
Keywords: neural regeneration, traditional Chinese medicine, rutaecarpine, cerebral ischemia reperfusion, learning and memory, infarct volume, free radical, glutathione peroxidase superoxide dismutase, malondialdehyde, grants-supported paper, neuroregeneration
Abstract
Rutaecarpine, an active component of the traditional Chinese medicine Tetradium ruticarpum, has been shown to improve myocardial ischemia reperfusion injury. Because both cardiovascular and cerebrovascular diseases are forms of ischemic vascular disease, they are closely related. We hypothesized that rutaecarpine also has neuroprotective effects on cerebral ischemia reperfusion injury. A cerebral ischemia reperfusion model was established after 84, 252 and 504 μg/kg carpine were given to mice via intraperitoneal injection, daily for 7 days. Results of the step through test, 2,3,5-triphenyl tetrazolium chloride dyeing and oxidative stress indicators showed that rutaecarpine could improve learning and memory ability, neurological symptoms and reduce infarction volume and cerebral water content in mice with cerebral ischemia reperfusion injury. Rutaecarpine could significantly decrease the malondialdehyde content and increase the activities of superoxide dismutase and glutathione peroxidase in mouse brain. Therefore, rutaecarpine could improve neurological function following injury induced by cerebral ischemia reperfusion, and the mechanism of this improvement may be associated with oxidative stress. These results verify that rutaecarpine has neuroprotective effects on cerebral ischemia reperfusion in mice.
INTRODUCTION
During rescue and treatment of ischemic disease, tissue injury was found not to be caused by ischemia itself, but after the restoration of blood supply. This phenomenon is called tissue ischemia reperfusion injury[1,2]. Cerebral ischemia reperfusion injury refers to severe brain dysfunction when blood supply returns to the tissue after a period of brain tissue ischemia[3]. There are a variety of factors involved in this pathological process, including excitatory amino acid poisoning, oxidative stress, intracellular calcium overload, inflammatory reaction and apoptosis. The free radical chain reaction is considered to be an important mechanism causing brain damage[4]. Therefore, scavenging free radicals and preventing their secondary damage in cerebral ischemia has become one form of neuroprotective therapy[5].
At present, neuroprotective agents that have a neuroprotective effect on cerebral ischemia in animal models include calcium channel blockers, free radical scavengers, excitatory amino acid antagonists, and leukocyte adhesion inhibitors[6]. Because of unsatisfactory effects or adverse reactions, drugs displaying strong developmental potential have been eliminated during clinical application. So far, no ideal neuroprotective agent has been discovered. Thus, finding an effective prevention and treatment medication for cerebral ischemia reperfusion injury is a pressing issue in stroke research.
Rutaecarpine is a component of the main alkaloid extracted from a Chinese medicine Evodia. Its structure is shown in Figure 1.
Figure 1.
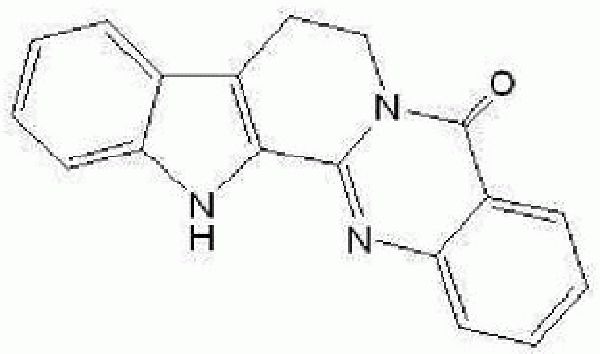
The chemical structure of rutaecarpine.
Rutaecarpine protects the heart, relaxes blood vessels, decreases blood pressure, resists platelet aggregation and thrombosis, and also blocks tumor cell growth, inflammation and ulcer formation[7,8]. Intravenous injection of rutaecarpine (100, 300 μg/kg) in rats has a protective effect on heart ischemia reperfusion injury[9,10]. Vascular lesions occur in both ischemic cardiovascular and cerebrovascular diseases. Therefore, they have common physiological and pathological characteristics, and are very closely related[11,12]. Based on results from a study on the effects of rutaecarpine on myocardial damage[9,10], active research concerning the effects of rutaecarpine on cerebral ischemic injury has vital significance and may develop the medicinal value of rutaecarpine.
Free radical damage is a major mechanism underlying cerebral ischemia reperfusion injury. Could rutaecarpine reduce cerebral ischemia reperfusion injury by decreasing the free radical damage and increasing learning and memory ability? We performed the step through test, and measured the content of malondialdehyde and the activity of superoxide dismutase and glutathione peroxidase in mouse brain to investigate this hypothesis. At the same time, the inclined board test and the measurement of infarct volume and brain water content were also conducted. These studies investigated the protective effects and mechanism of action of rutaecarpine on cerebral ischemia reperfusion injury.
The aims of this experiment included: (1) to establish a cerebral ischemia reperfusion injury model, (2) to study the protective effects and mechanism of action of rutaecarpine on nerve function and learning and memory in mice with cerebral ischemia reperfusion injury, and (3) to provide the experimental basis for rutaecarpine to become an ideal drug in the prevention and treatment of cerebral ischemia reperfusion injury.
