Keywords: neural regeneration, traditional Chinese medicine, olive leaf extract, lead, brain injury, superoxide dismutase, catalase, alkaline phosphatase, acid phosphatase, malondialdehyde, apoptosis, neuropathology, grants-supported paper, neuroregeneration
Abstract
Olive leaves have an antioxidant capacity, and olive leaf extract can protect the blood, spleen and hippocampus in lead-poisoned mice. However, little is known about the effects of olive leaf extract on lead-induced brain injury. This study was designed to determine whether olive leaf extract can inhibit lead-induced brain injury, and whether this effect is associated with antioxidant capacity. First, we established a mouse model of lead poisoning by continuous intragastric administration of lead acetate for 30 days. Two hours after successful model establishment, lead-poisoned mice were given olive leaf extract at doses of 250, 500 or 1 000 mg/kg daily by intragastric administration for 50 days. Under the transmission electron microscope, olive leaf extract attenuated neuronal and capillary injury and reduced damage to organelles and the matrix around the capillaries in the frontal lobe of the cerebral cortex in the lead-poisoned mice. Olive leaf extract at a dose of 1 000 mg/kg had the greatest protective effect. Spectrophotometry showed that olive leaf extract significantly increased the activities of superoxide dismutase, catalase, alkaline phosphatase and acid phosphatase, while it reduced malondialdehyde content, in a dose-dependent manner. Furthermore, immunohistochemical staining revealed that olive leaf extract dose-dependently decreased Bax protein expression in the cerebral cortex of lead-poisoned mice. Our findings indicate that olive leaf extract can inhibit lead-induced brain injury by increasing antioxidant capacity and reducing apoptosis.
INTRODUCTION
Can olive leaf extract promote lead excretion? Can it attenuate lead-induced brain injury? What are the chanisms underlying these effects? In recent years, neurotoxicity from exposure to low levels of lead in the environment has become increasingly prevalent. Therefore, the discovery of herbs that have lead-eliminating properties without harmful side effects is essential for the management of lead poisoning. Olive leaves and their main bioactive components have been extensively studied in China and abroad. Moreover, olive leaves can be used for medical purposes. Therefore, a thorough understanding of the protective effects of olive leaves in lead-poisoned mice can provide a rational basis for the use of the olive plant for the treatment of lead toxicity.
The nervous system of humans, particularly children, is susceptible to lead exposure[1,2,3,4,5,6,7]. Potentially toxic substances are activated and released in the injured brain, including free radicals and lipid peroxidases, which lead to decreased expression of voltage-dependent anion channels[8], altered DNA-binding activity and protein levels of Oct-2, inhibition of membrane depolarization and an elevation of intracellular Ca2+ concentration[9,10], changes in the microstructure of the brain[11,12,13], and alterations in gray and white matter architecture[14,15]. Calcium disodium edetate, penicillamine and dimercaprol are the Food and Drug Administration-approved compounds used clinically for the treatment of lead toxicity. However, these drugs have harmful side effects, including allergic reactions and renal damage[16]. Antioxidases, such as superoxide dismutase (SOD), catalase (CAT), alkaline phosphatase (AKP) and acid phosphatase (ACP), play a crucial role in the defense response. In addition, malondialdehyde (MDA) can be used as a marker for oxidative damage to the plasma membrane[17]. Therefore, these biochemical markers of antioxidant capacity have been frequently used in studies of lead-induced injury[18,19,20].
Olive leaf extract, which scavenges free radicals, can be applied in the treatment of heart disease, osteoporosis, skin disease, inflammation and diabetes mellitus[21,22,23,24,25]. Olive leaf contains the active iridoid constituent oleuropein (chief constituent). Other secoiridoids include 11-demethyloleuropein, 7,11-dimethyl ester of oleoside, ligustroside, oleuroside and unconjugated secoiridoid aldehydes. Triterpenes and flavonoids, including luteolin, apigenin, rutin and diosmetin, are also present. Oleasterol, leine and glycoside oleoside are also found in the leaves. Like many natural herbs, olive leaves contain some powerful antioxidants[26,27,28,29,30,31,32,33,34,35,36]. Esmaeili-Mahani and Ji showed that intraperitoneal injection of 50–400 mg/kg olive leaf extract or intragastric administration of 250–1 000 mg/kg of the extract produced dose-dependent effects[21,22]. In our previous studies, we found that olive leaf extract could protect the blood, spleen and hippocampus, and inhibit cell death[37,38,39]. The present study is the first to examine the effects of olive leaf extract on neuronal and capillary ultrastructure, antioxidant capacity (assessed using the markers SOD, CAT, ACP, AKP and MDA) and apoptosis (assessed by measuring Bax protein levels) in lead-poisoned mice. Our findings provide a rational bases for using olive leaf extract as a novel treatment strategy for lead toxicity.
The aim of this study is to answer the following: (1) Can olive leaf extract attenuate lead-induced brain injury? (2) Can olive leaf extract enhance antioxidant capacity in lead-poisoned mice? (3) Can olive leaf extract inhibit apoptosis?
RESULTS
Quantitative analysis of experimental animals
One hundred and fifty adult Kunming mice were included in this study and were randomly divided into normal control (n = 30) and experimental (n = 120; lead poisoning model was established with intragastric administration of lead acetate) groups. Furthermore, the mice in the experimental group were randomly divided into four subgroups (30 mice per group) according to a previously published method[22]: model, low-dose olive leaf extract (250 mg/kg), middle-dose olive leaf extract (500 mg/kg) and high-dose olive leaf extract (1 000 mg/kg). The model and normal control groups were treated with deionized water. No lead-poisoned mice displayed infection or died during the experiments. All 150 mice were included in the final analysis, without any dropout.
