Abstract
Resveratrol possesses beneficial biological effects, which include anti-oxidant, anti-inflammatory and anti-carcinogenic properties. Recently, resveratrol has been shown to exhibit neuroprotective effects in models of Parkinson's disease, cerebral ischemia and Alzheimer's disease. However, its effects on vascular dementia remain unclear. The present study established a rat model of vascular dementia using permanent bilateral common carotid artery occlusion. At 8–12 weeks after model induction, rats were intragastrically administered 25 mg/kg resveratrol daily. Our results found that resveratrol shortened the escape latency and escape distances in the Morris water maze, and prolonged the time spent percentage and swimming distance percentage in the target quadrant during the probe test, indicating that resveratrol improved learning and memory ability in vascular dementia rats. Further experiments found that resveratrol decreased malonyldialdehyde levels, and increased superoxide dismutase activity and glutathione levels in the hippocampus and cerebral cortex of vascular dementia rats. These results confirmed that the neuroprotective effects of resveratrol on vascular dementia were associated with its anti-oxidant properties.
Keywords: neural regeneration, traditional Chinese medicine, resveratrol, vascular dementia, cognitive function, learning and memory, oxidative stress, bilateral common carotid, artery occlusion, malonyldialdehyde, superoxide dismutase, neuroregeneration
Research Highlights
(1) To date, few studies have investigated the effect of resveratrol on vascular dementia. In this study, we confirm the protective effect of resveratrol on vascular dementia.
(2) Our results demonstrate that resveratrol improves learning and memory ability, and reduces oxidative stress following vascular dementia in rats, and provides an experimental basis and theoretical evidence for the clinical use of resveratrol in the treatment of vascular dementia.
INTRODUCTION
Following Alzheimer's disease, vascular dementia is the second most common type of dementia[1]. Coinciding with the increase in age of the population and improved survival rates from cardiovascular diseases and stroke, vascular dementia may affect more individuals in the coming decades[2]. 1–4% of people over 65 years suffer from vascular dementia, and every 5–10 years the morbidity rate increases by one-fold after the age of 65 years[3]. Post-stroke dementia is extremely widespread and occurs in up to 1/3 of ischemic stroke patients after 65 years[4]. The diagnosis of vascular dementia includes the presence of cognitive decline (loss of memory and deficits in at least two other domains), resulting in impaired functional abilities. Evidence of cerebrovascular disease must be confirmed by neuroimaging, and for the diagnosis of probable vascular dementia, dementia and cerebrovascular disease must be reasonably related in time or temporally. Because of the variety of pathogenic mechanisms, the clinical manifestations of vascular dementia are varied; they are also related to the size, and type of cerebral damage[3]. The clinical characteristics include abrupt onset, fluctuating course, and stepwise deterioration, which are often accompanied by focal sensory and motor abnormalities including early onset of urinary incontinence and gait disorders. However, subcortical vascular dementia can present with gradual onset and deterioration like Alzheimer's disease. Even within vascular dementia, the clinical features can be further subdivided[4]. Thus, it is very important to research the pathogenic mechanism and treatment of vascular dementia.
Resveratrol, a polyphenolic compound, was first reported in 1939 by a Japanese researcher, Dr. Michio Takaoka[5,6]. It is synthesized in several plants in response to adverse conditions such as stress, injury, ultraviolet irradiation and fungal infection[7]. Resveratrol has a wide range of biological effects, including anti-oxidative, anti-inflammatory[8,9,10,11] and anticarcinogenic properties[12], and is largely found in grapes and red wine.
Resveratrol exhibits comprehensive beneficial health effects, which include cardioprotective[13,14], antiproliferative[15] and neuroprotective properties[16]. Resveratrol was suggested to play a role in the prevention of heart disease as it modulated lipoprotein metabolism and inhibited platelet aggregation[17]. Zamora-Ros et al [18] carried out a large cross-sectional study using high cardiovascular risk individuals in Spain to investigate the association between total urinary resveratrol metabolites as biomarkers of wine and resveratrol consumption and cardiovascular risk factors. The research found that both resveratrol and wine intake were useful to decrease cardiovascular risk factors, through changing the lipid profiles in blood, fasting blood glucose (only resveratrol) and heart rate. Resveratrol has been proposed as a major constituent of the polyphenol fraction to which the health benefits of red wine consumption are attributed. In vivo and in vitro studies have also shown that resveratrol exhibited neuroprotective effects in models of many diseases, such as cerebral ischemia[19,20], kainic acid-induced excitotoxicity[21], Huntington's disease[22,23], Parkinson's disease[24,25] and Alzheimer's disease[26,27,28]. However, there is a lack of data evaluating the effect of resveratrol in vascular dementia.
When the blood supply to the brain is reduced by a blocked or diseased vascular system, vascular dementia occurs and leads to a progressive decline in memory and cognitive function[29]. Chronic cerebral hypoperfusion can be induced by permanent bilateral common carotid artery occlusion in rats, resulting in significant white matter lesions, learning and memory impairment[30], and hippocampal neuronal damage[31]. Ni et al [30] found that learning of the task was severely impaired in permanent bilateral common carotid artery occlusion rats that had not been pretrained. They also found some loss in hippocampal pyramidal neurons, which was observed at 1 month after permanent bilateral common carotid artery occlusion; however, the decrease was not significant. Moreover, significant loss of cells was observed in the hippocampal CA1 subregion at 4 months after the operation. Clinical evidence also supports the hypothesis that chronic cerebral hypoperfusion is associated with cognitive decline, in both aging and neurodegenerative disorders[32]. Thus, bilateral common carotid artery occlusion in rats is a useful model for researching the pathophysiology of chronic cerebrovascular hypoperfusion and for screening drugs to treat vascular dementia[33,34].
Oxidative stress occurs as a result of a shift in balance that favors the generation of oxygen-derived free radicals or reactive oxygen species over various anti-oxidant defense mechanisms. Malonyldialdehyde, superoxide dismutase, and glutathione assessment has been widely used to indicate oxidative stress in many studies[35,36]. In the present study, we used the permanent bilateral common carotid artery occlusion rat model of vascular dementia to study the effect of resveratrol on vascular dementia. The Morris water maze was used to test spatial learning and memory performance. Malonyldialdehyde levels, superoxide dismutase activity, and glutathione levels in the cortex and hippocampus were also measured.