RESULTS
Quantitative analysis of experimental animals
The 150 mice were equally and randomly assigned to five groups: the sham surgery group (the bilateral common carotid arteries were exposed, but not blocked), the model group (establishment of cerebral ischemia reperfusion injury), and the rutaecarpine 84, 252 and 504 μg/kg groups (cerebral ischemia reperfusion injury + 84, 252 and 504 μg/kg rutaecarpine, respectively)[13].
A total of three, six, two and three mice died in the model, 84, 252 and 504 μg/kg rutaecarpine groups, respectively. The mortality rate (between 6.6% and 20.0%) was less than that in a previous study[14]. According to the standard score system of Longa[15], 0-grade and dead mice (six, eight, four and four mice in the model group, 84, 252 and 504 μg/kg rutaecarpine groups, respectively) were removed at 48 hours after model induction. The mice graded 1 to 4 were considered as successful models of cerebral ischemia reperfusion injury. At 48 hours after cerebral ischemia reperfusion, 10 mice of the remaining mice in the sham surgery group (n = 30), model group (n = 24), 84 μg/kg rutaecarpine (n = 22), 252 μg/kg rutaecarpine (n = 26) and 504 μg/kg rutaecarpine (n = 26) groups were randomly selected for the step through test as well as measurement of malondialdehyde content and activities of superoxide dismutase and glutathione peroxidase in mouse brain. Another 10 mice from each group were randomly selected for the inclined board test and the measurement of infarct volume and brain water content. The remaining mice were not included, so 100 mice were included in the final analysis.
Influence of rutaecarpine on neurological function in mice following cerebral ischemia reperfusion injury
After 48 hours of cerebral ischemia reperfusion injury, the mice from the model group experienced severe loss of neurological function. Neurological symptom scores of the model group were significantly higher than those in the sham surgery group (P < 0.01). Scores from the 84, 252 and 504 μg/kg rutaecarpine groups were significantly lower than those of the model group (P < 0.01; Figure 2).
Figure 2.
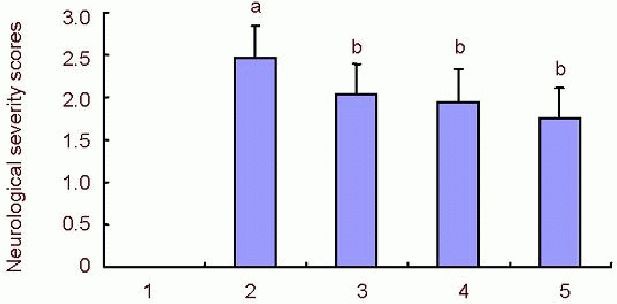
Effects of rutaecarpine on Longa neurological severity scores in mice with cerebral ischemia reperfusion injury.
Higher neurological function score indicates more severe cerebral ischemic reperfusion injury. aP < 0.01, vs. sham surgery group; bP < 0.01, vs. model group. Data are shown as mean ± SD (n = 30). Statistical analysis was determined by one-way analysis of variance and Dunnett's t-test between groups. 1: Sham surgery group; 2: model group; 3, 4, 5: 84, 252 and 504 μg/kg rutaecarpine groups.
Influence of rutaecarpine on learning and memory in mice with cerebral ischemia reperfusion injury
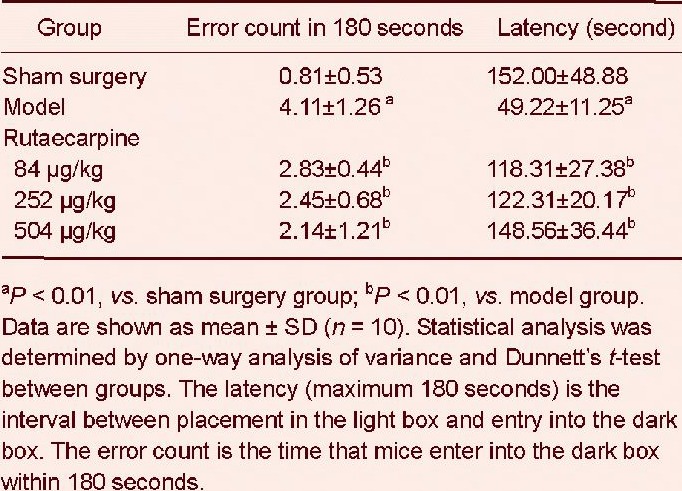
After 48 hours of cerebral ischemia reperfusion, mice were given step-through passive avoidance response training using a step through test, and 24 hours later, the test was repeated. The error count in 180 seconds and latency in the step through test are shown in Table 1. In the model group, the latency was significantly shorter (P < 0.01) and the number of errors was greater (P < 0.01) than in the sham surgery group. This observation indicated that learning and memory in the model group mice was impaired and that the model of cerebral ischemia reperfusion was established. Compared with the model group, the latency was significantly longer and the number of errors was significantly lower in each rutaecarpine group (P < 0.01), suggesting that rutaecarpine could significantly improve the learning and memory impairment caused by cerebral ischemia reperfusion injury.
Table 1.
