significantly diminished after treatment with various doses of Poxue Huayu and Tianjing Busui Decoction or Xingnao Jianshen Capsule (P < 0.05, P < 0.01; Table 3).
Table 3.
Effects of Poxue Huayu and Tianjing Busui Decoction on brain water content (%) in rats with cerebral hemorrhage
Poxue Huayu and Tianjing Busui Decoction increased brain-derived neurotrophic factor, tyrosine kinase B and vascular endothelial growth factor expression in brain tissue surrounding the hematoma in rats with cerebral hemorrhage
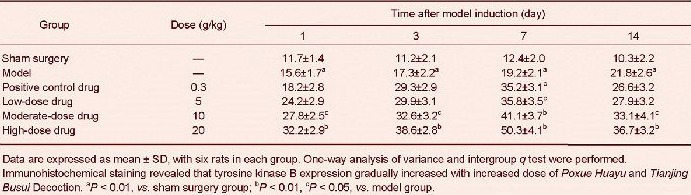
Immunohistochemical staining revealed that brain-derived neurotrophic factor, tyrosine kinase B and vascular endothelial growth factor expression increased in brain tissue surrounding the hematoma (P < 0.05). Brain-derived neurotrophic factor, tyrosine kinase B and vascular endothelial growth factor expression further increased after treatment with various doses of Poxue Huayu and Tianjing Busui Decoction (P < 0.05), and the effects were identical to that of Xingnao Jianshen Capsule (P > 0.05; Figure 2, Tables 4–6).
Figure 2.

Effects of Poxue Huayu and Tianjing Busui Decoction on brain-derived neurotrophic factor (A), tyrosine kinase B (B) and vascular endothelial growth factor (C) expression in brain tissue surrounding the hematoma in rats with cerebral hemorrhage (immunohistochemical staining, × 400).
Brain-derived neurotrophic factor, tyrosine kinase B and vascular endothelial growth factor expression further increased after treatment with various doses of Poxue Huayu and Tianjing Busui Decoction or Xingnao Jianshen Capsule. Arrows show positive expression. 7, 14 days: 7, 14 days after model induction.
Table 4.
Effects of Poxue Huayu and Tianjing Busui Decoction on brain-derived neurotrophic factor expression (absorbance) in brain tissue surrounding the hematoma in rats with cerebral hemorrhage
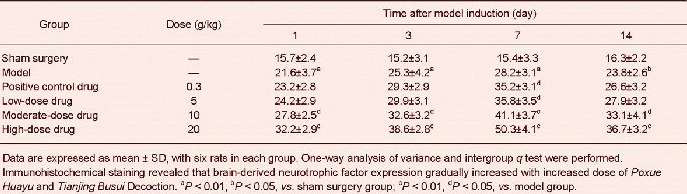
Table 6.
Effects of Poxue Huayu and Tianjing Busui Decoction on vascular endothelial growth factor expression (absorbance) in brain tissue surrounding the hematoma in rats with cerebral hemorrhage

Table 5.
Effects of Poxue Huayu and Tianjing Busui Decoction on tyrosine kinase B expression (absorbance) in brain tissue surrounding the hematoma in rats with cerebral hemorrhage
Poxue Huayu and Tianjing Busui Decoction increased brain-derived neurotrophic factor and tyrosine kinase B mRNA expression in brain tissue surrounding the hematoma in rats with cerebral hemorrhage
Reverse transcription-PCR results revealed that brain-derived neurotrophic factor and tyrosine kinase B mRNA expression significantly increased at 7 days after cerebral hemorrhage (P < 0.01). Various doses of Poxue Huayu and Tianjing Busui Decoction could increase brain-derived neurotrophic factor and tyrosine kinase B mRNA expression, especially at moderate and high doses (P < 0.05, P < 0.01). However, Xingnao Jianshen Capsule did not significantly affect brain-derived neurotrophic factor and tyrosine kinase B mRNA expression (P > 0.05; Figure 3).
Figure 3.