RESULTS
Quantitative analysis of experimental animals
A total of 80 rats were equally and randomly divided into four groups: normal control group (isolated bilateral common carotid arteries, but not ligated), model group (vascular dementia model established by permanent bilateral common carotid artery occlusion), resveratrol control group (sham operation with resveratrol treatment), resveratrol treatment group (vascular dementia model with resveratrol treatment). All rats were involved in the final analysis.
Resveratrol improved learning and memory abilities in vascular dementia rats
The Morris water maze was used to test and measure the spatial learning and memory performance of rats, and the escape latency (the amount of time spent finding and mounting the platform in the water maze) and escape distances (the swimming path before finding the platform in the water maze). In the first 3 days, all rats showed no difference in escape latency (Figure 1A; P > 0.05) and escape distances (Figure 1B; P > 0.05). At 4 days, no significant difference in the escape latency and escape distances was detected between the resveratrol control group and the normal control group (P > 0.05). However, the escape latency and escape distances were significantly longer in the model group than the normal control group (P < 0.05). All changes were partly reversed by resveratrol treatment. The escape latency and escape distances were significantly shorter in the resveratrol treatment group than the model group (P < 0.05; Figure 1).
Figure 1.
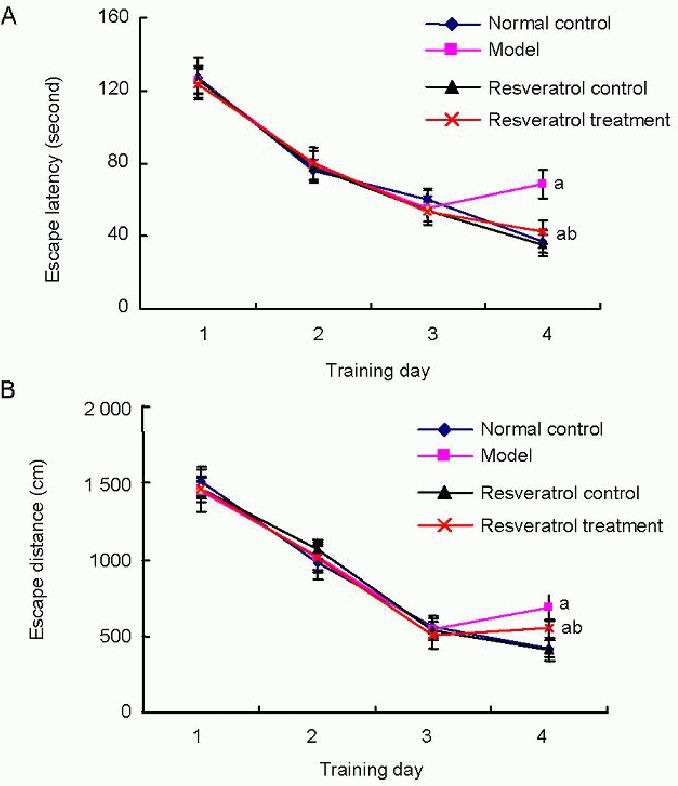
Effects of resveratrol on learning impairment in vascular dementia rats.
The Morris water maze was used to test the learning ability of rats. Escape latency (A) and escape distances (B) were measured. Data are expressed as the mean ± SD; n = 20 rats in each group. aP < 0.05, vs. normal control group; bP < 0.05, vs. model group using one-way analysis of variance, followed by Student-Newman-Keuls test.
After the water maze training test, we performed a probe test to analyze maintenance of memory, in which we removed the platform. The time spent percentage and swimming distance percentage in the target quadrant were recorded. During the probe test, the time spent percentage (Figure 2A) and swimming distance percentage (Figure 2B) in the target quadrant of the model group were significantly shorter than that of the normal control group (P < 0.05), and the changes were partly reversed by resveratrol treatment.
Figure 2.
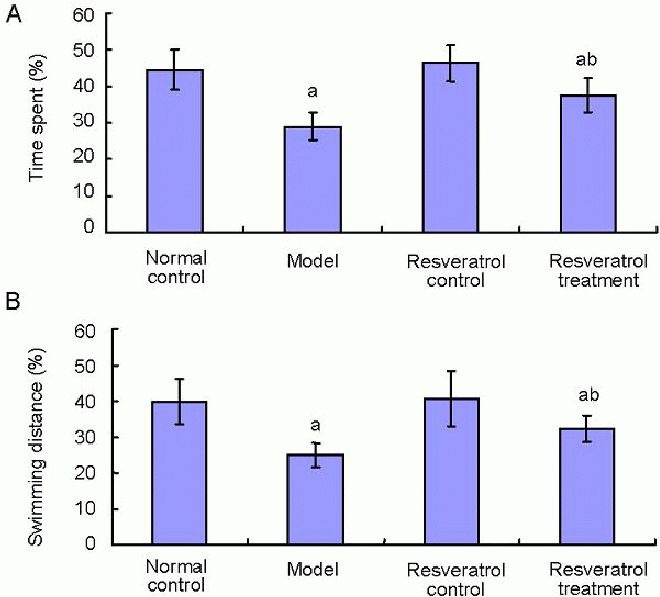
Effects of resveratrol on memory in vascular dementia rats.
A probe test to analyze the maintenance of memory in the Morris water maze was performed. The time spent (A) and swimming distance (B) in the target quadrant of rats were measured.
Data are expressed as mean ± SD; n = 20 rats in each group. aP < 0.05, vs. normal control group; bP < 0.05, vs. model group using one-way analysis of variance, followed by Student-Newman-Keuls test.
In the resveratrol treatment group, the time spent percentage (Figure 2A) and swimming distance percentage (Figure 2B) were significantly longer than that in the model group (P < 0.05; Figure 2).
Resveratrol decreased malonyldialdehyde levels in the cerebral cortex and hippocampus of vascular dementia rats
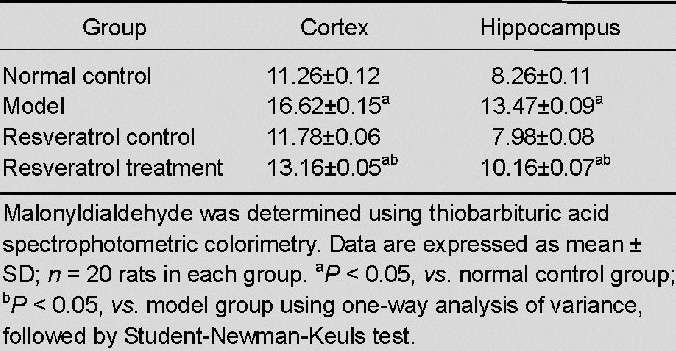
Malonyldialdehyde levels in the cerebral cortex and hippocampus were determined using thiobarbituric acid spectrophotometric colorimetry[37]. No significant difference in malonyldialdehyde levels in the cortex and hippocampus was observed between the resveratrol control group and normal control group (P > 0.05). Malonyldialdehyde levels were significantly increased in the model group when compared with the normal control group (P < 0.05). In contrast, malonyldialdehyde levels decreased significantly after treatment with resveratrol (P < 0.05) in the cortex and hippocampus as compared with the model group (P < 0.05; Table 1).