The effects of rutaecarpine on error count in 180 seconds and latency in mice with cerebral ischemia reperfusion injury in the step through test
Effects of rutaecarpine on the activities of superoxide dismutase, glutathione peroxidase and content of malondialdehyde in mice with cerebral ischemia reperfusion injury
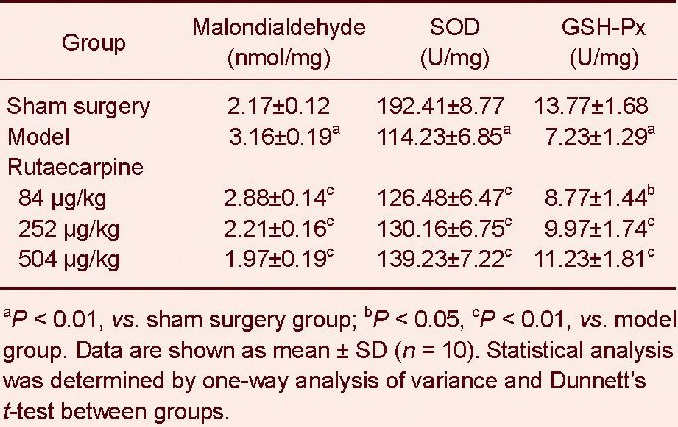
At 1 hour after the step through test, superoxide dismutase, glutathione peroxidase activity and malondialdehyde content in mouse brains were measured Table 2. Compared with the sham surgery group, malondialdehyde content was significantly higher, but the activities of superoxide dismutase and glutathione peroxidase were significantly lower in the model group (P < 0.01). Rutaecarpine doses of 84, 252 and 504 μg/kg significantly reduced malondialdehyde content, and significantly increased the activities of superoxide dismutase and glutathione peroxidase (P < 0.05 and P < 0.01, respectively). These findings suggested that rutaecarpine reduced free radical production in mice with cerebral ischemia reperfusion injury by increasing superoxide dismutase and glutathione peroxidase activities in mouse brain.
Table 2.
Effects of rutaecarpine on malondialdehyde content, superoxide dismutase (SOD) and glutathione peroxidase (GSH-Px) activities in the brain of mice with cerebral ischemia reperfusion injury
Effects of rutaecarpine on motor function, infarct volume and brain water content in mice with cerebral ischemia reperfusion injury
Motor function was measured using the inclined board test. Inclined angle was used as the major index. After the inclined board test, overdose of chloral hydrate was used for anesthesia, and mice were then sacrificed and brains quickly removed. The infarct volume and brain water content were determined (Table 3). In the inclined board test, inclined angle of the model group was significantly lower than that of the sham surgery group (P < 0.01). The inclined angles of the 252 and 504 μg/kg rutaecarpine groups were significantly higher than that of the model group (P < 0.01).
Table 3.
Effects of rutaecarpine on motor function, infarct volume and brain water content in the brain of mice with cerebral ischemia reperfusion injury
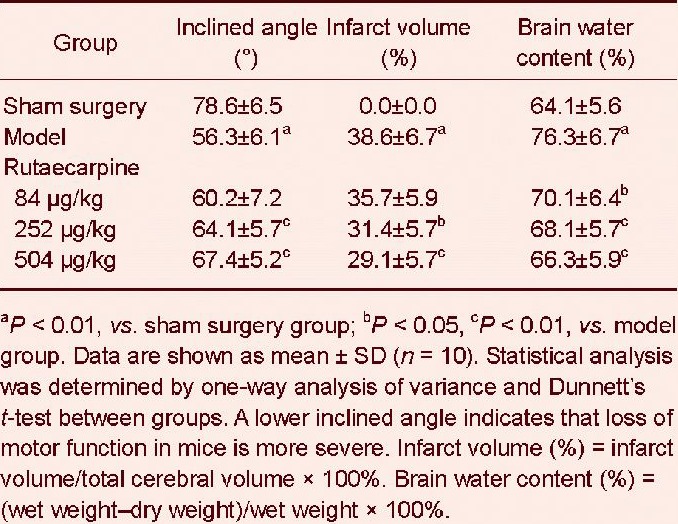
Figure 3.

Infarct volume at 48 hours after cerebral ischemia reperfusion injury.
The white area represents the infarct area, and the red area represents non-infarcted area. The sham surgery group has no infarction. Rutaecarpine groups display less of an infarct area than that of the model group.
Compared with the sham surgery group, infarct volume and brain water content were significantly greater in the model group (P < 0.01), showing that the model was successful. Compared with the model group, rutaecarpine could reduce infarct volume and brain water content of mice with cerebral ischemia reperfusion injury (P < 0.05 and P < 0.01, respectively), and the effect was enhanced with increased dose (P < 0.05 and P < 0.01, respectively).
DISCUSSION
This experiment adopted a modified method from Himori et al [14] in mice under anesthesia, causing cerebral ischemia for 5 minutes then perfusion, resulting in learning and memory impairment. Thus, a model of cerebral ischemia reperfusion injury was produced. This method can significantly improve the survival rate of mice, and ensures the smooth progress of future experiments.
Rutaecarpine is insoluble in water. In preliminary experiments, different proportions of dimethyl sulfoxide, twain-80, triple-distilled water and ethanol were used as the solvent to dissolve rutaecarpine, but the dissolving effect was not ideal. After exploration, rutaecarpine could be well dissolved in dimethyl sulfoxide. Because dimethyl sulfoxide is toxic, this experiment intraperitoneally injected 0.02 mL/kg using a microinjector to ensure accurate dose and medication safety.
Results of the preliminary study showed that > 700 μg/kg rutaecarpine could cause death in 50% of (3/6) mice. The mice intraperitoneally injected with dimethyl sulfoxide alone could not die, which illustrated that the death of the mice was caused by excessive dose of rutaecarpine. The damage to the mice caused by rutaecarpine needs further research. According to previous studies and preliminary experiments, doses of 84, 252, 504 μg/kg rutaecarpine were adopted[8].