Olive leaf extract attenuated neuronal and capillary injury in the frontal lobe of the cerebral cortex in lead-poisoned mice
Neuronal and capillary changes in the frontal cortical lobe were observed under a transmission electron microscope after 50 days of olive leaf extract administration. Neurons and blood capillaries in the normal group displayed a normal structure, with no signs of injury. In contrast, neurons in the model group showed severe injury, with irregularities in the nuclear membrane, rough endoplasmic reticulum dilation, and broken or vacuolated mitochondria. Moreover, the matrix around the capillaries appeared dissolved or fragmented, and the capillary lumen had narrowed. Interestingly, when lead-poisoned mice received olive leaf extract at any of the three doses for 50 days, neuronal and capillary injury was alleviated. In the high-dose olive leaf extract group, the ultrastructure of the cerebral cortex was comparatively normal (Figure 1).
Figure 1.
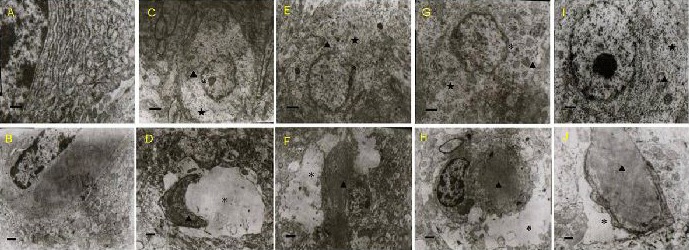
Effects of olive leaf extract (OLE) on neuronal and capillary injury in the frontal lobe of the cerebral cortex in lead-poisoned mice (transmission electron microscope).
Ultrastructure of neurons (A, C, E, G, I, scale bars: 1 μm) and a blood capillary (B, D, F, H, J, scale bars: 0.5 μm) was observed.
(A, B) Normal group; (C, D) model group. In the model group, the neurons and capillaries showed severe injury, including nuclear membrane irregularities and rough endoplasmic reticulum dilation. The mitochondria were broken and vacuolated, the matrix around the capillaries appeared dissolved or destroyed, and the capillary lumen had narrowed.
(E, F) Low-dose OLE (250 mg/kg) group; (G, H) middle-dose OLE (500 mg/kg) group; (I, J) high-dose OLE (1 000 mg/kg) group. In these OLE groups, the ultrastructure of the cerebral cortex appeared much better, compared with the model group. The three doses of OLE showed protective effects on injured neurons and capillaries.
“*”: Nuclear membrane; “★”: rough endoplasmic retriculum; “▲”: mitochondrial vacaoles.
Olive leaf extract enhanced SOD, CAT, ACP and AKP activities and reduced MDA content in the frontal lobe of the cerebral cortex in lead-poisoned mice
To evaluate antioxidant capacity in the lead-poisoned mice, SOD, CAT, ACP and AKP activities and MDA content in the frontal cortical lobe were measured after 50 days of olive leaf extract administration. SOD, CAT, ACP and AKP activities in the model group were significantly decreased, and MDA content was significantly increased, compared with the normal control group (P < 0.05 or P < 0.01). There was a significant increase in SOD, CAT, ACP and AKP activities, and a reduction in MDA content, in the olive leaf extract-treated groups compared with the model group (P < 0.05 or P < 0.01; Figure 2).
Figure 2.
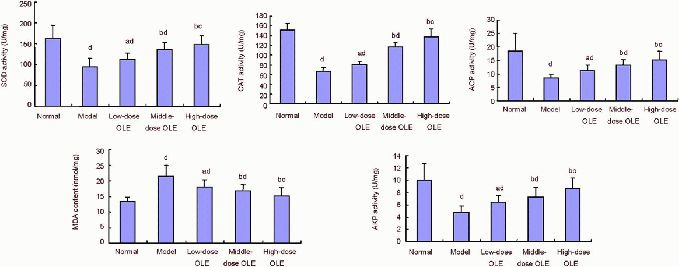
Effects of olive leaf extract (OLE) on SOD, CAT, ACP and AKP activities and MDA content in the frontal lobe of the cerebral cortex in lead-poisoned mice.
aP < 0.05, bP < 0.01, vs. model group; cP < 0.05, dP < 0.01, vs. normal group. Data are expressed as mean ± SD (n = 12), and differences between groups were compared with one-way analysis of variance and least significant difference t-test.
SOD: Superoxide dismutase; CAT: catalase; ACP: acid phosphatase; MDA: malondialdehyde; AKP: alkaline phosphatase; low-, middle-, high-dose OLE groups: 250, 500, 1 000 mg/kg OLE treatment.
Olive leaf extract inhibited expression of Bax in the cerebral cortex of lead-poisoned mice
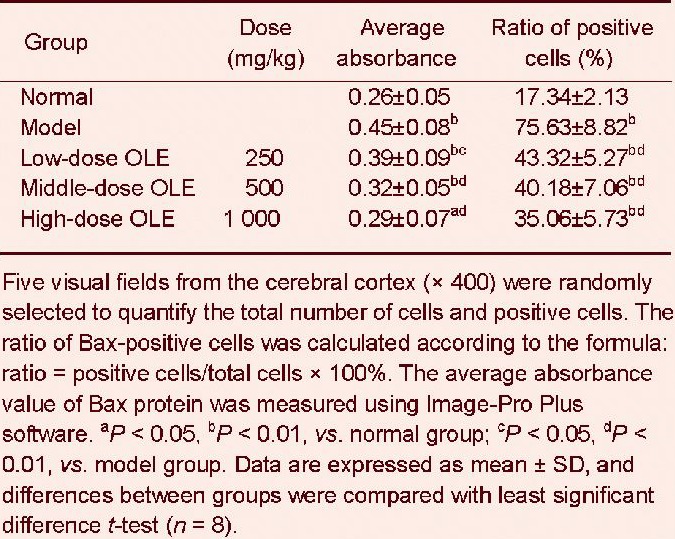
After 50 days of olive leaf extract administration, Bax-positive cells were observed by immunohistochemical staining, and were primarily found to be located in the cerebral cortex. Immunoreactive particles were located in the cell membrane and cytoplasm. A small number of Bax-positive cells were observed in the cerebral cortex of the normal group and the immunoreactive particles were lightly stained. Bax-positive cells displayed strong immunoreactivity in the cerebral cortex of the model group. Compared with the model group, there were significantly fewer Bax-positive cells in the olive leaf extract-treated groups (Figure 3).