Table 1.
Effect of resveratrol on malonyldialdehyde levels (nmol/mg) in the cortex and hippocampus of vascular dementia rats
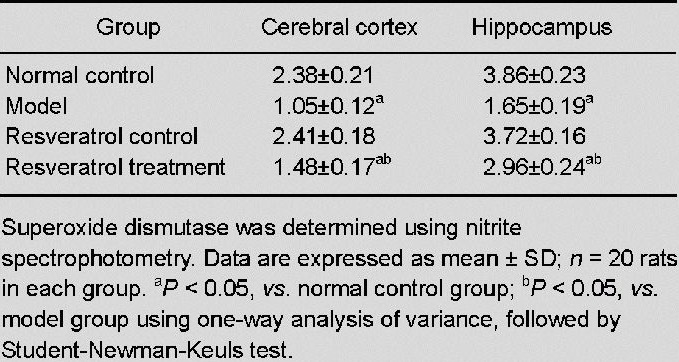
Resveratrol elevated superoxide dismutase activity in the cerebral cortex and hippocampus of vascular dementia rats
Superoxide dismutase activity in the cerebral cortex and hippocampus was determined using nitrite spectrophotometry. No significant difference in superoxide dismutase activity in the cortex and hippocampus was detectable between the resveratrol control group and the normal control group (P > 0.05). Superoxide dismutase activities were significantly lower in the model group as compared with the normal control group (P < 0.05). After treatment with resveratrol, the superoxide dismutase activities in the cortex and hippocampus were higher as compared with the model group (P < 0.05; Table 2).
Table 2.
Effect of resveratrol on superoxide dismutase activity (U/mg) in the cerebral cortex and hippocampus of vascular dementia rats
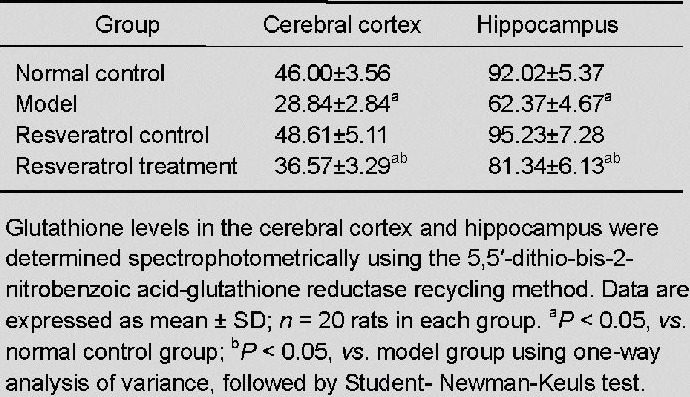
Resveratrol elevated glutathione levels in the cerebral cortex and hippocampus of vascular dementia rats
Glutathione levels in the cerebral cortex and hippocampus were determined spectrophotometrically using the 5,5’-dithio-bis-2-nitrobenzoic acid-glutathione reductase recycling method[38]. No significant difference in glutathione levels in the cerebral cortex and hippocampus was detected between the resveratrol control group and the normal control group (P > 0.05). Glutathione levels were significantly lower in the model group as compared with the normal control group (P < 0.05). After treatment with resveratrol, glutathione levels were significantly increased in the cortex and hippocampus when compared with the model group (P < 0.05; Table 3).
Table 3.
Effect of resveratrol on glutathione levels (nmol/mg) in the cerebral cortex and hippocampus of vascular dementia rats
DISCUSSION
Dementia is a syndrome of acquired intellectual deficit resulting in significant impairment of social or occupational functions. Vascular dementia has been recognized as the second common dementia, which comprises dementias resulting from all types of vascular pathologies[4]. Its prevalence is increased in the elderly population. Etiopathogenic mechanisms leading to vascular dementia include oxidative stress, cytotoxicity of reactive oxygen species, mitochondrial dysfunction and apoptosis[39,40].
Oxidative stress occurs as the result of a shift in balance that favors the generation of oxygen-derived free radicals or reactive oxygen species over various antioxidant defense mechanisms[41]. Reactive oxygen species can damage proteins, nucleic acids, and membrane polyunsaturated fatty acids, causing lipid peroxidation and leading to loss of membrane integrity, reduction of mitochondrial membrane potential, and increased permeability to Ca2+ in the plasma membrane[41]. In the central nervous system, neurons are especially vulnerable to insults induced by neurotoxins, ischemia/stroke, and seizure/excitotoxic injury, and oxidative stress is a common underlying factor for these injuries. In vascular diseases, reactive oxygen species have direct effects on vascular tone but also impair vasomotor responses to other stimuli[42]. The effects of reactive oxygen species are prominent in cerebral circulation. Cerebral blood vessels have the capacity to generate high levels of superoxide when compared with peripheral blood vessels, and are particularly sensitive to the effects of reactive oxygen species[43]. Many polyphenolic compounds from fruits and vegetables are known for their antioxidant properties, and thus have been considered as therapeutic agents, for example resveratrol[41].
Resveratrol is a phytoalexin that is naturally synthesized in response to fungal attack[44]. There are two isomeric forms of resveratrol: biologically inactive cis-resveratrol and active trans-resveratrol(3,5,4’-trihydroxystilbene). In vivo and in vitro studies have also shown that resveratrol plays a role in the prevention of inflammation, atherosclerosis[45,46] and carcinogenesis[47]. Matos et al [48] found that resveratrol had significant anti-atherogenic and anti-inflammatory effects in an animal model with rabbits fed a hypercholesterolemic diet (1% (w/v) cholesterol). Back et al [47] used human A431 SCC cells to study the effect of resveratrol and found that resveratrol could induce premature senescence in human A431 SCC cells, and that resveratrol-induced premature senescence was associated with blockade of autolysosome formation, as assessed by the absence of colocalization of microtubule-associated protein 1 light chain 3, a specific marker of autophagy, and Lamp-2, markers for autophagosomes and lysosomes, respectively. Resveratrol also downregulates the level of Rictor, a component of the mammalian target of rapamycin complex 2, leading to a decrease in RhoA-GTPase and altered actin cytoskeleton organization. Resveratrol has also estrogenic, vaso-relaxing activity and cardiovascular benefits[49].