Ischemic cardiovascular disease and cerebrovascular disease are similar vascular lesions. According to clinical occurrence and development of ischemic cardiovascular disease and cerebrovascular disease, the mechanism of ischemic cardiovascular disease is similar to that of cerebrovascular disease[16,17,18]. Recent studies have found that rutaecarpine strengthened the function of the heart, dilation of blood vessels, and protected against myocardial ischemia reperfusion injury[19,20]. Neurological function abnormality, brain edema, and brain tissue morphological changes all occur during cerebral injury caused by cerebral ischemia reperfusion[21,22]. Neurological symptom scores, brain water content and cerebral infarction volume were measured in this experiment to explore whether the cerebral ischemia reperfusion injury model was successful. Learning and memory ability, superoxide dismutase, malondialdehyde and other indexes in mouse brain were measured to explore the mechanism of protective effects of rutaecarpine on cerebral ischemia reperfusion injury in mice.
Brain tissue damage is also reflected in behavior disorders. Ischemic animals often have convulsions, contralateral falling, and rotary motion, to varying degrees. The severity of symptoms and signs is directly associated with the degree of cerebral tissue damage. Neurological scores, however, are strongly subjective, and the grades used are not very prescriptive. Using this system means it is sometimes difficult to exactly reflect the treatment effect, and so neurological scores may be considered a supplementary index[23,24,25].
In this experiment, comprehensive infarction volume and behavior evaluation results have shown that rutaecarpine had a neuroprotective effect, and could relieve brain tissue damage caused by ischemia reperfusion injury in mice. In the improvement of neurological symptoms, the therapeutic effects of rutaecarpine (84, 252 and 504 μg/kg) were evident. In the inclined angle test, rutaecarpine (84 μg/kg) had no obvious effect. However, there was a significant difference between the model group and rutaecarpine (252 and 504 μg/kg) groups (P < 0.01). In the analysis of brain morphology and water content, rutaecarpine significantly decreased cerebral infarct volume and brain water content, which indicated protective effects of rutaecarpine in ischemic brain injury. In the biochemical analysis of brain tissue, the oxidative reduction system of the mouse brain in the model group was obviously damaged, suggesting that cerebral ischemia reperfusion induced oxidative damage in the brain, which was consistent with a previous report[22]. When brain ischemia occurs, xanthine oxidase production increases rapidly, and a lot of hypoxanthine is produced. When blood flow is restored, hypoxanthine and molecular oxygen generate a large amount of oxygen free radicals under the catalysis of xanthine oxidase, which causes lipid peroxidation of the cell membrane[21]. The increases in oxygen free radical and lipid peroxidation are important mechanisms of cerebral ischemia reperfusion injury[25,26]. During cerebral ischemia reperfusion, numerous free radicals cause biological membrane unsaturated fatty acid and lipid peroxidation, which damage the structure and the function of the cell membrane[27,28,29]. This study found that rutaecarpine significantly improved learning and memory of cerebral ischemia reperfusion mice in a dose-dependent manner. Rutaecarpine (84, 252 and 504 μg/kg) decreased the malondialdehyde content significantly and increased the activities of superoxide dismutase and glutathione peroxidase in mouse brain. In this experiment, the effects of rutaecarpine (84 μg/kg) on cerebral ischemia reperfusion injury in mice are not obvious, while the effects of rutaecarpine (252 and 504 μg/kg) are more obvious.
A previous study indicated that rutaecarpine promoted the activation of the calcitonin gene to alleviate cerebral ischemia reperfusion injury in rats[12], which further indicated that the pathogenesis of cerebral ischemia reperfusion injury is diverse, and that rutaecarpine can improve many aspects of cerebral ischemia reperfusion. Rutaecarpine was the first alkaloid drug used for treating cerebral ischemia reperfusion injury, and provides a new direction for the treatment of cerebral ischemia reperfusion injury. Therefore, rutaecarpine may be developed to become a new drug for vascular dementia[30,31].
MATERIALS AND METHODS
Design
A randomized, controlled, in vivo study.
Time and setting
Experiments were performed at the Department of Pharmacology of Hebei North University in China from November 2011 to June 2012.
Materials
Animals
A total of 150 clean, healthy, male Kunming mice weighing 26–30 g were provided by the Animal Research Center of Chinese Academy of Medical Sciences in China (animal license No. SCXK (Jing) 2006-0008). The mice were raised in animal housing for 1 week, without exposure to strong light, at 22°C and 50% humidity under a 12-hour day/night cycle. Animals were fed normal diets and allowed free access to water.
Drugs
Rutaecarpine is extracted from the traditional Chinese medicine Tetradium ruticarpum. It is a white or pale yellow crystalline substance that has effects on cardiovascular function, platelet aggregation and thrombosis. It is also anti-inflammatory, analgesic, antihypoxic, anticancer, and can influence body temperature regulation. Rutaecarpine (98.5%, batch No. 20110611) was supplied by Nanjing Zelang Pharmaceutical Technology Co., Ltd., Nanjing, China.
Methods
Preparation of cerebral ischemia reperfusion model
Each rutaecarpine group was intraperitoneally injected with 84, 252 or 504 μg/kg rutaecarpine supplemented with dimethyl sulfoxide[9,13]. After drugs were administered for 7 consecutive days, the cerebral ischemia reperfusion model was established as previously described[32].