Figure 3.
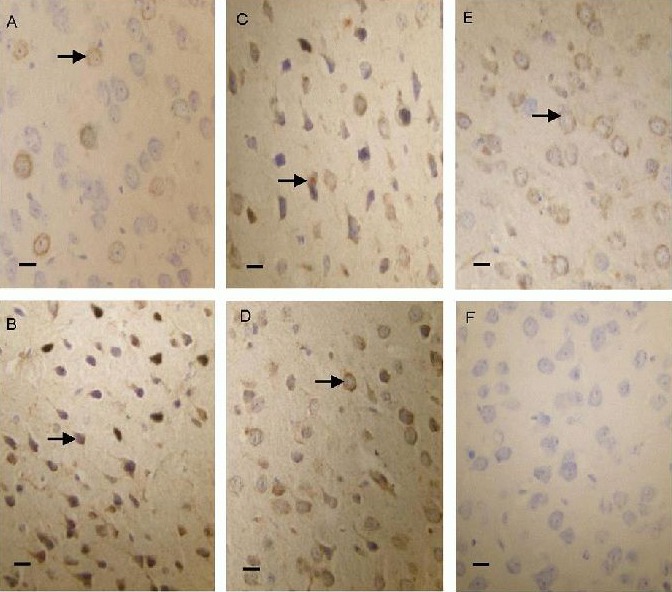
Bax protein expression in the cerebral cortex of lead-poisoned mice (immunohistochemical staining, optical microscope, scale bars: 20 μm).
Bax protein was expressed predominantly in the cell membrane and cytoplasm. In the normal group (A), Bax-positive products were stained light yellow. In the low-dose olive leaf extract (250 mg/kg) group (C), middle-dose olive leaf extract (500 mg/kg) group (D) and high-dose olive leaf extract (1 000 mg/kg) group (E), the positive products were brown, and their levels were lower than in the model group (B). (F) Negative control group.
After 50 days of the respective treatment, the average absorbance value and the number of Bax-positive cells were significantly increased in the cerebral cortex of mice in the model group compared with the normal control group (P < 0.01).
These values were significantly lower in the olive leaf extract-treated groups compared with the model group (P < 0.05 or P < 0.01). In particular, the high-dose olive leaf extract group exhibited the greatest reduction in the number of Bax-positive cells (P < 0.01; Table 1).
Table 1.
Effects of olive leaf extract (OLE) on Bax immunoreactivity in the cerebral cortex of lead-poisoned mice
DISCUSSION
The experimental model of lead-induced brain injury used in this study has a similar pathophysiology to lead toxicity in humans, and is thus commonly used[18,19]. The liquid olive leaf extract was given to the mice via intragastric administration at a dose of 100–1 000 mg/kg per day for 20–60 days[21,22,40]. In addition, control mice were used to control for potential side effects.
There is increasing evidence that many natural products and food have lead-eliminating properties. For example, Li et al[41] demonstrated that a compound preparation of hoelen, sweet root and flos lonicerae can promote lead excretion. Many crude plant extracts, such as chrysophanol, salicylic acid, Haierful Oral Liquid, tea polyphenols and Hippophae rhamnoides, also exhibit an antioxidant capacity. These extracts improve SOD activity in the liver, kidney and brain tissue, reduce MDA content in a lead-poisoned mouse model, and can prevent lead from being accumulated[16,42,43,44,45]. Actinidia chinensis, Coriandrum sativum and Allium sativum appear to contain natural chelating agents for the treatment of lead toxicity[46,47,48]. Olive leaf extract can increase the activity of antioxidant enzymes and reduce MDA accumulation[37,38,39]. The present study is the first investigation of the action of olive leaf extract on brain histological structure and antioxidant capacity in lead-poisoned mice using transmission electron microscopy, spectrophotometry and immunohistochemical staining.
As modern transportation and industry develop, environmental lead pollution is becoming more severe. In the current study, SOD, CAT, ACP and AKP activities after lead administration were significantly lower than those in the normal control group. These findings indicate that lead might inhibit SOD, CAT, ACP and AKP activities. It has been documented that lead competes with metal ions (such as Cu2+, Zn2+, Fe2+ and Mg2+) that are essential for the activity of antioxidant enzymes, resulting in a loss or decrease in SOD, CAT, ACP and AKP activities[19,49]. In addition, MDA content, which is a marker for oxidative damage to the plasma membrane[50,51], increased significantly after lead administration. In the present study, olive leaf extract reduced the levels of MDA, and enhanced the activities of SOD, CAT, ACP and AKP, which is consistent with previous reports[37,38,39]. Our findings provide support for the natural protective effects of olive leaf extract against oxidative stress-induced injury.