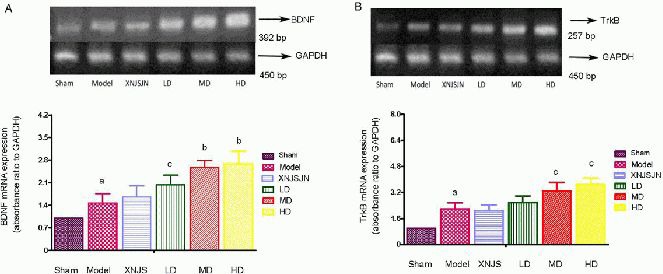
Effects of Poxue Huayu and Tianjing Busui Decoction on brain-derived neurotrophic factor (BDNF; A) and tyrosine kinase B (TrkB; B) mRNA expression in brain tissue surrounding the hematoma in rats at 7 days after cerebral hemorrhage.
Data are expressed as mean ± SD, with six rats in each group. One-way analysis of variance and intergroup q test were performed. aP < 0.01, vs. sham surgery group; bP < 0.01, cP < 0.05, vs. model group. Sham: Sham surgery group; Model: model group; XNJS: Xingnao Jianshen Capsule group (positive control drug group); LD: low-dose drug group; MD: moderate-dose drug group; HD: high-dose drug group.
DISCUSSION
Cerebral hemorrhage induces a series of pathological reactions in local brain tissue and the body, including reduction of regional cerebral blood flow, intracerebral hematoma, cerebral edema, increased intracranial pressure and delayed neuronal damage[4]. The above-mentioned changes directly affect the prognosis of the cerebral hemorrhage. However, to date, an effective therapeutic drug that can decrease the mortality and disability rate remains unavailable.
Brain-derived neurotrophic factor is a polypeptide growth factor that is ubiquitously found in the cerebral cortex, hippocampus, and corpus striatum. Under physiological conditions, brain-derived neurotrophic factor in the central nervous system regulates neuronal survival, differentiation, and axon guidance. Under pathological conditions, brain-derived neurotrophic factor participates in the protective process of brain injury[5,6,7,8]. Brain-derived neurotrophic factor protein and mRNA expression have been suggested to increase at early stages of cerebral ischemia. In particular, brain-derived neurotrophic factor protein and receptor tyrosine kinase B expression was detected in granulocytes of the dentate gyrus. These cells produce brain-derived neurotrophic factor and resist ischemic and hypoxic injury[9]. Cheng et al [10] discovered that brain-derived neurotrophic factor had noticeable protective effects on neurons against ischemic-hypoxic brain injury, and early application of brain-derived neurotrophic factor had an optimal effect. Brain-derived neurotrophic factor upregulated tyrosine kinase B receptor expression, blocked the deactivation of intracellular damage factors to protein kinase C, and prevented the death of neural cells after ischemia[9]. Results from this study demonstrated that brain-derived neurotrophic factor expression markedly increased in tissue surrounding the hematoma at 3 days after consecutive administration of Poxue Huayu and Tianjing Busui Decoction, and peaked at 7 days. This study assumed that on one hand, Poxue Huayu and Tianjing Busui Decoction promoted the synthesis and secretion of brain-derived neurotrophic factor in neurons or glial cells in the cerebral cortex, hippocampus and surrounding the hematoma, and protected damaged neurons. On the other hand, Poxue Huayu and Tianjing Busui Decoction elevated the expression of endogenous brain-derived neurotrophic factor, which bound to its specific receptor and initiated intracellular signal conduction. This signal conduction has been shown to produce corresponding effector molecules and the regulation of neuronal structure, which contributes to neuronal survival and axonal regeneration[11,12]. A clinical study verified that Poxue Huayu and Tianjing Busui Decoction improved syndromes of hemorrhagic stroke, relieved neurological functions of patients, elevated patient quality of life, and it had increased survival by 84%[3].
Brain-derived neurotrophic factor mRNA is mainly expressed in neurons, and is partially expressed in activated microglial cells surrounding the hematoma[13]. Conventional ideas suggest that brain-derived neurotrophic factor is a target-derived nerve growth factor. That is, brain-derived neurotrophic factor synthesized and secreted in the cortex and hippocampus acts on receptor tyrosine kinase B, forms a ligand-receptor complex, retrogradely transfers to the neuronal body and promotes neuronal survival. Nevertheless, recent studies confirmed that brain-derived neurotrophic factor was secreted into the extracellular matrix in an exocytosis-like formation and exerted trophic action on neural cells[14,15]. Results from the present study showed that compared with the sham surgery group, brain-derived neurotrophic factor expression increased in tissue surrounding the hematoma, and further increased after treatment with various doses of Poxue Huayu and Tianjing Busui Decoction or Xingnao Jianshen Capsule. Therefore, we presumed that Poxue Huayu and Tianjing Busui Decoction contributed to brain-derived neurotrophic factor synthesis and secretion possibly by promoting brain-derived neurotrophic factor mRNA expression in neurons or glial cells in the cortex and hippocampus, resulting in exertion of neurotrophic action and promotion of neuronal survival.