The sham surgery group and model group received intraperitoneal injection of 0.02 mL/10 g dimethyl sulfoxide daily. Mice were anesthetized with an intraperitoneal injection of 3.5% chloral hydrate (10 mL/kg). A 5–8 mm incision was made in the middle of the neckline to separate both sides of the carotid artery. Liquid paraffin was used to soak black and white surgical lines, one black line was put above the trachea and passed through both sides of the carotid artery, and a pine backhand knot was tied. Two white lines were taken above and across the black line on each side of the common carotid artery, and then the black surgical line was pulled tightly to block both sides of the common carotid artery to cause ischemia. The thread was maintained for 5 minutes and subsequently white lines were pulled on both sides to relax the black line and restore blood flow to the carotid artery. The incision was sutured. The sham surgery group received identical treatment except blood flow was not blocked. Blood flow was blocked in the remaining groups for 5 minutes[33].
Longa neurological symptom scores and criteria of model success
Neurological score was determined within 48 hours after cerebral ischemia reperfusion according to the method of Longa[15]. Neurological function was graded as previously described, grade 0: no symptoms; grade 1: when rats were suspended by the tail, at the time of injury, the contralateral forelimb could not unbend; grade 2: rotation to the injured side; grade 3: falling to the side; grade 4: no spontaneous activity or unconsciousness; grade 5: death. Five points are a full mark. The higher the score, the more serious the animal behavior disorder was. Every mouse received a score, and then a mean and standard deviation was calculated for every group. Mice graded 0 and dead mice were not included in the study. Mice graded 1 to 4 were considered a successfully established cerebral ischemia reperfusion model.
Learning and memory ability of mice detected with the step through test
At 48 hours after cerebral ischemia reperfusion, the step through test was measured with a SBA-2 programmed step through box (Institute of Materia Medica, Chinese Academy of Medical Sciences, Beijing, China), as previously described[34]. The SBA-2 programmed step through box has two boxes: a bright and a dark box. Each mouse was placed in the bright box. A 36V power source was connected to the dark box, which was also connected to a timer. This timer was used to induce an electric shock as soon as the mouse entered the dark room. The timer would simultaneously stop the clock[35,36]. A PCLab Biological Signal Acquisition System (Micro-channel StarTech Beijing Science and Technology Development Co., Ltd., Beijing, China) was used to collect the signals. Twenty-four hours later, the test was conducted in the same way. The latency and error count of the animals entering the dark room within 180 seconds were recorded. The interval between entry into the light box and entry into the dark box represented the latency (maximum 180 seconds), and the time to enter the dark box represented the error count during 180 seconds.
Detection of biochemical indexes in mouse brain
After the step through test, an overdose of chloral hydrate was administered to each mouse. The mice were sacrificed and the brains were removed. The cerebellum and olfactory bulb were quickly taken out and accurately weighed. The brains were rinsed in 4°C normal saline, and placed on filter paper to drain excess water. Subsequently, a nine-fold volume of pre-cooled normal saline (weight/volume) was added, and brain tissue was homogenized using a high-speed homogenizer. The homogenate was centrifuged at a speed of 68.6 × g for 15 minutes. The supernatant was then taken to an alternate 0.5 mL tube. Superoxide dismutase and glutathione peroxidase activity, as well as malondialdehyde content and total protein in brain tissues, were measured according to individual kit instructions[37,38]. All kits were purchased from Nanjing Jiancheng Bioengineering Institute, Nanjing, China.
Detection of motor function of mice using the inclined board test
Motor function was measured by inclined board test. The incline angle was the major index. After neurological scoring, mice were placed on a board (25 cm × 15 cm) in accordance with the Yonemori method[39]. The board was then slowly tilted from the flat to vertical, until mice dropped from the board. A lower inclined angle indicates that cerebral ischemic reperfusion injury is more severe (Figure 4).
Figure 4.

A schematic view of the inclined angle.
The maximum angle was the inclined angle. The vertical distance from the desktop to the highest point of the board was measured, and recorded as X. The length of the board is Y, which is 25 cm. The inclined angle was recorded as α, so sinα = X/Y; α can be calculated by the inverse function of sinα. This experiment was repeated three times in each mouse, and the average value of the inclined angle was calculated. A lower inclined angle indicates that the loss of motor function in mice is more severe.
Determination of infarct volume and brain water content
Infarct volume was measured using the 2,[3]5-triphenyl tetrazolium chloride method[40,41,42,43,44]. Briefly, after an overdose of chloral hydrate, mice were sacrificed and brains were quickly removed and accurately weighed. Each brain was cut into 2-mm coronal slices, giving five sections in total. Sections were incubated in 2% 2,3,5-triphenyl tetrazolium chloride (Sigma, St Louis, MO, USA; Shanghai Chemical Reagent Company Import repacking, Shanghai, China, batch No. 110710) at 37°C for 30 minutes and then fixed with 10% formalin for 12 hours. Changes in color of brain tissue were observed. Normal brain tissue was red, while infarcted brain tissue was pale.
The image was scanned by Image J analysis software and the infarct volume was measured. The percentage of infarcted brain tissue was calculated by infarct volume/total cerebral volume × 100%[45,46,47]. After the measurement of infarct volume, brain tissue was dried in an oven at 100°C for 24 hours and reweighed to obtain the dry weight. Brain water content was expressed as a percentage of the wet mass = (wet weight–dry weight)/wet weight × 100%[48,49,50].
