Keywords: neural regeneration, traditional Chinese medicine, combination of He-Mu points, electroacupuncture, irritable bowel syndrome, visceral hypersensitivity, P2X4 receptor, acupuncture, grants-supported paper, neuroregeneration
Abstract
Electroacupuncture at Shangjuxu (ST37) and Tianshu (ST25) was reported to improve visceral hypersensitivity in rats. Colorectal distension was utilized to generate a rat model of chronic visceral hypersensitivity in irritable bowel syndrome. Results showed that abdominal withdrawal reflex scores noticeably increased after model establishment. Simultaneously, P2X4 receptor immureactivity significantly increased in the colon and spinal cord. Electroacupuncture and pinaverium bromide therapy both markedly decreased abdominal withdrawal reflex scores in rats with visceral hypersensitivity, and significantly decreased P2X4 receptor immunoreactivity in the colon and spinal cord. These data suggest that electroacupuncture treatment can improve visceral hypersensitivity in rats with irritable bowel syndrome by diminishing P2X4 receptor immunoreactivity in the colon and spinal cord.
INTRODUCTION
Irritable bowel syndrome is a common chronic functional intestinal disease, mainly presenting with abdominal pain and abnormal defecation, but minimal morphological and biochemical changes[1]. Irritable bowel syndrome belongs to the category of diarrhea, constipation and abdominal pain in Chinese medicine[2]. Presently, the onset mechanism of irritable bowel syndrome remains unclear. Chronic visceral hypersensitivity has been shown to be the main pathophysiological mechanism underlying abdominal pain in patients with irritable bowel syndrome[3,4]. Moreover, irritable bowel syndrome patients extensively suffer from chronic visceral hypersensitivity, with involvement of all levels of the brain-gut axis, as well as various neurotransmitters. Chronic visceral hypersensitivity can occur in the periphery, spinal cord and central nervous system[5,6,7]. P2 purinergic receptor family members are membrane receptors expressed on various cells, and can selectively combine with extracellular adenosine triphosphate to produce a wide range of biological effects. The P2X receptor is a ligand-gated ion channel that plays an important role in visceral pain, and has been extensively investigated. Acupuncture has been shown to be an effective method for treatment of irritable bowel syndrome[8,9]. However, the mechanism of action of acupuncture on irritable bowel syndrome, and whether acupuncture adjusts visceral hypersensitivity in rats and the potential mechanism, are unknown.
Adenosine triphosphate plays a regulatory role in visceral pain signal transduction and visceral hyperalgesia through P2X and P2Y receptors, and regulates intestinal movement and gastrointestinal secretion[10,11]. Adenosine triphosphate transfers intercellular information via P2 purinergic receptors[12,13]. Burnstock et al[14] proposed that purine signaling may explain the acupuncture mechanism. Adenosine triphosphate injected into human skin can stimulate sensory neurons[15]. A recent study confirmed that subcutaneous injection of adenosine triphosphate activated the P2X receptor in nerve endings[16], and contributed to the release of neurotransmitter from neurons[17]. Acupuncture was also reported to induce skin deformation by lifting, thrusting, and twirling, which did not injure cells, but caused release of adenosine triphosphate from various cell types including osteoblasts, endothelial cells, epithelial cells and glial cells[14]. The combination of thermal and electrical stimulation can reinforce the effects of acupuncture, and lead to the release of adenosine triphosphate. Signals enter the spinal cord through the dorsal root ganglion, and then reach the brain stem via interneurons, including motor neurons of the intestine, lung, heart, artery and reproductive system. Signals are also transmitted to the pain area of the cerebral cortex, resulting in pain suppression[18,19,20]. The analgesic effect of acupuncture may relate to binding of adenosine triphosphate with purinergic receptors of sensory nerve endings of the skin, causing activation of a signal conduction pathway and regulated pain perception in the cerebral cortex. Recent studies also suggest that P2X may participate in the occurrence of visceral hypersensitivity. For example, P2X4 mRNA expression was detected in the rat intestine, and P2X4 receptor expression was increased in the dorsal commissural nucleus and spinal cord posterior horn in rats with visceral noxious stimulation and colorectal distension-induced irritable bowel syndrome[21,22], indicating that P2X4 may be essential for the increase of visceral hypersensitivity in irritable bowel syndrome. Furthermore, Burnstock et al[14] reported that acupuncture therapy was effective for the treatment of visceral hypersensitivity. Thus, in the present study, we used acupuncture to regulate P2X4 receptor as a potential treatment for visceral hypersensitivity in irritable bowel syndrome patients.