Simao et al [50] found that resveratrol can prevent damage to CA1 neurons against ischemic injury and that this neuroprotective effect of resveratrol may be mediated by the activation of the PI3-K/Akt signaling pathway, which subsequently downregulates the expression of glycogen synthase kinase-3 beta and cAMP-response element binding protein. Li et al [20] used a rat brain ischemia model, induced by middle cerebral artery occlusion, and found that resveratrol treatment significantly reduced brain infarct volume and decreased the expression of the pro-apoptotic protein Bax and increased the expression of the anti-apoptotic protein Bcl-2. Resveratrol was also found to provide protection against kainic acid-induced excitotoxicity in the cortex and hippocampus of Wistar rats[51]. In a global cerebral ischemia model induced by four-vessel occlusion for 10 minutes, resveratrol could modulate membrane lipid composition, as well as the ganglioside profile in ischemia/reperfusion injury[19]. Resveratrol also had a neuroprotective effect following spontaneous seizures[52], Huntington's disease[53], Parkinson's disease[54,55,56,57,58] and Alzheimer's disease[59,60,61] through its anti-oxidative properties.
The present study found that at 4 days, the escape latency and escape distances of the model group were significantly higher than that of the normal control group. During the probe test, the time spent percentage and swimming distance percentage in the target quadrant of the model group were significantly shorter than that of the normal control group. All changes were partly reversed by resveratrol treatment. This suggested that resveratrol can improve memory impairment in vascular dementia rats.
Lipid peroxidation is often assayed by measuring thiobarbituric acid reactive substances. The end products of lipid peroxidation, such as malonyldialdehyde assessment, have been widely used to indicate oxidative stress in many studies[35,36]. Greilberger et al [36] used malonyldialdehyde, carbonyl proteins and albumin-disulphide as oxidative stress parameters to test whether oxidative stress had a primary role in neurodegeneration or a secondary end-stage epiphenomenon in patients with neurodegenerative diseases, namely mild cognitive impairment and Alzheimer's disease. Malonyldialdehyde, carbonyl proteins and albumin-disulphide were significantly increased in the neurodegenerative diseases group when compared to controls. Significant correlations were found between carbonyl proteins and albumin-disulphide, carbonyl proteins and malonyldialdehyde and between malonyldialdehyde and albumin-disulphide in both patients with neurodegenerative diseases and the control group. These results support the hypothesis that oxidative damage to lipids and proteins is an important early event in the pathogenesis of neurodegenerative diseases. Resveratrol possesses a wide range of biological effects, including antioxidation. Using malonyldialdehyde as a marker for oxidative stress, the present study found that malonyldialdehyde levels in the cerebral cortex and hippocampus were significantly increased in the model group when compared with the normal control group, which showed that permanent bilateral common carotid artery occlusion induced oxidative stress injury. In contrast, malonyldialdehyde levels were decreased significantly after treatment with resveratrol in the cerebral cortex and hippocampus as compared with the model group, suggesting that resveratrol could protect vascular dementia rats through regulating malonyldialdehyde levels.
The potential toxicity of free radicals is counteracted by a number of cytoprotective enzymes and antioxidants that limit the damage. This protective mechanism functions cooperatively in the form of a cascade, which includes various antioxidant enzymes such as superoxide dismutase[62]. Superoxide dismutase is involved in the regulation of antioxidant defenses by catalyzing the dismutation of superoxide anion into H2O2 and O2. In the present study, superoxide dismutase activity was significantly decreased in the model group as compared with the normal control group in the cerebral cortex and hippocampus, indicating that permanent bilateral common carotid artery occlusion induced oxidative stress injury by reducing antioxidant enzymes, and superoxide dismutase activity. After treatment with resveratrol, superoxide dismutase activity was increased when compared with the model group, indicating that resveratrol could protect vascular dementia rats by increasing the activity of superoxide dismutase.
Glutathione is a tripeptide thiol synthesized by glutamic acid, cysteine and glycine in the pathway of γ-glutamyl cycle[63], and is present in either a reduced (GSH) or oxidized (GSSG) form. Glutathione acts as a strong reducing agent as well as serving as an electron donor for glutathione peroxidase. Glutathione is known to have an important role in maintaining the intracellular redox state and protecting cells against damage caused by oxidative stress[63]. This role suggests that permanent bilateral common carotid artery occlusion-induced oxidative stress injury occurs by reducing glutathione levels, and that resveratrol could protect vascular dementia rats by increasing glutathione levels.
In conclusion, resveratrol suppressed memory impairment, decreased malonyldialdehyde levels, increased superoxide dismutase activity and glutathione levels in our vascular dementia rat model. Our results confirmed the neuroprotective effects of resveratrol on vascular dementia, and provided novel insights into the neuroprotective effects of resveratrol and its possible therapeutic role in vascular dementia.
MATERIALS AND METHODS
Design
A randomized, controlled animal study.
Time and setting
This experiment was performed at the First Affiliated Hospital of Zhengzhou University and Henan Provincial People's Hospital, China, from February 2011 to October 2012.
Materials
Animals
A total of 80 Wistar rats of either gender, aged 12–14 months, weighing 300–400 g, were provided by Henan Laboratory Animal Research Center, Zhengzhou, China (license No. SCXK (Yu) 2010-0002). Rats were housed in standard cages at a constant temperature of 22 ± 2°C and humidity of 55 ± 5%, and allowed free access to food and water, with a 12-hour light/dark cycle (lights off at 7:00 a.m.). All protocols were conducted in accordance with the Guidance Suggestions for the Care and Use of Laboratory Animals, formulated by the Ministry of Science and Technology of China[64].
Drugs
Resveratrol (≥ 99% gas chromatography) was obtained from Sigma Chemical Company (Sigma Prod. No. R5010, St. Louis, MO, USA). Resveratrol was administered with 0.5% (w/v) aqueous methylcellulose (Sigma Chemical Company); a dosing volume of 5 mL/kg per day was used.