Statistical analysis
All data were statistically analyzed using SPSS 16.0 software (SPSS, Chicago, IL, USA), and were expressed as mean ± SD. One-way analysis of variance and Dunnett's t-test were used for comparison of the difference between groups. A value of P < 0.05 was considered statistically significant.
Research background: Cerebral ischemia reperfusion injury is a common clinical pathological process. This type of injury often appears after recanalization, which is used in relieving tumor compression, cardiocerebral resuscitation, and cerebral embolism. Cerebral ischemia reperfusion injury is a serious threat to the lives of patients. At present, there are no precise methods for treating cerebral ischemia reperfusion injury. Rutaecarpine is extracted from the traditional Chinese medicine Tetradium ruticarpum. It has effects on cardiovascular function, platelet aggregation and thrombosis, and is also anti-inflammatory, analgesic, antihypoxic, anticancer, and is involved in body temperature regulation.
Research frontiers: The pathological process of cerebral ischemia reperfusion injury is very complex and not yet clear. It is generally believed that the pathological process involves a number of aspects including disorder in brain tissue energy metabolism, toxicity of excitatory amino acids, intracellular calcium overload, free radical injury, inflammatory reaction, the cytotoxicity of nitric oxide, and abnormal blood-brain barrier permeability. In recent years, nitric oxide-induced cell toxicity and abnormal blood-brain barrier permeability, free radical injury, and the inflammatory reaction have been areas of focus in research concerning the mechanisms of cerebral ischemia reperfusion injury.
Clinical significance: This study provides a novel treatment of cerebral ischemia reperfusion injury, and has provided a theoretical basis for the application of rutaecarpine in the treatment of cerebrovascular diseases.
Academic terminology: Cerebral ischemia reperfusion injury refers to severe brain dysfunction when blood supply returns to the tissue after a period of brain tissue ischemia.
Peer review: The study investigates the neuroprotective effect of rutaecarpine on cerebral ischemia reperfusion injury in mice, focusing on the effects of rutaecarpine on free radical injury and pathological changes. This study is innovative and somewhat advanced.
Footnotes
Funding: This study was financially supported by Zhangjiakou Science and Technology Commission Foundation, No. 1021095D; and the Hebei North University Foundation, No. Q2010018.
Conflicts of interest: None declared.
Ethical approval: The use of animals was approved by the Institutional Animal Care and Use Committee of Hebei North University in China.
REFERENCES
- [1].Tian JZ, Wang YY, Haworth J, et al. The review of research on vascular dementia. Beijing Zhongyiyao Daxue Xuebao. 1997;38(4):2–7. 72. [Google Scholar]
- [2].Kanoski SE, Meisel RL, Mullins AJ, et al. The effects of energy-rich diets on discrimination reversal learning and on BDNF in the hippocampus and prefrontal cortex of the rat. Behav Brain Res. 2007;182(1):57–66. doi: 10.1016/j.bbr.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Cheng ZC, Sun BL, Yang MF, et al. Changes of cerebral blood flow following subarachnoid hemorrhage after blockade of cerebral lymphatic drainage and the influence of ginkgolide and ginkgetin. Zhongguo Weixunhuan. 2009;13(5):355–358. [Google Scholar]
- [4].Paciaroni M, Caso V, Agnelli G. The concept of ischemic penumbra in acute stroke and therapeutic opportunities. Eur Neurol. 2009;61(6):321–330. doi: 10.1159/000210544. [DOI] [PubMed] [Google Scholar]
- [5].Kaushal V, Schlichter LC. Mechanisms of microglia mediated neurotoxicity in a new model of the stroke penumbra. J Neurosci. 2008;28(9):2221–2230. doi: 10.1523/JNEUROSCI.5643-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Jain KK. Neuroprotection in cerebrovascular disease. Exp Opin Invest Drugs. 2000;9:695–711. doi: 10.1517/13543784.9.4.695. [DOI] [PubMed] [Google Scholar]
- [7].Yu Q, Guo C, Cheng Z. Current advances in the study on rutaecarpine. Yaoxue Shijian Zazhi. 2007;25(6):353–357. [Google Scholar]
- [8].Hu CP, Li YJ. Research progress in pharmacological actions of evodiamine and rutaecarpine. Zhongguo Yaolixue Tongbao. 2003;19(10):1084–1087. [Google Scholar]
- [9].Hu CP, Li NS, Xiao L, et al. Involvement of capsaicin-sensitive sensory nerves in cardioprotection of rutaecarpine in rats. Zhongnan Yaoxue. 2003;1(2):67–70. doi: 10.1016/s0167-0115(03)00087-9. [DOI] [PubMed] [Google Scholar]