Tyrosine kinase B is a high-affinity receptor for brain-derived neurotrophic factor that is mainly expressed in the cerebral cortex, hippocampal dentate gyrus, substantia nigra-corpus striatum, hypothalamus, cerebellum, tectum of midbrain and brain stem. Its positive particles are not only distributed in neuronal cell bodies, but also in fibers, mainly in the hippocampal dentate gyrus and cortex[16,17]. Immunohistochemical staining for tyrosine kinase B revealed that compared with the sham surgery group, tyrosine kinase B expression increased in tissue surrounding the hematoma, and further increased after treatment with Poxue Huayu and Tianjing Busui Decoction or Xingnao Jianshen Capsule.
Vascular endothelial growth factor is a sub-family of growth factors and a signal protein produced by cells that stimulate vasculogenesis and angiogenesis[18]. Vascular endothelial growth factor expression was very low under normal conditions, but vascular endothelial growth factor receptor expression was high under pathological conditions. Numerous studies have confirmed that vascular endothelial growth factor expression is upregulated after cerebral ischemia, mainly via the protein kinase C pathway[19]. Vascular endothelial growth factor induces neovascularization in the ischemic region by promoting endothelial cell proliferation, improved microcirculation, increased reperfusion and oxygen supply in the involved brain tissue, and contributes to the repair of injured tissue by reinforcing the recovery of neuronal function[20,22]. Previous studies concerning pathological mechanisms of hemorrhagic cerebrovascular disease mainly focused on the effects of vascular endothelial growth factor on blood-brain barrier, cerebral edema, brain injury and cerebral protection. Some scholars thought that vascular endothelial growth factor was involved in blood-brain barrier injury and promoted cerebral edema formation after cerebral hemorrhage[23,24], thereby accelerating the damage to ischemic neurons[25,26]. While other scholars thought that vascular endothelial growth factor protected the blood-brain barrier after cerebral hemorrhage, reduced brain edema and brain injury, and exerted a neuroprotective effect[27,28,29,30]. Results from this study suggest that compared with the sham surgery group, vascular endothelial growth factor expression increases in tissue surrounding the hematoma, and further increases after treatment with various doses of Poxue Huayu and Tianjing Busui Decoction or Xingnao Jianshen Capsule, indicating that Poxue Huayu and Tianjing Busui Decoction significantly increases the number of vascular endothelial growth factor-positive cells in the injured region.
In summary, Poxue Huayu and Tianjing Busui Decoction in the treatment of acute cerebral hemorrhage obviously improves neurological function, reduces intracerebral hematoma, and accelerates the absorption of cerebral edema. The mechanism of action is most likely associated with the promotion of brain-derived neurotrophic factor expression and activation of the brain-derived neurotrophic factor-tyrosine kinase B pathway, resulting in protection and repair of neuronal cells.
MATERIALS AND METHODS
Design
A randomized, controlled animal study.
Time and setting
Experiments were performed at the Animal Experimental Center, Norman Bethune College of Medicine, Jilin University, China from March 2011 to March 2012.
Materials
Animals
A total of 150 clean Wistar rats aged 8 weeks old, of both genders, weighing 150–160 g, were provided by the Experimental Animal Center, Jilin University, China, animal license No. SCXK (Ji) 2011-0063. The rats were housed in individual cages at 20–25°C, humidity 40–70%, 15–20 Ix, and allowed free access to food and water. The protocols were conducted in accordance with the Guidance Suggestions for the Care and Use of Laboratory Animals, formulated by Ministry of Science and Technology of China[31].
Drugs
Poxue Huayu and Tianjing Busui Decoction was composed of 8 g Hirudo, 10 g raw rhubarb, 15 g raw Pollen Typhae, 5 g gadfly, 20 g Fructrs Trichosanthis, 10 g Radix Notoginseng, 15 g Rhizoma Acori Talarinowii, and 10 g glue of tortoise plastron. All ingredients were identified by Beijing Tong Ren Tang Group Co., Ltd., Beijing, China. All ingredients were placed in a 4 L-casserole by the decocting method. Purified water (2 L) was added for decocting each time and the ingredients were immersed for 30 minutes. The decoction was performed at 120°C on an electric stove (Midea, Guangzhou, Guangdong Province, China). After boiling, the decoction was cooled for 30 minutes, and then the liquid was filtered using a 120-mesh silk fabric. The remaining residue was decocted with water twice. The three extractions of liquid were mixed, heated, for 1 g of crude drug in 1 mL of liquid, and then stored at 4°C.