Histological examination revealed that lead produced prominent tissue damage characterized by nuclear membrane fragmentation, mitochondrial destruction, rough endoplasmic reticulum dilation and capillary lumen narrowing. Lead exposure can lead to impaired coordination and cause glioblastoma multiforme and meningioma [52]. Some scholars demonstrated that the activities of SOD, CAT, ACP and AKP are temporarily inhibited by lead[19], thereby inducing an oxidative stress reaction, and ultimately resulting in changes to the histological structure of the brain. Intragastric administration of olive leaf extract may reduce neural and capillary injury. This indicates that olive leaf extract can protect damaged neurons and capillaries.
Reactive oxygen species include molecules such as hydroxyl radical (•OH), superoxide anion (O2•-) and nitric oxide (NO•). Reactive oxygen species can rapidly induce the expression of apoptotic genes[53]. The Bcl-2 family proteins regulate a distal step in an evolutionarily conserved pathway for programmed cell death[54,55,56]. In the nervous system, Bax and Bcl-2 genes primarily regulate apoptosis[57]. The Bax/Bcl-2 ratio determines the survival or death of cells following an apoptotic insult, and an elevated Bax/Bcl-2 ratio can trigger apoptosis and target the cells for death[58]. In the present study, we detected altered Bax protein expression following lead and olive leaf extract treatments. Olive leaf extract treatment decreased Bax protein expression, thereby reducing cellular apoptosis and protecting the injured cerebral cortical neurons. It is well known that oleuropein, hydroxytyrosol, tyrosol and caffeic acid are the main constituents of olive leaves, and are thought to be responsible for the pharmacological effects. Furthermore, olive leaves contain p-coumaric acid, vanillic acid, vanillin, luteolin, diosmetin, rutin, luteolin-7-glucoside, apigenin-7-glucoside and diosmetin-7-glucoside[26,27,28,29,30,31,32,33,34,35,36]. Olive leaf extract can inhibit or reduce the severity of heart disease, osteoporosis, viral infections and skin disorders, and can regulate body antioxidant capacity and lipid metabolism[30]. In the present study, olive leaf extract protected the brain. It increased SOD, CAT, ACP and AKP activities, and significantly decreased MDA content and Bax protein expression. It is likely that olive leaf extract contains numerous bioactive substances, such as oleuropein and flavonoids. Mohagheghi showed that the major constituent of olive leaf extract is oleuropein, comprising 35.6% of the extract, using high performance liquid chromatography analysis[59]. Oleuropein can enhance antioxidant capacity or combine with Pb2+, thereby possibly decreasing systemic lead absorption and lead accumulation in the brain.
In summary, olive leaf extract rescues neurons and capillaries from lead-induced damage, and attenuates oxidative stress reactions and diminishes apoptosis. However, this is a preliminary study on the protective effects of olive leaf extract. Further experimental studies on olive leaf extract are required, especially those focusing on the molecular mechanisms underlying the protective effects of the natural medicine.
MATERIALS AND METHODS
Design
A randomized, controlled animal experiment.
Time and setting
This experiment was performed at the Animal Laboratory of Longnan Teachers College, Scientific Experimental Center of Lanzhou University Medical College, China from May 2011 to September 2012.
Materials
Animals
A total of 150 healthy adult Kunming mice, of both genders, 30 days of age, weighing 20–22 g, were provided by the Experimental Animal Center of Lanzhou University, China (certification No. 14-006). The experimental procedures were conducted in accordance with the Guidance Suggestions for the Care and Use of Laboratory Animals, formulated by the Ministry of Science and Technology of China[60].
Herbs
Olive leaves were collected from the Wudu area of Gansu Province, China, in September when the plant grows wildly under natural conditions. In brief, air-dried slices of olive leaves were immersed in a five-fold volume of water for 1 hour, and processed into a decoction by boiling and simmering for 1 hour. Following three extractions, the filtrate was centrifuged at 3 000 r/min for 10 minutes, filtered, and condensed into aqueous extracts. The resultant aqueous extracts were incubated at 4°C for more than 24 hours, and subsequently centrifuged and mixed with 95% ethanol. At a final concentration of 80% ethanol, the extracts were rinsed five times with dehydrated alcohol and acetone. The extracts were deproteinized, followed by a 45-minute reflow. The filtrate solution was repeatedly harvested, and supernatants were dialyzed for 48 hours against distilled water to eliminate pigment, followed by precipitation. Quantification of some identified phenolic compounds by high performance liquid chromatography showed that oleuropein (356 mg/g), tyrosol (3.73 mg/g), hydroxytyrosol (4.89 mg/g) and caffeic acid (49.41 mg/g) were the main phenolic components of the olive leaf extract[21,60]. The solid residues of the olive leaf extracts were obtained after solvent evaporation. Therefore, we had no significant solvent in our extract. The extract was dissolved in deionized water to make the required concentration for use on each day of our experiments.
Methods
Establishment of lead-poisoned mouse model
Under aseptic conditions, lead acetate (Tianxiang Chemical Co., Ltd., Zhengzhou, Henan Province, China) was soaked in normal saline, which was given to the mice via intragastric administration at a dose of 10 mg/kg per day for 30 consecutive days as previously described [18]. After 30 days, successful generation of the lead poisoning model was confirmed by observing abnormal behaviors and measuring blood lead levels. Mice exhibiting the abnormal behaviors of irritability and hyperactivity, with a blood lead level ≥ 2 mg/dl, were considered to have undergone successful modeling[18,40].