Methods
Bilateral common carotid artery occlusion
Bilateral common carotid arteries were occluded as previously described by Ni et al [30]. Rats were anesthetized with 10% (v/v) chloral hydrate (0.3 mL/100 g; Sigma) via intraperitoneal injection. To prevent respiratory distress, the rats were also administered atropine sulfate (0.1 mg/kg; intramuscular injection). A median incision was made to expose the bilateral common carotid arteries, which were carefully separated from the surrounding tissues, including the vagus nerve, and ligated with coated Vicryl Plus antibacterial/polyglactin 910 3/0 absorbable surgical suture (Ethicon, Johnson & Johnson, Scotland, UK), approximately 1 cm inferior to the origin of the external carotid artery. Control rats were subjected to the same surgical procedure without occlusion of arteries.
Drug treatment
Rats from the resveratrol control group and resveratrol treatment group received a daily oral dose of 25 mg/kg resveratrol from 8 weeks after the operation to 12 weeks. Rats in the normal control group and model group received 5 mL vehicle (0.5% (w/v) aqueous methylcellulose) for the same period.
Morris water maze test
Spatial learning and memory performance of rats was measured using the Morris water maze task (provided by the Chinese Academy of Medical Sciences, Beijing, China)[65] at 12 weeks after operation. The test was administered by an operator blinded to group conditions. The Morris water maze consisted of a painted circular pool (120 cm in diameter and 30 cm in depth) in which rats were trained to escape from the water by swimming to a hidden platform (9 cm in diameter) 1.5 cm beneath the surface, the location of which could only be identified by using distal extra-maze cues attached to the room walls. The water was kept at 22°C and made opaque with titanium dioxide throughout the training and testing period. The pool was divided into four quadrants: north (Target), south (Opposite), east (Adjacent 1) and west (Adjacent 2). The experiments were recorded using a camera connected to a video recorder and a computerized tracking system (Tianhua Zhongwei Technology, Beijing, China).
The Morris water maze test continued for 5 days. The first 4 days were the reference memory test phase, which consisted of 16 training trials: 4 training trials per day for 4 training days with an intertrial interval of 30–40 minutes. At the beginning of each trial, the rat was placed into one of four quadrants facing the wall. Although the starting point was randomly selected, the protocol was fixed at the beginning of each trial and was maintained throughout all trials. Each rat was given 180 seconds to find and mount the platform. At 30 seconds after the rat mounted the platform, the rat was removed, placed in a holding cage and warmed with a heating lamp. Rats that failed to locate and mount the platform within 180 seconds were gently guided to the platform and required to remain there for 30 seconds before they were transferred to the holding cage. A video camera mounted above the pool was used to track the rats. The amount of time spent finding and mounting the platform (escape latency) and the swimming pathway before finding the platform (escape distances) were calculated from the recorded videos using the Morris water maze software (Shanghai Jiliang Software Technology Co., Ltd., Shanghai, China). At 5 days, a probe test was performed in which the platform was removed. Rats were placed into the water in one of two quadrants adjacent to the platform (Adjacent 1 and Adjacent 2 quadrant) and animals were allowed to swim freely for 120 seconds. The time spent percentage and swimming distance percentage in the target quadrant were recorded.
Sample preparation and biochemical evaluations
Rats were sacrificed using zoletil (zolazepam + tiletamine, 1:1) (50 mg/kg; intraperitoneal injection) for biochemical evaluations. Brains were quickly removed and the cerebral cortex and hippocampus were separated on ice. Tissues were homogenated in 0.1 mol/L PBS (pH 7.4) and centrifuged at 10 000 × g at 4°C for 15 minutes to remove cellular debris. Supernatants were used for the estimation of malonyldialdehyde levels, superoxide dismutase activity and glutathione levels. Protein concentrations of samples were determined using the bicinchoninic acid protein assay kit (Pierce Biotechnology, Rockford, IL, USA) with bovine serum albumin used as a standard[66].
Measurement of malonyldialdehyde levels
Malonyldialdehyde was determined using thiobarbituric acid spectrophotometric colorimetry[37]. The kit was purchased from Thermo Fisher Scientific (Shanghai, China). The assay measures malonyldialdehyde, a degraded product of peroxidized lipids, which forms a red product when condensed with thiobarbituric acid, and displays a maximum absorption peak at 532 nm. After the reaction, the SP-75 ultraviolet spectrophotometer (Shanghai Spectrum, Shanghai, China) was adjusted to 532 nm, zeroed with distilled water, and the absorbance was measured. Malonyldialdehyde content was calculated according to the formula[67]:

Measurement of superoxide dismutase activity
Superoxide dismutase activity was based on the inhibitory effect of superoxide dismutase on the reduction of nitroblue tetrazolium by the superoxide anion generated by xanthine/xanthine oxidase[68]. Superoxide dismutase was determined using nitrite spectrophotometry. Reagents were purchased from Thermo Fisher Scientific. The SP-75 ultraviolet spectrophotometer was used to determine the absorbance at 550 nm based on the fact that the determination tube absorbance values were less than the control tube during superoxide dismutase colorimetry using the xanthine and xanthine oxidase reaction system. Superoxide dismutase activity was calculated according to the formula[67]:

Measurement of glutathione levels
Glutathione levels were determined spectrophotometrically using the 5,5’-dithio-bis-2-nitrobenzoic acid-glutathione reductase recycling method[38]. The assay kit was purchased from Cayman Chemical (Ann Arbor, MI, USA), and samples were measured at 405 nm. Glutathione levels were calculated according to the formula:

Statistical analysis
Data were expressed as mean ± SD and were analyzed using SPSS 16.0 statistical software (SPSS, Chicago, IL, USA). Each procedure was performed in duplicate using 3–5 independent experiments. Statistical analyses were performed using one-way analysis of variance, followed by Student-Newman-Keuls test. A value of P < 0.05 was considered statistically significant.
Acknowledgments
We thank Chen SD from Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, China, for the help of manuscript revision.
Footnotes
Conflicts of interest: None declared.
Ethical approval: This study was conducted with permission from the Animal Care and Research Committee of Zhengzhou University, China.