Xingnao Jianshen Capsule, lot No. 111216, specification: 0.25 g/pill, was composed of Calculus Bovis, Radix Curcumae, Acorus calamus, Arisaema Cum Bile, gradfly and Rhizoma Chuanxiong and prepared by the Manufacturing Laboratory, Jilin Provincial Hospital of Traditional Chinese Medicine, Jilin Province, China[32].
Methods
Establishment of cerebral hemorrhage rat model
In accordance with a previously published method[33,34,35,36], the rats were intraperipherally anesthetized with 10% (v/v) chloral hydrate (350 mg/kg), and fixed on a stereotaxic instrument (Stoelting, New York, USA). A 1 mm-diameter hole was drilled at 3 mm left of the midline and 0.2 mm anterior to the anterior fontanelle[37,38]. A total of 50 μL autologous anticoagulated blood (Tianjin Hongri Pharmaceutics Co., Ltd., Tianjin, China) was slowly injected into the left caudate nucleus along the direction of drilling with a microsyringe (Shanghai Anting Factory, Shanghai, China). The needle was maintained in place for 10 minutes. The sham surgery group did not receive injection of autologous anticoagulated blood.
Rats that experienced left hemiplegia, mainly forelimb, and cycling to the right during walking, indicated successful model induction[33].
Drug treatment
Rats in the low-, moderate- and high-dose drug groups were intragastrically administered 5, 10 and 20 g/kg Poxue Huayu and Tianjing Busui Decoction, respectively, once a day. Xingnao Jianshen Capsule was dissolved in purified water, and made into 100 g/mL solution for further use. The rats in the positive control drug group were administered 0.3 g/kg Xingnao Jianshen Capsule, once a day, for 14 consecutive days. The rats in the sham surgery and model groups were intragastrically administered 10 mL/kg saline for 14 consecutive days. Rats in the normal control group were allowed free access to food and water.
Evaluation of neurological function
At 1, 3, 7 and 14 days after model induction, neurological function was assessed by Longa's method[39]: a score of 0 indicated no neurologic deficit; a score of 1 indicated failure to extend left forepaw fully; a score of 2 indicated spontaneous turning to the left; a score of 3 indicated spontaneous circling to the left; and a score of 4 indicated failure to spontaneous walking, loss of consciousness.
Sample collection
At 1, 3, 7 and 14 days after model establishment, the rats were deeply anesthetized with 10% (v/v) chloral hydrate and decapitated. Right brain tissue was obtained on a diethyl pyrocarbonate-treated Petri dish in an ice plate. Brain tissue (100 mg) was collected and placed in a frozen tube at –80°C.
Measurement of intracerebral hematoma volume
At 3 and 7 days after model induction, rats were decapitated. The cerebrum was sliced into 2 mm-thick coronal sections, and photographed with a digital camera (Canon, Tokyo, Japan). Intracerebral hematoma volume was analyzed using pathological image analysis software (Hubei Huida Instrument Co., Ltd., Hubei Province, China). The area of the hematoma in each layer of the cranial CT was measured with Image-Pro Plus software, and the areas of all layers were added and multiplied by the thickness of each layer, i.e., intracerebral hematoma volume.
Measurement of brain water content
At 3 and 7 days after model induction, brain water content was measured by the dry-wet method[40]. Wet weight of unilateral focal tissue (2 mm thick, coronal) was accurately weighed. The above-mentioned tissue was dried in an oven at 100–110°C for 24 hours until the weight was constant. The dry weight was weighed. Brain water content (%) was equal to (wet weight – dry weight)/wet weight × 100%[39].