Intragastric administration of olive leaf extract
Two hours after establishment of the lead poisoning model, mice from the three treatment groups received olive leaf extract at a dose of 250, 500 or 1 000 mg/kg (dissolved in deionized water, 5 mL/kg) by intragastric administration. The model and normal groups were treated with deionized water (5 mL/kg). The dosage was based on a previous report[22]. Each group received intragastric administration once daily for 50 consecutive days.
Observation of neurons and capillaries in the frontal lobe of the cerebral cortex in lead-poisoned mice
Three mice randomly taken from each group were sacrificed under anesthesia. Four to six tissue samples from the frontal lobe of the cerebral cortex were fixed for 24 hours at 4°C with 4% glutaraldehyde in 0.1 mol/L phosphate buffer (pH 7.2–7.4), then post-fixed for 2 hours in 1% osmium tetroxide and dehydrated in a graded acetone series. The samples were washed with propylene oxide and embedded in Epon 812. The sections were obtained with an RMC ultramicrotome (RMC, Chicago, IL, USA). Semi-thin sections of 60-nm thickness were stained with toluidine blue, and thin sections were stained with uranyl acetate and lead citrate, and examined with a JEM-1230 electron microscope (JEOL, Japan).
Detection of antioxidant capacity in the frontal lobe of the cerebral cortex in lead-poisoned mice
The frontal cortical tissue was quickly put into an ice bath immediately after the blood was soaked up with filter paper. Samples were then homogenized with a glass homogenizer. The homogenates were centrifuged for 10 minutes at 3 000 r/min, and the supernatant was collected and stored at –20°C for determining the antioxidant defense capacities[19]. SOD, CAT, ACP and AKP activities and MDA content were determined with a method established by Nanjing Jiancheng Bioengineering Institute, China. The measured sample was compared with the absorbance of a blank control liquid, and the SOD, CAT, ACP and AKP activities and MDA content were calculated on the basis of protein levels.
Immunochemical staining for Bax in the cerebral cortex of lead-poisoned mice
The cerebral tissue was fixed with 10% formalin, followed by dehydration, waxing, embedding, and sectioning (6-μm thickness). The slices were dewaxed with xylene, hydrated in a graded alcohol series, boiled in citrate buffer solution (pH 6.0 ± 0.1) in a heated pressure cooker for 1.5 minutes to retrieve antigen, and incubated for 10 minutes at room temperature with 3% hydrogen peroxide to block endogenous peroxidase activity. The slices were incubated with rabbit anti-Bax protein polyclonal antibody (1:200; Wuhan Boster Bioengineering Institute, Wuhan, Hubei Province, China) at 4°C overnight. Then, sections were incubated with biotin-labeled secondary antibody (goal anti-rabbit IgG; Boster) at 37°C for 30 minutes and horseradish peroxidase-conjugated streptavidin (Boster) at 37°C for 30 minutes, and subjected to 3,3’-diaminobenzidine coloration (Boster) under the optical microscope (Olympus, Tokyo, Japan). Between each incubation, the specimens were rinsed with PBS three times for 5 minutes each time. After termination of the color reaction, the specimens were counterstained with hematoxylin, rinsed until blue, dehydrated in an alcohol series, cleared with xylene, and mounted with neutral gum. The negative control was treated with PBS rather than primary antibodies. The appearance of brownish yellow particles or fine particles in a diffuse distribution was considered a positive reaction.
A total of five visual fields from the cerebral cortex (× 400) were randomly selected to quantify the total number of cells and positive cells. The ratio of Bax-positive cells was calculated as follows: positive cells/total cells × 100%. The average absorbance value of Bax was measured using the Image-Pro Plus software (Media Cybernetics Co., Bethesda, MD, USA).
Statistical analysis
All data were analyzed with SPSS 16.0 software (SPSS, Chicago, IL, USA) and expressed as mean ± SD. One-way analysis of variance and least significant difference t-test was used for comparison between two groups. P < 0.05 was considered statistically significant.
Research background: Lead has been known as a human health hazard for a long time. In particular, damage to the brain and nervous system is a major feature of lead toxicity. Existing lead-removing drugs may produce side effects. The identification of herbs that have lead-eliminating properties without hazardous side effects is essential for lead management.
Research frontiers: Olive leaves contain antioxidants and have been recommended for the treatment of heart disease, osteoporosis, skin disorder, inflammation and diabetes. Olive leaf extract also improves the antioxidant capacity of blood, spleen and hippocampus in lead-poisoned mice.
Clinical significance: The present report addressing the effects of olive leaf extract on brain histological structure and antioxidant capacity in lead-poisoned mice can provide an empirical basis for the development and use of olive leaves for enhancing antioxidant capacity and removing toxins.
Academic terminology: Olive leaf extract–alcohol-dissolved active component extracted from natural olive leaves after removing chlorophyll and other matter. It has good anti-oxidative capacity.
Peer review: The present study showed that olive leaf extract inhibits lead-induced brain damage by increasing antioxidant capacity, reducing neural apoptosis, and by scavenging free radicals. The experiments were performed using transmission electron microscopy, spectrophotometry and immunohistochemical staining. The findings provide a new treatment strategy for lead excretion.
Footnotes
Funding: This study was supported by the Natural Science Foundation of Gansu Province, No. 1107RJZK243, and a grant from Gansu Provincial Education Committee, No. 1128B-01.
Conflicts of interest: None declared.
Ethical approval: This experiment was approved by the Animal Ethics Committee of Lanzhou University Medical College in China.