(Reviewed by Diwakarla S, Haase R, Zhang JW, Yang HQ)
(Edited by Wang LM, Qiu Y, Li CH, Song LP, Liu WJ, Zhao M)
REFERENCES
- [1].Rockwood K, Wentzel C, Hachinski V, et al. Prevalence and outcomes of vascular cognitive impairment. Vascular Cognitive Impairment Investigators of the Canadian Study of Health and Aging. Neurology. 2000;54(2):447–451. doi: 10.1212/wnl.54.2.447. [DOI] [PubMed] [Google Scholar]
- [2].Levine DA, Langa KM. Vascular cognitive impairment: disease mechanisms and therapeutic implications. Neurotherapeutics. 2011;8(3):361–373. doi: 10.1007/s13311-011-0047-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].McVeigh C, Passmore P. Vascular dementia: prevention and treatment. Clin Interv Aging. 2006;1(3):229–235. doi: 10.2147/ciia.2006.1.3.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Román GC. Vascular dementia may be the most common form of dementia in the elderly. J Neurol Sci. 2002;203-204:7–10. doi: 10.1016/s0022-510x(02)00252-6. [DOI] [PubMed] [Google Scholar]
- [5].Villaflores OB, Chen YJ, Chen CP, et al. Curcuminoids and resveratrol as anti-Alzheimer agents. Taiwan J Obstet Gynecol. 2012;51(4):515–525. doi: 10.1016/j.tjog.2012.09.005. [DOI] [PubMed] [Google Scholar]
- [6].Takaoka M. Resveratrol, a new phenolic compound from Veratrum grandiflorum. Nippon Kagaku Kaishi. 1939;60:1090–1100. [Google Scholar]
- [7].Floreani M, Napoli E, Quintieri L, et al. Oral administration of trans-resveratrol to guinea pigs increases cardiac DT-diaphorase and catalase activities, and protects isolated atria from menadione toxicity. Life Sci. 2003;72(24):2741–2750. doi: 10.1016/s0024-3205(03)00179-6. [DOI] [PubMed] [Google Scholar]
- [8].Wood LG, Wark PA, Garg ML. Antioxidant and anti-inflammatory effects of resveratrol in airway disease. Antioxid Redox Signal. 2010;13(10):1535–1548. doi: 10.1089/ars.2009.3064. [DOI] [PubMed] [Google Scholar]
- [9].Chang CC, Chang CY, Huang JP, et al. Effect of resveratrol on oxidative and inflammatory stress in liver and spleen of streptozotocin-induced type 1 diabetic rats. Chin J Physiol. 2012;55(3):192–201. doi: 10.4077/CJP.2012.BAA012. [DOI] [PubMed] [Google Scholar]
- [10].Spanier G, Xu H, Xia N, et al. Resveratrol reduces endothelial oxidative stress by modulating the gene expression of superoxide dismutase 1 (SOD1), glutathione peroxidase 1 (GPx1) and NADPH oxidase subunit (Nox4) J Physiol Pharmacol. 2009;60(Suppl 4):111–116. [PubMed] [Google Scholar]
- [11].Orsu P, Murthy BV, Akula A. Cerebroprotective potential of resveratrol through anti-oxidant and anti-inflammatory mechanisms in rats. J Neural Transm. doi: 10.1007/s00702-013-0982-4. in press. [DOI] [PubMed] [Google Scholar]
- [12].Athar M, Back JH, Kopelovich L, et al. Multiple molecular targets of resveratrol: Anti-carcinogenic mechanisms. Arch Biochem Biophys. 2009;486(2):95–102. doi: 10.1016/j.abb.2009.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Sebai H, Sani M, Aouani E, et al. Cardioprotective effect of resveratrol on lipopolysaccharide-induced oxidative stress in rat. Drug Chem Toxicol. 2011;34(2):146–150. doi: 10.3109/01480545.2010.494666. [DOI] [PubMed] [Google Scholar]
- [14].Wu JM, Hsieh TC. Resveratrol: a cardioprotective substance. Ann N Y Acad Sci. 2011;1215:16–21. doi: 10.1111/j.1749-6632.2010.05854.x. [DOI] [PubMed] [Google Scholar]
- [15].Cui J, Sun R, Yu Y, et al. Antiproliferative effect of resveratrol in pancreatic cancer cells. Phytother Res. 2010;24(11):1637–1644. doi: 10.1002/ptr.3157. [DOI] [PubMed] [Google Scholar]
- [16].Richard T, Pawlus AD, Iglésias ML, et al. Neuroprotective properties of resveratrol and derivatives. Ann N Y Acad Sci. 2011;1215:103–108. doi: 10.1111/j.1749-6632.2010.05865.x. [DOI] [PubMed] [Google Scholar]
- [17].Yashiro T, Nanmoku M, Shimizu M, et al. Resveratrol increases the expression and activity of the low density lipoprotein receptor in hepatocytes by the proteolytic activation of the sterol regulatory element-binding proteins. Atherosclerosis. 2012;220(2):369–374. doi: 10.1016/j.atherosclerosis.2011.11.006. [DOI] [PubMed] [Google Scholar]
- [18].Zamora-Ros R, Urpi-Sarda M, Lamuela-Raventós RM, et al. High urinary levels of resveratrol metabolites are associated with a reduction in the prevalence of cardiovascular risk factors in high-risk patients. Pharmacol Res. 2012;65(6):615–620. doi: 10.1016/j.phrs.2012.03.009. [DOI] [PubMed] [Google Scholar]
- [19].Simão F, Matté A, Breier AC, et al. Resveratrol prevents global cerebral ischemia-induced decrease in lipid content. Neurol Res. 2013;35(1):59–64. doi: 10.1179/1743132812Y.0000000116. [DOI] [PubMed] [Google Scholar]
- [20].Li Z, Pang L, Fang F, et al. Resveratrol attenuates brain damage in a rat model of focal cerebral ischemia via up- regulation of hippocampal Bcl-2. Brain Res. 2012;1450:116–124. doi: 10.1016/j.brainres.2012.02.019. [DOI] [PubMed] [Google Scholar]
- [21].Wang Q, Yu S, Simonyi A, et al. Resveratrol protects against neurotoxicity induced by kainic acid. Neurochem Res. 2004;29(11):2105–2112. doi: 10.1007/s11064-004-6883-z. [DOI] [PubMed] [Google Scholar]
- [22].Pasinetti GM, Wang J, Marambaud P, et al. Neuroprotective and metabolic effects of resveratrol: therapeutic implications for Huntington's disease and other neurodegenerative disorders. Exp Neurol. 2011;232(1):1–6. doi: 10.1016/j.expneurol.2011.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Maher P, Dargusch R, Bodai L, et al. ERK activation by the polyphenols fisetin and resveratrol provides neuroprotection in multiple models of Huntington's disease. Hum Mol Genet. 2011;20(2):261–270. doi: 10.1093/hmg/ddq460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Khan MM, Ahmad A, Ishrat T, et al. Resveratrol attenuates 6-hydroxydopamine-induced oxidative damage and dopamine depletion in rat model of Parkinson's disease. Brain Res. 2010;1328:139–151. doi: 10.1016/j.brainres.2010.02.031. [DOI] [PubMed] [Google Scholar]