Hematoxylin-eosin staining for pathological morphology of brain tissue surrounding the hematoma
Rat brain tissue was fixed in 10% (v/v) formalin, embedded in paraffin, and sliced into 2 mm-thick sections. The sections were heated at 65°C for 30 minutes, dewaxed in xylene for 20 minutes, immersed in alcohol for 10 minutes, hydrated and stained with hematoxylin for 3–5 minutes. After washing, the sections were immersed in 1% (v/v) hydrogen ethanol for 10–30 seconds, washed in running water, treated with ammonia water for 10–30 seconds, and immersed in running water for 5–10 minutes. Subsequently, the sections were stained with eosin for 20 minutes, washed with running water, dehydrated with alcohol for 10 minutes, permeabilized in xylene for 10 minutes, mounted with neutral resin, and observed using a light microscope (Nanjing Jiangnan Yongxin Optics Co., Ltd., Nanjing, Jiangsu Province, China)[41,42].
Immunohistochemical staining for brain-derived neurotrophic factor, tyrosine kinase B and vascular endothelial growth factor in brain tissue surrounding the hematoma
At 1, 3, 7 and 14 days after model establishment, the sections were dewaxed in xylene, hydrated with alcohol, retrieved with citric acid buffer for 30 minutes, cooled at room temperature, incubated in 3% (v/v) H2O2 at room temperature for 5 minutes to block endogenous peroxidase, and then washed with PBS, 5 minutes × 3. The sections were incubated with rabbit anti-rat brain-derived neurotrophic factor, tyrosine kinase B and vascular endothelial growth factor antibodies (1:50–200; Zhong Shan Golden Bridge Biological Technology Co., Ltd., Beijing, China) at 4°C overnight, washed with PBS for 5 minutes, 3 times, and then incubated with IgG antibody-horseradish peroxidase (Boster, Wuhan, Hubei Province, China) at 37°C for 15 minutes. Sections were then washed with PBS for 5 minutes, 3 times. The sections were visualized with 3,3’-diaminobenzidine, washed with distilled water, counterstained with hematoxylin, washed with running water, treated with hydroxyacetic acid, washed with running water, dehydrated with alcohol, permeabilized with xylene, and mounted with resin. The negative control was not treated with primary antibody. Cell staining was observed under a high-power light microscope (Nanjing Jiangnan Yongxin Optics Co., Ltd.), and results were photographed and recorded. Absorbance values were measured with an image analyzer (Beijing Yijialin Technology Co., Ltd., Beijing, China). Absorbance values represented protein expression.
Reverse transcription-PCR for brain-derived neurotrophic factor and receptor tyrosine kinase B mRNA expression in brain tissue surrounding the hematoma
At 7 days after model induction, 100 mg tissue surrounding the hematoma was placed in a diethyl pyrocarbonate-treated homogenizer. The tissues were homogenized with 1 mL Trizol (Invitrogen, Carlsbad, CA, USA) at room temperature for 5 minutes. Homogenates were stored in an Eppendorf tube at 4°C, and centrifuged at 12 000 r/min for 10 minutes. The supernatant was placed in an additional Eppendorf tube. Chloroform (0.2 mL) was added to each Eppendorf tube at room temperature for 2–3 minutes, without stirring, followed by centrifugation at 12 000 r/min and 4°C for 15 minutes. The colorless upper layer was moved to a clean Eppendorf tube, and an equal volume of isopropyl alcohol was added and shaken uniformly, without stirring, for 10 minutes at room temperature, followed by centrifugation at 12 000 r/min for 10 minutes. After removal of the supernatant, 1 mL 75% (v/v) cold alcohol was added to each Eppendorf tube, followed by centrifugation at 7 500 r/min at 4°C for 5 minutes. After removal of the supernatant, the samples were dried and precipitated at room temperature for 3–5 minutes. RNase-free water (0.2 mL) was added to prepare total RNA solution. RNA was stored in 50 μL RNase-free water at –80°C. RNA integrity was detected using 2% (w/v) agarose gel electrophoresis. RNA was measured with an ultraviolet spectrophotometer (Shanghai Yuanxi Instrument Co., Ltd., Shanghai, China). The ratio of absorbance at 260 nm to absorbance at 280 nm was between 1.8 and 2.0, indicating a high purity of RNA, which could be used in reverse transcription-PCR. The primers were synthesized by Beijing Aoke Biotechnology Co., Ltd., Beijing, China.
Primer sequences are shown as follows:
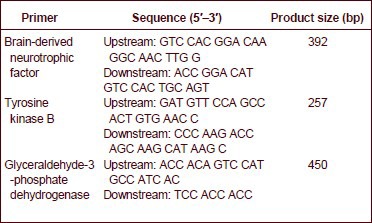
2 μL cDNA solution, 5 μL 10 × Taq enzyme buffer, 2 μL dNTPs (10 mmol/L), 10 μL upstream primer of target gene, 10 μL downstream primer of target gene, 5 μL upstream primer of internal reference, 5 μL downstream primer of internal reference, 5 μL cDNA solution, 3 μL Taq enzyme, and 3 μL diethyl pyrocarbonate-treated water were added in each 0.2 mL reaction tube, in a total volume of 50 μL.