(Reviewed by Patel B, Norman C, Chen X, Hu LM)
(Edited by Wang J, Yang Y, Li CH, Song LP, Liu WJ, Zhao M)
REFERENCES
- [1].Peters JL, Kubzansky LD, Ikeda A, et al. Childhood and adult socioeconomic position, cumulative lead levels, and pessimism in later life: the VA normative aging study. Am J Epidemiol. 2011;174(12):1345–1353. doi: 10.1093/aje/kwr269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Lasky RE, Luck ML, Parikh NA, et al. The effects of early lead exposure on the brains of adult rhesus monkeys: a volumetric MRI study. Toxicol Sci. 2005;85(2):963–975. doi: 10.1093/toxsci/kfi153. [DOI] [PubMed] [Google Scholar]
- [3].Zhu ZW, Yang RL, Dong GJ, et al. Study on the neurotoxic effects of low-level lead exposure in rats. J Zhejiang Univ Sci B. 2005;6(7):686–692. doi: 10.1631/jzus.2005.B0686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Zhang Y, Ye LP, Wang B, et al. Chelation of grp78 with lead and its localization changes in the astroglia of rats exposed to lead. J Huazhong Univ Sci Technolog Med Sci. 2009;29(4):492–497. doi: 10.1007/s11596-009-0420-x. [DOI] [PubMed] [Google Scholar]
- [5].Suresh C, Dennis AO, Heinz J, et al. Melatonin protection against lead-induced changes in human neruoblastoma cell cultures. Int J Toxicol. 2006;25(6):459–464. doi: 10.1080/10915810600959576. [DOI] [PubMed] [Google Scholar]
- [6].Meng XM, Zhu DM, Ruan DY, et al. Effects of chronic lead exposure on 1H MRS of hippocampus and frontal lobes in children. Neurology. 2005;64(9):1644–1647. doi: 10.1212/01.WNL.0000160391.58004.D4. [DOI] [PubMed] [Google Scholar]
- [7].Chiu Y, Chuang H, Chen Y, et al. The effect of chronic lead exposure on brain magnetic resonance spectroscopy. Epidemiology. 2008;19(6):90–95. [Google Scholar]
- [8].John MP, Sunyoung P, Diana IL. Decreased expression of the voltage-dependent anion channel in differentiated pc-12 and sh-sy5y cells following low-level Pb exposure. Toxicol Sci. 2010;113(1):169–176. doi: 10.1093/toxsci/kfp249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Bakheet SA, Basha MR, Cai H, et al. Lead exposure: expression and activity levels of oct-2 in the developing rat brain. Toxicol Sci. 2007;95(2):436–442. doi: 10.1093/toxsci/kfl163. [DOI] [PubMed] [Google Scholar]
- [10].Wang W, Duan B, Xu H, et al. Calcium-permeable acid-sensing ion channel is a molecular target of the neurotoxic metal ion lead. J Biol Chem. 2006;281(5):2497–2505. doi: 10.1074/jbc.M507123200. [DOI] [PubMed] [Google Scholar]
- [11].Gao S, Wen F, Sun LG, et al. Effect of chronic lead exposure on expression of PKC-γ in mice hippocampus. Zhongguo Gonggong Weisheng. 2008;24(7):793–794. [Google Scholar]
- [12].Deveci E. Ultrastructural effects of lead acetate on brain of rats. Toxicol Ind Health. 2006;22(10):419–422. doi: 10.1177/07482337060220100101. [DOI] [PubMed] [Google Scholar]
- [13].Zhang TB, Gao MQ, Sun LG, et al. Effect of lead exposure on the concentrations of blood lead and brain lead in mice and the related microstructural changes in hippocampus. Zhongguo Yike Daxue Xuebao. 2005;34(3):195–196. [Google Scholar]
- [14].Brubaker CJ, Dietrich KN, Lanphear BP, et al. The influence of age of lead exposure on adult gray matter volume. Neurotoxicology. 2010;31(3):259–266. doi: 10.1016/j.neuro.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Brubaker CJ, Schmithorst VJ, Haynes EN, et al. Altered myelination and axonal integrity in adults with childhood lead exposure: a diffusion tensor imaging study. Neurotoxicology. 2009;30(6):867–875. doi: 10.1016/j.neuro.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Zhang J, Yan CL, Zhang DS, et al. Effects of chrysophanol on learning and memory impairment induced by lead in mice and the study of its mechanisms. Zhongguo Yaolixue Tongbao. 2011;27(11):1614–1618. [Google Scholar]
- [17].Wang JQ, Wu JH, Zhang ZM. Oxidative stress in mouse brain exposed to lead. Ann Occup Hyg. 2006;50(4):405–409. doi: 10.1093/annhyg/mei079. [DOI] [PubMed] [Google Scholar]
- [18].Zhang XZ, Su YS, Yu DJ, et al. Effects of acute exposure to lead on behaviors and lipid peroxidation in mice. Guangdong Weiliang Yuansu Kexue. 2007;14(12):30–35. [Google Scholar]
- [19].Wang Y, Wang SQ. Effects of lead exposure on histological structure and antioxidant capacity in the cerebellum of 30-day-old mice. Neural Regen Res. 2011;6(14):1077–1081. [Google Scholar]
- [20].Cabral M, Dieme D, Verdin A, et al. Low-level environmental exposure to lead and renal adverse effects: a cross-sectional study in the population of children bordering the mbeubeuss landfill near Dakar, Senegal. Hum Exp Toxicol. 2012;31(12):1280–1291. doi: 10.1177/0960327112446815. [DOI] [PubMed] [Google Scholar]