- [25].Jin F, Wu Q, Lu YF, et al. Neuroprotective effect of resveratrol on 6-OHDA-induced Parkinson's disease in rats. Eur J Pharmacol. 2008;600(1-3):78–82. doi: 10.1016/j.ejphar.2008.10.005. [DOI] [PubMed] [Google Scholar]
- [26].Li F, Gong Q, Dong H, et al. Resveratrol, a neuroprotective supplement for Alzheimer's disease. Curr Pharm Des. 2012;18(1):27–33. doi: 10.2174/138161212798919075. [DOI] [PubMed] [Google Scholar]
- [27].Vingtdeux V, Dreses-Werringloer U, Zhao H, et al. Therapeutic potential of resveratrol in Alzheimer's disease. BMC Neurosci. 2008;9(Suppl 2):S6. doi: 10.1186/1471-2202-9-S2-S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Karuppagounder SS, Pinto JT, Xu H, et al. Dietary supplementation with resveratrol reduces plaque pathology in a transgenic model of Alzheimer's disease. Neurochem Int. 2009;54(2):111–118. doi: 10.1016/j.neuint.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Jellinger KA. The enigma of vascular cognitive disorder and vascular dementia. Acta Neuropathol. 2007;113(4):349–388. doi: 10.1007/s00401-006-0185-2. [DOI] [PubMed] [Google Scholar]
- [30].Ni J, Ohta H, Matsumoto K, et al. Progressive cognitive impairment following chronic cerebral hypoperfusion induced by permanent occlusion of bilateral carotid arteries in rats. Brain Res. 1994;653(1-2):231–236. doi: 10.1016/0006-8993(94)90394-8. [DOI] [PubMed] [Google Scholar]
- [31].Kim SK, Cho KO, Kim SY. White matter damage and hippocampal neurodegeneration induced by permanent bilateral occlusion of common carotid artery in the rat: comparison between Wistar and Sprague-Dawley strain. Korean J Physiol Pharmacol. 2008;12(3):89–94. doi: 10.4196/kjpp.2008.12.3.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Cechetti F, Worm PV, Pereira LO, et al. The modified 2VO ischemia protocol causes cognitive impairment similar to that induced by the standard method, but with a better survival rate. Braz J Med Biol Res. 2010;43(12):1178–1183. doi: 10.1590/s0100-879x2010007500124. [DOI] [PubMed] [Google Scholar]
- [33].Stasiak A, Mussur M, Unzeta M, et al. The central histamine level in rat model of vascular dementia. J Physiol Pharmacol. 2011;62(5):549–558. [PubMed] [Google Scholar]
- [34].Gong X, Ma M, Fan X, et al. Down-regulation of IGF-1/IGF-1R in hippocampus of rats with vascular dementia. Neurosci Lett. 2012;513(1):20–24. doi: 10.1016/j.neulet.2012.01.077. [DOI] [PubMed] [Google Scholar]
- [35].Padurariu M, Ciobica A, Dobrin I, et al. Evaluation of antioxidant enzymes activities and lipid peroxidation in schizophrenic patients treated with typical and atypical antipsychotics. Neurosci Lett. 2010;479(3):317–320. doi: 10.1016/j.neulet.2010.05.088. [DOI] [PubMed] [Google Scholar]
- [36].Greilberger J, Koidl C, Greilberger M, et al. Malondialdehyde, carbonyl proteins and albumin-disulphide as useful oxidative markers in mild cognitive impairment and Alzheimer's disease. Free Radic Res. 2008;42(7):633–638. doi: 10.1080/10715760802255764. [DOI] [PubMed] [Google Scholar]
- [37].Yang LJ, You YH. Test method of cell in jury induced by oxidative stress. Yixue Zongshu. 2010;16(6):924–927. [Google Scholar]
- [38].Monostori P, Wittmann G, Karg E, et al. Determination of glutathione and glutathione disulfide in biological samples: an in-depth review. J Chromatogr B Analyt Technol Biomed Life Sci. 2009;877(28):3331–3346. doi: 10.1016/j.jchromb.2009.06.016. [DOI] [PubMed] [Google Scholar]
- [39].Wang J, Zhang HY, Tang XC. Cholinergic deficiency involved in vascular dementia: possible mechanism and strategy of treatment. Acta Pharmacol Sin. 2009;30(7):879–888. doi: 10.1038/aps.2009.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Bennett S, Grant MM, Aldred S. Oxidative stress in vascular dementia and Alzheimer's disease: a common pathology. J Alzheimers Dis. 2009;17(2):245–257. doi: 10.3233/JAD-2009-1041. [DOI] [PubMed] [Google Scholar]
- [41].Wassmann S, Wassmann K, Nickenig G. Modulation of oxidant and antioxidant enzyme expression and function in vascular cells. Hypertension. 2004;44(4):381–386. doi: 10.1161/01.HYP.0000142232.29764.a7. [DOI] [PubMed] [Google Scholar]
- [42].Faraci FM. Reactive oxygen species: influence on cerebral vascular tone. J Appl Physiol. 2006;100(2):739–743. doi: 10.1152/japplphysiol.01044.2005. [DOI] [PubMed] [Google Scholar]
- [43].Faraci FM. Protecting against vascular disease in brain. Am J Physiol Heart Circ Physiol. 2011;300(5):H1566–1582. doi: 10.1152/ajpheart.01310.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].de la Lastra CA, Villegas I. Resveratrol as an antioxidant and pro-oxidant agent: mechanisms and clinical implications. Biochem Soc Trans. 2007;35(Pt 5):1156–1160. doi: 10.1042/BST0351156. [DOI] [PubMed] [Google Scholar]
- [45].Agarwal B, Campen MJ, Channell MM, et al. Resveratrol for primary prevention of atherosclerosis: Clinical trial evidence for improved gene expression in vascular endothelium. Int J Cardiol. 2012;pii:S0167. doi: 10.1016/j.ijcard.2012.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Novaes RD, Peluzio Mdo C, Maldonado IR. Resveratrol causes antiatherogenic effects in an animal model of atherosclerosis. Arq Bras Cardiol. 2012;98(6):571–572. doi: 10.1590/s0066-782x2012000600014. [DOI] [PubMed] [Google Scholar]