PCR reaction conditions are shown as follows:
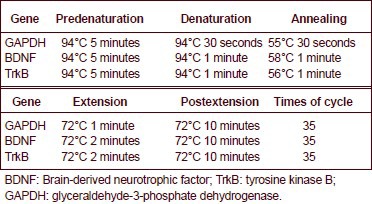
Images were collected with a gel image analyzer (Hangzhou Tianneng Biotechnology Co., Ltd., Hangzhou, Zhejiang Province, China). Mean absorbance values were analyzed with a gel image analyzer. The expression of brain-derived neurotrophic factor and tyrosine kinase B was expressed as the ratio to the absorbance of GAPDH mRNA.
Statistical analysis
The data were analyzed using SPSS 17.0 (SPSS, Chicago, IL, USA). Measurement data were expressed as mean ± SD. A comparison among multiple groups was performed using one-way analysis of variance. Paired comparison q test was used if statistical significance was present. A value of P < 0.05 was considered statistically significant.
Acknowledgments
We thank all research participators for their hard work.
Footnotes
Funding: This study was supported by the National Chinese Medicine Research Center Foundation (Stroke), No. 2012B02, and Jilin Provincial Natural Science Foundation in China, No. 201015211.
Conflicts of interest: None declared.
Ethical approval: This study was approved by the Animal Ethics Committee, Norman Bethune College of Medicine, Jilin University, China.
(Reviewed by Diwakarla S, Robens J, Zou LB, Chen L)
(Edited by Yu J, Qiu Y, Li CH, Song LP, Liu WJ, Zhao M)
REFERENCES
- [1].Jiang AJ, Hu JP, Shen GM, et al. Effect of two kinds of Chinese herbal compound on expression of BDNF and bFGF in the brain of cerebral ischemia rat. Liaoning Zhongyiyao Daxue Xuebao. 2009;11(7):188–190. [Google Scholar]
- [2].Ren JX. Expounding of brains essence. Zhongguo Zhongyi Jichu Yixue Zazhi. 2003;9(3):161–164. [Google Scholar]
- [3].Zhao DX, Yu L. Clinical research of “promoting blood circulation to remove blood stasis” on the treatment of hemorrhagic stroke. Changchun Zhongyiyao Daxue Xuebao. 2012;28(6):974–975. [Google Scholar]
- [4].Kim JS. Cytokines and adhesion molecules in stroke and related diseases. J Neurol Sci. 1996;137(2):69–78. doi: 10.1016/0022-510x(95)00338-3. [DOI] [PubMed] [Google Scholar]
- [5].Schäbitz WR, Berger C, Kollmar R, et al. Effect of brain-derived neurotrophic factor treatment and forced arm use on functional motor recovery after small cortical ischemia. Stroke. 2004;35(4):992–997. doi: 10.1161/01.STR.0000119754.85848.0D. [DOI] [PubMed] [Google Scholar]
- [6].Nawa H, Takei N. BDNF as an anterophin; a novel neurotrophic relationship between brain neurons. Trends Neurosci. 2001;24(12):683–685. doi: 10.1016/s0166-2236(00)01955-x. [DOI] [PubMed] [Google Scholar]
- [7].Takei N, Nawa H. Roles of neurotrophins on synaptic development and functions in the central nervous system. Hum Cell. 1998;11(3):157–165. [PubMed] [Google Scholar]
- [8].Hou LJ, Qiao DC. The research advance of brain derived neurotrophic factor. Zhongguo Kangfu Yixue Zazhi. 2005;20(12):940–942. [Google Scholar]
- [9].Zou W, Wang HF, Kuang HY, et al. Effect of acupuncture on endothelin levels in the plasma of rat with acute cerebral hemorrhage. Zhongguo Zhongyiyao Keji. 1998;5(5):302. [Google Scholar]
- [10].Cheng Y, Gidday JM, Yan Q, et al. Marked age-dependent neuroprotection by brain-derived neurotrophic factor against neonatal hypoxic-ischemic brain injury. Ann Neurol. 1997;41(4):521–529. doi: 10.1002/ana.410410416. [DOI] [PubMed] [Google Scholar]
- [11].Jin Y, Fischer I, Tessler A, et al. Transplants of fibroblasts genetically modified to express BDNF promote axonal regeneration from supraspinal neurons following chronic spinal cord injury. Exp Neurol. 2002;177(1):265–275. doi: 10.1006/exnr.2002.7980. [DOI] [PubMed] [Google Scholar]