- [21].Esmaeili-Mahani S, Rezaeezadeh-Roukerd M, Esmaeilpour K, et al. Olive (Olea europaea L.) leaf extract elicits antinociceptive activity, potentiates morphine analgesia and suppresses morphine hyperalgesia in rats. J Ethnopharmacol. 2010;132(1):200–205. doi: 10.1016/j.jep.2010.08.013. [DOI] [PubMed] [Google Scholar]
- [22].Ji CM, Wu GQ, Shen ZL. Effects of olive leaf extract on glycemia and lipidemia in normal and diabetic mice induced by streptozocin. Dongnan Daxue Xuebao: Yixue-Ban. 2003;22(4):236–238. [Google Scholar]
- [23].Kaeidi A, Esmaeili-Mahani S, Sheibani V, et al. Olive (Olea europaea L.) leaf extract attenuates early diabetic neuropathic pain through prevention of high glucose-induced apoptosis: in vitro and in vivo studies. J Ethnopharmacol. 2011;136(1):188–196. doi: 10.1016/j.jep.2011.04.038. [DOI] [PubMed] [Google Scholar]
- [24].Susalit E, Agus N, Effendi I, et al. Olive (Olea europaea) leaf extract effective in patients with stage-1 hypertension: comparison with Captopril. Phytomedicine. 2011;18(4):251–258. doi: 10.1016/j.phymed.2010.08.016. [DOI] [PubMed] [Google Scholar]
- [25].Ortega-garcía F, Blanco S, Peinado MA, et al. Polyphenol oxidase and its relationship with oleuropein concentration in fruits and leaves of olive (Olea europaea) cv. ‘Picual’ trees during fruit ripening. Tree Physiol. 2008;28(1):45–54. doi: 10.1093/treephys/28.1.45. [DOI] [PubMed] [Google Scholar]
- [26].Turkez H, Togar B, Polat E. Olive leaf extract modulates permethrin induced genetic and oxidative damage in rats. Cytotechnology. 2012;64(4):459–464. doi: 10.1007/s10616-011-9424-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Papoti VT, Tsimidou MZ. Impact of sampling parameters on the radical scavenging potential of olive (olea europaea L.) Leaves. J Agric Food Chem. 2009;57(9):3470–3477. doi: 10.1021/jf900171d. [DOI] [PubMed] [Google Scholar]
- [28].Bouaziz M, Grayer RJ, Simmonds MS, et al. Identification and antioxidant potential of flavonoids and low molecular weight phenols in olive cultivar chemlali growing in Tunisia. J Agric Food Chem. 2005;53(2):236–241. doi: 10.1021/jf048859d. [DOI] [PubMed] [Google Scholar]
- [29].Jemai H, Feki AE, Sayadi S. Antidiabetic and antioxidant effects of hydroxytyrosol and oleuropein from olive leaves in alloxan-diabetic rats. J Agric Food Chem. 2009;57(19):8798–8804. doi: 10.1021/jf901280r. [DOI] [PubMed] [Google Scholar]
- [30].El SN, Karakaya S. Olive tree (Olea europaea) leaves: potential beneficial effects on human health. Nutr Rev. 2009;67(11):632–638. doi: 10.1111/j.1753-4887.2009.00248.x. [DOI] [PubMed] [Google Scholar]
- [31].Lee OH, Lee BY, Lee J, et al. Assessment of phenolics-enriched extract and fractions of olive leaves and their antioxidant activities. Bioresour Technol. 2009;100(23):6107–6113. doi: 10.1016/j.biortech.2009.06.059. [DOI] [PubMed] [Google Scholar]
- [32].Lee OH, Lee BY. Antioxidant and antimicrobial activities of individual and combined phenolics in Olea europaea leaf extract. Bioresour Technol. 2010;101(10):3751–3754. doi: 10.1016/j.biortech.2009.12.052. [DOI] [PubMed] [Google Scholar]
- [33].Kontogianni VG, Gerothanassis IP. Phenolic compounds and antioxidant activity of olive leaf extracts. Nat Prod Res. 2012;26(2):186–189. doi: 10.1080/14786419.2011.582842. [DOI] [PubMed] [Google Scholar]
- [34].Lalasa S, Athanasiadisa V, Gortzia O, et al. Enrichment of table olives with polyphenols extracted from olive leaves. Food Chem. 2011;127(4):1521–1525. [Google Scholar]
- [35].Goulas V, Papoti VT, Exarchou V, et al. Contribution of flavonoids to the overall radical scavenging activity of olive (olea europaea L.) leaf polar extracts. J Agric Food Chem. 2010;58(6):3303–3308. doi: 10.1021/jf903823x. [DOI] [PubMed] [Google Scholar]
- [36].Omagari K, Kato S, Tsuneyama K, et al. Olive leaf extract prevents spontaneous occurrence of non-alcoholic steatohepatitis in SHR/NDmcr-cp rats. Pathology. 2010;42(1):66–72. doi: 10.3109/00313020903434389. [DOI] [PubMed] [Google Scholar]
- [37].Wang Y. Effects of olive leaf extract on biochemical indexes of blood in mice with lead poisoned. Ningxia Daxue Xuebao: Ziran Kexue Ban. 2012;33(3):279–282. [Google Scholar]
- [38].Wang Y. Effects of olive leaf extract on antioxidant enzyme, NOS activity and NO content in spleen of mice with lead poisoning. Dongbei Nongye Daxue Xuebao. 2012;43(9):86–89. [Google Scholar]
- [39].Wang Y. Effects of olive leaf extract on antioxidant enzyme, NOS activity and NO content in hippocampus of mice with lead poisoning. Gansu Nongye Daxue Xuebao. 2012;47(2):21–24. [Google Scholar]