- [10].Liu Y, Cui YP, Song T, et al. Rutaecarpine ameliorates cerebral ischemia-reperfusion injury by stimulating calcitonin gene-related peptide release in the rat brain. Zhongguo Yishi Zazhi. 2005;7(5):589–591. [Google Scholar]
- [11].Li Y, Qian XH, Zhang RF, et al. A clinical analysis of 102 cases with coronary heart disease and brain infarction. Nao yu Shenjing Jibing Zazhi. 1997;5(3):159–161. [Google Scholar]
- [12].Zeng JY, Li YR, Chen JM. Clinical analysis of 36 cerebral infarction patients accompanied with coronary heart disease. Zhongguo Xiandai Yixue Zazhi. 1993;3(3):5–6. 78. [Google Scholar]
- [13].Tang JS, Pei QH. Neuroprotective role of panax notoginseng saponins on cerebral ischemia-reperfusion injury in rats. Zhongguo Shiyan Fangjixue Zazhi. 2011;17(15):210–213. [Google Scholar]
- [14].Himori N, Watanabe H, Akaike N, et al. Cerebral ischemia model with conscious mice: involvement of NMDA receptor activation and derangement of learning 0and memory ability. J Pharmacol Methods. 1990;23(4):311–327. doi: 10.1016/0160-5402(90)90059-t. [DOI] [PubMed] [Google Scholar]
- [15].Longa EZ, Weinstein PR, Carlson S, et al. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20(1):84–91. doi: 10.1161/01.str.20.1.84. [DOI] [PubMed] [Google Scholar]
- [16].Tao LL, Liu K. Experimental research progress on chinese medicine monome protection of cerebral ischemia reperfusion injury. Shizhen Guoyi Guoyao. 2012;23(12):3107–3109. [Google Scholar]
- [17].Hu XM, Zhou MM, Hu XM, et al. The Effects of sodium β-aescinate on inflammatory process induced by focal cerebral ischemia-reperfusion in rats. Zhongguo Yaolixue Tongbao. 2006;21(4):436–440. [Google Scholar]
- [18].Fang SD, Zhu YS. Recent advances in pathophysiology mechanisms of cerebral ischemic reperfusion injury. Yixue Zongshu. 2006;12(18):1114–1116. [Google Scholar]
- [19].Li D, Luo D, Guo R, et al. Calcitonin gene-related peptide mediated depressor effect of rutaecarpine in spontaneously hypertensive rats. Zhongnan Yaoxue. 2007;5(5):420–423. [Google Scholar]
- [20].Duan X, Ling F. The Pharmacologic action of rutacarpine. Zhonghua Zhongyiyao Xuekan. 2007;25(9):1857–1859. [Google Scholar]
- [21].Ran YH, Wang H. Lptakalim, an ATP-sensitive potassium channel opener, confers neuroprotection against cerebral ischemia/reperfusion injury in rats by protecting neurovascular unit cells. Zhejiang Daxue Xuebao B: Shengwu Yixue yu Shengwu Jishu. 2011;12(10):835–845. doi: 10.1631/jzus.B1100067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Shen JG. Reactive nitrogen species: dual roles for blood brain barrier disruption and brain repairs in cerebral ischemia and reperfusion injury. Acta Biophysica Sinica. 2012;28(4):295–306. [Google Scholar]
- [23].Gunther A. Reduced activation hyperbaric volume and differential effects on glial cell treatment in rat permanent focal cerebral ischaemia. Eur J Neurosci. 2005;21(11):3189–3194. doi: 10.1111/j.1460-9568.2005.04151.x. [DOI] [PubMed] [Google Scholar]
- [24].Zhan HT, Wu JF, Li HY, et al. Protective effect effect of flavonoids flavonoids from polygala polygala hongkongensis on the blood blood brain brain barrier barrier in rats rats with focal focal cerebral cerebral ischemia ischemia reperfusion reperfusion. Zhongfeng yu Shenjing Jibing Zazhi. 2012;29(11):1004–1007. [Google Scholar]
- [25].Yuan Y, Yang JQ, Zhou QX. Level of free radicals and expression of SOD-1 in neuronal degeneration mice due to cerebral ischemia-reperfusion. Disan Junyi Daxue Xuebao. 2011;33(12):1211–1215. [Google Scholar]
- [26].Ye NH, Lin YY, Liu ST, et al. Protection of fusion protein PTD-SOD by oral on rats of focal cerebral ischemia/reperfusion injury. Zhongguo Xiandai Yingyong Yaoxue. 2011;28(7):602–606. [Google Scholar]
- [27].Li ZY, Liu B, Yu J, et al. Ischaemic postconditioning rescues brain injury caused by focal ischaemia/reperfusion via attenuation of protein oxidization. J Int Med Res. 2012;40(3):954–966. doi: 10.1177/147323001204000314. [DOI] [PubMed] [Google Scholar]
- [28].Shen LH, Ye M, Ding XS. Protective effects of MCI-186 on transplantation of bone marrow stromal cells in rat ischemic stroke model. Neuroscience. 2012;223:315–324. doi: 10.1016/j.neuroscience.2012.08.001. [DOI] [PubMed] [Google Scholar]
- [29].Chen L, Liang JH, Wei LY. Protective effect of danhong Injection on cerebral ischemia-reperfusion injury in rats. Zhongguo Dongmai Yinghua Zazhi. 2012;20(3):239–242. [Google Scholar]
- [30].Warner DS, Sheng H, Batinic-Haberle I. Oxidants, antioxidants and the ischemic brain. J Exp Biol. 2004;207(18):3221–3231. doi: 10.1242/jeb.01022. [DOI] [PubMed] [Google Scholar]