- [12].Liu Y, Himes BT, Murray M, et al. Grafts of BDNF-producing fibroblasts rescue axotomized rubrospinal neurons and prevent their atrophy. Exp Neurol. 2002;178(2):150–164. doi: 10.1006/exnr.2002.7977. [DOI] [PubMed] [Google Scholar]
- [13].Wu H, Li XQ, Tang T, et al. Effect of Naoyi-an on the expression of brain-derived neurotrophic factor protein in rats with cerebral hemorrhage. Hunan Yike Daxue Xuebao. 2003;28(5):485–489. [Google Scholar]
- [14].Schütte A, Yan Q, Mestres P, et al. The endogenous survival promotion of axotomized rat corticospinal neurons by brain-derived neurotrophic factor is mediated via paracrine, rather than autocrine mechanisms. Neurosci Lett. 2000;290(3):185–188. doi: 10.1016/s0304-3940(00)01351-3. [DOI] [PubMed] [Google Scholar]
- [15].Zou W, Liu F, Sun XW, et al. Effects of scalp-acupuncture on expressions of BDNF and NGF in acute intercerebral hemorrhage. Zhongyiyao Xuebao. 2010;38(6):14–17. [Google Scholar]
- [16].Ivanova T, Beyer C. Pre- and postnatal expression of brain-derived neurotrophic factor mRNA/protein and tyrosine protein kinase receptor B mRNA in the mouse hippocampus. Neurosci Lett. 2001;307(1):21–24. doi: 10.1016/s0304-3940(01)01905-x. [DOI] [PubMed] [Google Scholar]
- [17].Pencea V, Bingaman KD, Wiegand SJ, et al. Infusion of brain-derived neurotrophic factor into the lateral ventricle of the adult rat leads to new neurons in the parenchyma of the striatum, septum, thalamus, and hypothalamus. J Neurosci. 2001;21(17):6706–6717. doi: 10.1523/JNEUROSCI.21-17-06706.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Zhang X, Li H, Hu S, et al. Brain edema after intracerebral hemorrhage in rats: the role of inflammation. Neurol India. 2006;54(4):402–407. doi: 10.4103/0028-3886.28115. [DOI] [PubMed] [Google Scholar]
- [19].Sun J, Zhou W, Ma D, et al. Endothelial cells promote neural stem cell proliferation and differentiation associated with VEGF activated Notch and Pten signaling. Dev Dyn. 2010;239(9):2345–2353. doi: 10.1002/dvdy.22377. [DOI] [PubMed] [Google Scholar]
- [20].Zhang ZG, Zhang L, Tsang W, et al. Correlation of VEGF and angiopoietin expression with disruption of blood-brain barrier and angiogenesis after focal cerebral ischemia. J Cereb Blood Flow Metab. 2002;22(4):379–392. doi: 10.1097/00004647-200204000-00002. [DOI] [PubMed] [Google Scholar]
- [21].Marti HJ, Bernaudin M, Bellail A, et al. Hypoxia-induced vascular endothelial growth factor expression precedes neovascularization after cerebral ischemia. Am J Pathol. 2000;156(3):965–976. doi: 10.1016/S0002-9440(10)64964-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Byzova TV, Goldman CK, Jankau J, et al. Adenovirus encoding vascular endothelial growth factor-D induces tissue-specific vascular patterns in vivo. Blood. 2002;99(12):4434–4442. doi: 10.1182/blood.v99.12.4434. [DOI] [PubMed] [Google Scholar]
- [23].Senger DR, Van de Water L, Brown LF, et al. Vascular permeability factor (VPF, VEGF) in tumor biology. Cancer Metastasis Rev. 1993;12(3-4):303–324. doi: 10.1007/BF00665960. [DOI] [PubMed] [Google Scholar]
- [24].Kanazawa M, Igarashi H, Kawamura K, et al. Inhibition of VEGF signaling pathway attenuates hemorrhage after tPA treatment. J Cereb Blood Flow Metab. 2011;31(6):1461–1474. doi: 10.1038/jcbfm.2011.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Dietrich WD, Nakayama H, Watson BD, et al. Morphological consequences of early reperfusion following thrombotic or mechanical occlusion of the rat middle cerebral artery. Acta Neuropathol. 1989;78(6):605–614. doi: 10.1007/BF00691287. [DOI] [PubMed] [Google Scholar]