- [40].Bunn TL, Parsons PJ, Kao E, et al. Exposure to lead during critical windows of embryonic development: differential immunotoxic outcome based on stage of exposure and gender. Toxicol Sci. 2001;64(1):57–66. doi: 10.1093/toxsci/64.1.57. [DOI] [PubMed] [Google Scholar]
- [41].Li CG, Zhang SQ, Liu Y, et al. Influence of compound preparation of hoelen, sweet root and flos lonicerae on the levels of lead, calcium and zinc in mice with lead poisoning. Zhongguo Zuzhi Gongcheng Yanjiu yu Linchuang Kangfu. 2006;10(35):115–117. [Google Scholar]
- [42].Chen J, Zhu C, Li LP, et al. Effects of exogenous salicylic acid on growth and H2O2-metabolizing enzymes in rice seedlings under lead stress. J Environ Sci. 2007;19(1):44–49. doi: 10.1016/s1001-0742(07)60007-2. [DOI] [PubMed] [Google Scholar]
- [43].Zhang SQ, Li CG, Huang XM, et al. Effect of Haierful oral liquid on cerebral lead content in lead exposed mice. Zhongguo Gongye Yixue Zazhi. 2008;21(3):184–185. [Google Scholar]
- [44].Zhou BT, Ding ZG, Li XZ, et al. Effects of tea polyphenol on NOS, NO, SOD and MDA in rats of lead exposure. Zhongguo Yiyuan Yaoxue Zazhi. 2008;28(18):1563–1565. [Google Scholar]
- [45].Xu Y, Li G, Han C, et al. Protective effects of Hippophae rhamnoides L. juice on lead-induced neurotoxicity in mice. Biol Pharm Bull. 2005;28(3):490–494. doi: 10.1248/bpb.28.490. [DOI] [PubMed] [Google Scholar]
- [46].Li JX, Chen SP, Qin Y, et al. Studies on Pb-discharging function promoted by Kiwi Juice. Zhongguo Shipin Xuebao. 2005;5(4):22–27. [Google Scholar]
- [47].Sharma V, Kansal L, Sharma A, et al. Ameliorating effect of coriandrum sativum extracts on hematological and immunological variables in an animal model of lead intoxication. J Pharm Allied Health Sci. 2011;1(1):16–29. [Google Scholar]
- [48].Sharma V, Sharma A, Kansal L. The effect of oral administration of Allium sativum extracts on lead nitrate induced toxicity in male mice. Food Chem Toxicol. 2010;48(3):928–936. doi: 10.1016/j.fct.2010.01.002. [DOI] [PubMed] [Google Scholar]
- [49].Shao P, Yuan X, Liu R, et al. Effects of nitrobenzene on liver antioxidant defense system of Carassius auratus. Chem Res Chin Univ. 2010;26(2):204–209. [Google Scholar]
- [50].Xiao X, Liu J, Hu J, et al. Protective effects of protopine on hydrogen peroxide-induced oxidative injury of PC12 cells via Ca2+ antagonism and antioxidant mechanisms. Eur J Pharmacol. 2008;591(1-3):21–27. doi: 10.1016/j.ejphar.2008.06.045. [DOI] [PubMed] [Google Scholar]
- [51].Annabi Berrahal A, Nehdi A, Hajjaji N, et al. Antioxidant enzymes activities and bilirubin level in adult rat treated with lead. Comptes Rendus Biologies. 2007;330(8):581–588. doi: 10.1016/j.crvi.2007.05.007. [DOI] [PubMed] [Google Scholar]
- [52].Bhatti P, Stewart PA, Hutchinson A, et al. Lead exposure, polymorphisms in genes related to oxidative stress, and risk of adult brain tumors. Cancer Epidemiol Biomarkers Prev. 2009;18(6):1841–1848. doi: 10.1158/1055-9965.EPI-09-0197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Deng FJ, Li RB, Yang YB, et al. Neuroprotective effect of epigallocatechin-3-gallate on hemisection-induced spinal cord injury in rats. Neural Regen Res. 2011;6(6):405–411. [Google Scholar]
- [54].Li A, Ojogho O, Escher A. Saving death: apoptosis for intervention in transplantation and autoimmunity. Clin Dev Immunol. 2006;13(2-4):273–282. doi: 10.1080/17402520600834704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Wei J, Kitada S, Rega MF, et al. Apogossypol derivatives as antagonists of antiapoptotic. Mol Cancer Ther. 2009;8(4):904–913. doi: 10.1158/1535-7163.MCT-08-1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Merry DE, Korsmeyer SJ. Bcl-2 gene family in the nervous system. Annu Rev Neurosci. 1997;20:245–267. doi: 10.1146/annurev.neuro.20.1.245. [DOI] [PubMed] [Google Scholar]
- [57].Sharifi AM, Mousavi SH, Jorjani M. Effect of chronic lead exposure on pro-apoptotic Bax and anti-apoptotic Bcl-2 protein expression in rat hippocampus in vivo. Cell Mol Neurobiol. 2010;30(5):769–774. doi: 10.1007/s10571-010-9504-1. [DOI] [PubMed] [Google Scholar]
- [58].Oltersdorf T, Elmore SW, Shoemaker AR, et al. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature. 2005;435(7042):677–681. doi: 10.1038/nature03579. [DOI] [PubMed] [Google Scholar]
- [59].Mohagheghi F, Bigdeli MR, Rasoulian B, et al. The neuroprotective effect of olive leaf extract is related to improved blood–brain barrier permeability and brain edema in rat with experimental focal cerebral ischemia. Phytomedicine. 2010;15(2-3):170–175. doi: 10.1016/j.phymed.2010.06.007. [DOI] [PubMed] [Google Scholar]
- [60].The Ministry of Science and Technology of the People's Republic of China. Guidance Suggestions for the Care and Use of Laboratory Animals. 2006 Sep 30; [Google Scholar]