- [31].Harukuni I, Bhardwaj A. Mechanisms of brain injury after global cerebral ischemia. Neurol Clin. 2006;24:1–21. doi: 10.1016/j.ncl.2005.10.004. [DOI] [PubMed] [Google Scholar]
- [32].Zhang XT, Liang J, Liu HX, et al. Protective effects of ginkgolide N on focal cerebral ischemia reperfusion injury in rats. Zhongguo Shiyan Fangjixue Zazhi. 2012;18(1):141–144. [Google Scholar]
- [33].Ma SW, Zhang XT, He SJ. Protective effects of ginkgolide B on cerebral ischemia reperfusion injury in rats. Zhongguo Yaoxue Zazhi. 2011;46(13):993–997. [Google Scholar]
- [34].Lee JH, Kim KY, Lee YK, et al. Cilostazol prevents focal cerebral ischemic injury by enhancing casein kinase 2 phosphorylation and suppression of phosphatase and tensin homolog deleted from chromosome 10 phosphorylation in rats. J Pharmacol Exp Ther. 2004;308(3):896–903. doi: 10.1124/jpet.103.061853. [DOI] [PubMed] [Google Scholar]
- [35].Liang H, Wang B, Li WP, et al. Protective effects of Tea ployphenol injection on cerebral ischemia reperfusion injury in mice. Dalian Yike Daxue Xuebao. 2009;26(3):112–115. [Google Scholar]
- [36].Liu D, Wang X, Luo ZY, et al. A study on the effect of acupuncture on the learning and memory abilities of chronic cerebral ischemia. Zhongyiyao Xuebao. 2011;39(1):61–62. [Google Scholar]
- [37].Dai Z, Xiao J, Liu SY, et al. Rutaecarpine inhibits hypoxia/reoxygenation induced apoptosis in rat hippocampal neurons. Neuropharmacology. 2008;55(8):1307–1312. doi: 10.1016/j.neuropharm.2008.08.030. [DOI] [PubMed] [Google Scholar]
- [38].Jiang LY, Tang SS, Wang XY. PPARγ agonist pioglitazone reverses memory impairment and biochemical changes in a mouse model of type 2 diabetes. Zhongguo Yaolixue yu Dulixue Zazhi. 2012;26(3):437. doi: 10.1111/j.1755-5949.2012.00341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Yonemori F, Yamaguchi T, Yamada H, et al. Evaluation of a motor deficit after chronic focal cerebral ischemia in rats. J Cereb Blood Flow Metab. 1998;18(10):1099–1106. doi: 10.1097/00004647-199810000-00006. [DOI] [PubMed] [Google Scholar]
- [40].Jiao CA, Song B, Chen ZW. Effects of exogenous endothelium-dependent hyperpolarizing factor on cerebral ischemia reperfusion injury. Zhongguo Linchuang Yaolixue yu Zhiliaoxue. 2012;17(6):648–653. [Google Scholar]
- [41].Zausinger S, Hungerhuber E, Baethmann A, et al. Neurological impairment in rats after transient middle cerebral artery occlusion:a comparative study under varioustreatment paradigms. Brain Res. 2000;86(3):94–105. doi: 10.1016/s0006-8993(00)02100-4. [DOI] [PubMed] [Google Scholar]
- [42].Yu XY, Zhong Y, Zuo CX, et al. Effects of hydroxyethylpuerarin on focal brain ischemia/reperfusion oxidant injury in rats. Zhongguo Shenghua Yaowu Zazhi. 2012;33(1):4–8. [Google Scholar]
- [43].Bederson JB, Pitts LH, Germano SM, et al. Evaluation of 2,3,5-triphenyltrazolium chloride as a stain for detection andquantification of experimental cerebral infarction in rats. Stroke. 1986;17(6):1304–1308. doi: 10.1161/01.str.17.6.1304. [DOI] [PubMed] [Google Scholar]
- [44].Li Q, Li Z, Xu XY, et al. Neuroprotective properties of picroside II in a rat model of focal cerebral ischemia. Int J Mol Sci. 2010;11(11):4580–4590. doi: 10.3390/ijms11114580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Chang Y, Hsieh CY, Peng ZA, et al. Neuroprotective mechanisms of puerarin in middle cerebral artery occlusion-induced brain infarction in rats. J Biomed Sci. 2009;16(9):1–13. doi: 10.1186/1423-0127-16-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Wu XA, Fu GF, Dou MQ, et al. Protective effects of xinnaotong oral liquid on global cerebral ischemia reperfusion injury. Jiefangjun Yaoxue Xuebao. 2012;28(2):113–115. [Google Scholar]
- [47].Scott W, Kirkland Y, Adrian K, et al. Delayed recovery and exaggerated infarct size by post-lesion stress in a rat model of focal cerebral stroke. Brain Res. 2008;1201:151–160. doi: 10.1016/j.brainres.2008.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Ma XH, Lv FR, Lv FJ, et al. Evaluating rat model of acute cerebral ischemia-reperfusion by computed tomography perfusion imaging. Disan Junyi Daxue Xuebao. 2009;31(6):506–509. [Google Scholar]
- [49].Wang DM, Zhao SZ, Feng WY, et al. Protection effect of Yinao Huoxue capsules on cerebral ischemia. Huaxi Yaoxue Zazhi. 2004;19(6):427. [Google Scholar]
- [50].Gupta YK, Briyal S, Sharma U. Effect of endothelin antagonist(TAK-044) on cerebral ischemic volume, oxidative stress markers and neurobehavioral parameters in the middle cerebral artery occlusion model of stroke in rats. Life Sci. 2005;77(1):15–27. doi: 10.1016/j.lfs.2004.11.025. [DOI] [PubMed] [Google Scholar]