- [26].Dietrich WD, Busto R, Halley M, et al. The importance of brain temperature in alterations of the blood-brain barrier following cerebral ischemia. J Neuropathol Exp Neurol. 1990;49(5):486–497. doi: 10.1097/00005072-199009000-00004. [DOI] [PubMed] [Google Scholar]
- [27].Kanazawa M, Igarashi H, Kawamura K, et al. Inhibition of VEGF signaling pathway attenuates hemorrhage after tPA treatment. J Cereb Blood Flow Metab. 2011;31(6):1461–1474. doi: 10.1038/jcbfm.2011.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Jin KL, Mao XO, Greenberg DA. Vascular endothelial growth factor: direct neuroprotective effect in in vitro ischemia. Proc Natl Acad Sci U S A. 2000;97(18):10242–10247. doi: 10.1073/pnas.97.18.10242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Sun Y, Jin K, Xie L, et al. VEGF-induced neuroprotection, neurogenesis, and angiogenesis after focal cerebral ischemia. J Clin Invest. 2003;111(12):1843–1851. doi: 10.1172/JCI17977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Chowdhury P. Endocrine and metabolic regulation of body mass by nicotine: role of growth hormone. Ann Clin Lab Sci. 1990;20(6):415–419. [PubMed] [Google Scholar]
- [31].The Ministry of Science and Technology of the People's Republic of China. Guidance Suggestions for the Care and Use of Laboratory Animals. 2006 Sep 30; [Google Scholar]
- [32].Zhang QJ, Gong LT, Gao BH, et al. Experimental and clinical studies of Xingnaojinshen capsules, Zhongfengnaodeping granules and Qingkailing Injection. Shenzhen Zhongxiyi Jiehe Zazhi. 1999;9(1):13–16. [Google Scholar]
- [33].Deinsberger W, Vogel J, Fuchs C, et al. Fibrinolysis and aspiration of experimental intracerebral hematoma reduces the volume of ischemic brain in rats. Neurol Res. 1999;21(5):517–523. [PubMed] [Google Scholar]
- [34].Zhang H, Ma XY, Lv Y, et al. Model of cerebral hemorrhage. Zhongguo Yiyao Zhinan. 2012;10(34):88–89. [Google Scholar]
- [35].Yu Z, Chen LF, Li XF, et al. A double-injection model of intracerebral hemorrhage in rabbits. J Clin Neurosci. 2009;16(4):545–548. doi: 10.1016/j.jocn.2008.04.026. [DOI] [PubMed] [Google Scholar]
- [36].Tian ZY, Meng LL, Yang SS, et al. Progress of cerebral hemorrhage modeling method. Hebei Lianhe Daxue Xuebao: Yixue Ban. 2012;14(6):809–810. [Google Scholar]
- [37].Bao XM, Shu SY. Beijing: People's Medical Publishing House; 1991. The Rat Brain in Stereotaxic Coordinates. [Google Scholar]
- [38].Blandini F, Armentero MT. Animal models of Parkinson's disease. FEBS J. 2012;279(7):1156–1166. doi: 10.1111/j.1742-4658.2012.08491.x. [DOI] [PubMed] [Google Scholar]
- [39].Longa EZ, Weinstein PR, Carlson S, et al. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20(1):84–91. doi: 10.1161/01.str.20.1.84. [DOI] [PubMed] [Google Scholar]
- [40].Abe K, Yuki S, Kogure K. Strong attenuation of ischemic and postischemic brain edema in rats by a novel free radical scavenger. Stroke. 1988;19(4):480–485. doi: 10.1161/01.str.19.4.480. [DOI] [PubMed] [Google Scholar]
- [41].Chang YH, Fan XN, Shi XM. HE staining and light microscope observation method used in researches of brain tissue. Liaoning Zhongyi Zazhi. 2010;37(5):777–779. [Google Scholar]
- [42].Zhang M. HE staining in clinical and pathological diagnosis. Qiqihar Yixueyuan Xuebao. 2011;32(4):552–553. [Google Scholar]