- [47].Back JH, Zhu Y, Calabro A, et al. Resveratrol-mediated downregulation of Rictor attenuates autophagic process and suppresses UV-induced skin carcinogenesis. Photochem Photobiol. 2012;88(5):1165–1172. doi: 10.1111/j.1751-1097.2012.01097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Matos RS, Baroncini LA, Précoma LB, et al. Resveratrol causes antiatherogenic effects in an animal model of atherosclerosis. Arq Bras Cardiol. 2012;98(2):136–142. doi: 10.1590/s0066-782x2012005000006. [DOI] [PubMed] [Google Scholar]
- [49].Xu Q, Si LY. Resveratrol role in cardiovascular and metabolic health and potential mechanisms of action. Nutr Res. 2012;32(9):648–658. doi: 10.1016/j.nutres.2012.07.002. [DOI] [PubMed] [Google Scholar]
- [50].Simão F, Matté A, Pagnussat AS, et al. Resveratrol prevents CA1 neurons against ischemic injury by parallel modulation of both GSK-3β and CREB through PI3-K/Akt pathways. Eur J Neurosci. 2012;36(7):2899–2905. doi: 10.1111/j.1460-9568.2012.08229.x. [DOI] [PubMed] [Google Scholar]
- [51].Gupta YK, Briyal S, Chaudhary G. Protective effect of trans-resveratrol against kainic acid-induced seizures and oxidative stress in rats. Pharmacol Biochem Behav. 2002;71(1-2):245–249. doi: 10.1016/s0091-3057(01)00663-3. [DOI] [PubMed] [Google Scholar]
- [52].Wu Z, Xu Q, Zhang L, et al. Protective effect of resveratrol against kainate-induced temporal lobe epilepsy in rats. Neurochem Res. 2009;34(8):1393–1400. doi: 10.1007/s11064-009-9920-0. [DOI] [PubMed] [Google Scholar]
- [53].Ho DJ, Calingasan NY, Wille E, et al. Resveratrol protects against peripheral deficits in a mouse model of Huntington's disease. Exp Neurol. 2010;225(1):74–84. doi: 10.1016/j.expneurol.2010.05.006. [DOI] [PubMed] [Google Scholar]
- [54].Lu KT, Ko MC, Chen BY, et al. Neuroprotective effects of resveratrol on MPTP-induced neuron loss mediated by free radical scavenging. J Agric Food Chem. 2008;56(16):6910–6913. doi: 10.1021/jf8007212. [DOI] [PubMed] [Google Scholar]
- [55].Albani D, Polito L, Batelli S, et al. The SIRT1 activator resveratrol protects SK-N-BE cells from oxidative stress and against toxicity caused by alpha-synuclein or amyloid-beta (1-42) peptide. J Neurochem. 2009;110(5):1445–1456. doi: 10.1111/j.1471-4159.2009.06228.x. [DOI] [PubMed] [Google Scholar]
- [56].Zhang F, Shi JS, Zhou H, et al. Resveratrol protects dopamine neurons against lipopolysaccharide-induced neurotoxicity through its anti-inflammatory actions. Mol Pharmacol. 2010;78(3):466–477. doi: 10.1124/mol.110.064535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Bournival J, Quessy P, Martinoli MG. Protective effects of resveratrol and quercetin against MPP+-induced oxidative stress act by modulating markers of apoptotic death in dopaminergic neurons. Cell Mol Neurobiol. 2009;29(8):1169–1180. doi: 10.1007/s10571-009-9411-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Kumar A, Naidu PS, Seghal N, et al. Neuroprotective effects of resveratrol against intracerebroventricular colchicine-induced cognitive impairment and oxidative stress in rats. Pharmacology. 2007;79(1):17–26. doi: 10.1159/000097511. [DOI] [PubMed] [Google Scholar]
- [59].Mishra R, Sellin D, Radovan D, et al. Inhibiting islet amyloid polypeptide fibril formation by the red wine compound resveratrol. Chembiochem. 2009;10(3):445–449. doi: 10.1002/cbic.200800762. [DOI] [PubMed] [Google Scholar]
- [60].Marambaud P, Zhao H, Davies P. Resveratrol promotes clearance of Alzheimer's disease amyloid-beta peptides. J Biol Chem. 2005;280(45):37377–37382. doi: 10.1074/jbc.M508246200. [DOI] [PubMed] [Google Scholar]
- [61].Kim D, Nguyen MD, Dobbin MM, et al. SIRT1 deacetylase protects against neurodegeneration in models for Alzheimer's disease and amyotrophic lateral sclerosis. EMBO J. 2007;26(13):3169–3179. doi: 10.1038/sj.emboj.7601758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Ciobica A, Padurariu M, Dobrin I, et al. Oxidative stress in schizophrenia - focusing on the main markers. Psychiatr Danub. 2011;23(3):237–245. [PubMed] [Google Scholar]
- [63].Takahashi M. Oxidative stress and redox regulation on in vitro development of mammalian embryos. J Reprod Dev. 2012;58(1):1–9. doi: 10.1262/jrd.11-138n. [DOI] [PubMed] [Google Scholar]
- [64].The Ministry of Science and Technology of the People's Republic of China. Guidance Suggestions for the Care and Use of Laboratory Animals. 2006 Sep 30; [Google Scholar]
- [65].Su D, Zhao Y, Xu H, et al. Isoflurane exposure during mid-adulthood attenuates age-related spatial memory impairment in APP/PS1 transgenic mice. PLoS One. 2012;7(11):e50172. doi: 10.1371/journal.pone.0050172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Huang WH, Li YD, Lin YF, et al. Effects of leukemia inhibitory factor and basic fibroblast growth factor on free radicals and endogenous stem cell proliferation in a mouse model of cerebral infarction. Neural Regen Res. 2012;7(19):1469–1474. doi: 10.3969/j.issn.1673-5374.2012.19.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Zheng SZ, Song L, Lu L, et al. Granulocyte colony-stimulating factor increases extracellular glutamic acid uptake and suppresses free radicals in an experimental model of amyotrophic lateral sclerosis. Neural Regen Res. 2011;6(2):107–111. [Google Scholar]
- [68].Winerdal M, Winerdal ME, Kinn J, et al. Long lasting local and systemic inflammation after cerebral hypoxic ischemia in newborn mice. PLoS One. 2012;7(5):e36422. doi: 10.1371/journal.pone.0036422. [DOI] [PMC free article] [PubMed] [Google Scholar]