Many studies have also examined the potential mechanisms of acupuncture in relieving visceral hypersensitivity. For example, acupuncture at Tianshu (ST25) and Shangjuxu (ST37) were reported to provide significant curative effects for irritable bowel syndrome[23]. Tianshu and Shangjuxu are acupoints indicated for intestinal diseases such as diarrhea and abdominal pain[24]. Tianshu belongs to Mu points, which are the twelve points located at the chest or abdomen, and which are closely related to the zang-fu organ. Shangjuxu belongs to the lower confluent point, which is the confluent point of the three yang meridians of the hand located at the lower extremities. A combination of He-Mu points refers to the clinical point selection methods that combine points of the lower He-sea point of six-fu-organs (a collective term for gall bladder, stomach, small intestine, large intestine, urinary bladder, and sanjiao) with the front Mu point. Yang and Yan[25] investigated the spectral peak of differential protein expression in the stomach after acupuncture at He point and Mu point using nano-two dimensional-liquid chromatography, and concluded that the therapeutic mechanism of acupuncture on stress ulcers may relate to a decrease in the contents of five types of polypeptides. Electroacupuncture at Shangjuxu has therapeutic effects on irritable bowel syndrome and reduces visceral hypersensitivity. Moreover, the effects were better than that of single electroacupuncture and single sham acupuncture, which was identical to the results of abdominal withdrawal reflex scores[26]. Acupuncture at Tianshu and Shangjuxu reduced visceral hypersensitivity in a rat model of colorectal stimulation-induced irritable bowel syndrome, elevated pain threshold and diminished abdominal withdrawal reflex scores in rats with visceral hypersensitivity[27,28]. Acupuncture exerted therapeutic effects by altering 5-hydroxytryptamine[29] and c-Fos gene[30] expression in the colon, spinal cord and brain of rats with irritable bowel syndrome, downregulating adrenocorticotropic hormone[31] levels, vasoactive intestinal peptide[32] and enkephalin[33] in the hypothalamus, and reducing the number of mast cells and substance P[34] and prokineticin-1/prokineticin receptor-1[35,36] expression in the mucous membrane of the colon. Positron emission tomography revealed that electroacupuncture at Tianshu also reduced abdominal pain, abdominal distension and abdominal discomfort by diminishing the glucose metabolic rate in the brain[37].
Only a few studies have addressed the regulatory effect of acupuncture on purinergic receptors (no studies for the P2X4 receptor), and it remains unclear whether Burnstock's hypothesis can be verified. In the present study, we sought to explore the mechanism of electroacupuncture at He-Mu points in the prevention and treatment of visceral hypersensitivity in irritable bowel syndrome. We examined: (1) whether the P2X4 receptor is involved in visceral hypersensitivity in irritable bowel syndrome, (2) whether the P2X4 receptor is expressed in the rat colon and spinal cord, and (3) the regulatory effect of acupuncture on P2X4 receptor expression. Pinaverium bromide has frequently been used to treat irritable bowel syndrome-related abdominal pain, defecation disorder and intestinal discomfort[38]. Thus, the present study utilized pinaverium bromide as a reference treatment.
RESULTS
Quantitative analysis of experimental animals
A total of 32 neonatal rats were equally and randomly assigned to control, model, electroacupuncture and pinaverium bromide groups. A rat model of chronic visceral hypersensitivity was produced in the model, electroacupuncture and pinaverium bromide groups. After model induction, rats of the electroacupuncture group underwent electroacupuncture bilaterally at Shangjuxu and Tianshu. Rats of the pinaverium bromide group were intragastrically administered pinaverium bromide. All rats were included in the final analysis.
Electroacupuncture at He-Mu points decreased chronic visceral hypersensitivity
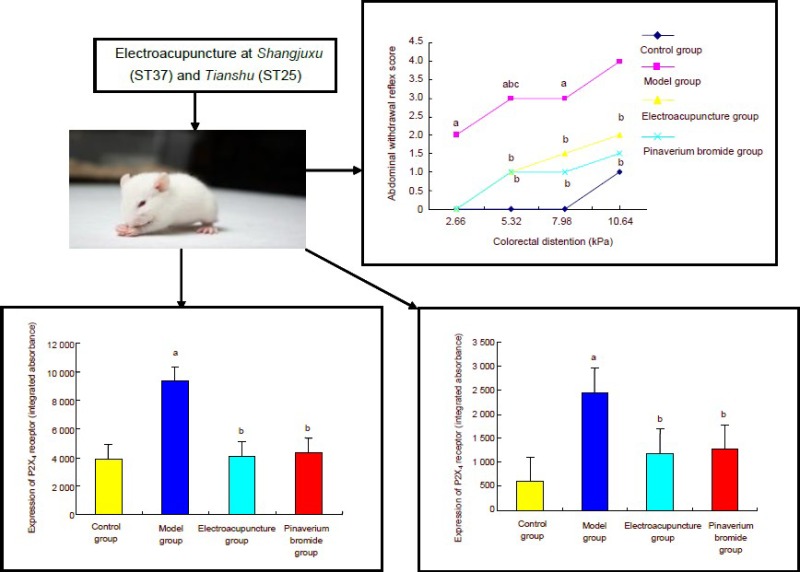
Abdominal withdrawal reflex scores were significantly increased with increasing colorectal distension (2.66, 5.32, 7.98 and 10.64 kPa; P < 0.01). After electroacupuncture at Shangjuxu and Tianshu, abdominal withdrawal reflex scores were significantly lower compared with the model group (P < 0.05), and were similar to those in rats undergoing intragastric administration of pinaverium bromide (P > 0.05; Figure 1).
Figure 1.
The effect of electroacupuncture at He-Mu points on abdominal withdrawal reflex (AWR) scores in rats with chronic visceral hypersensitivity.
As data were not normally distributed and the median values were used. Intergroup comparison was performed using the least significant difference t-test. aP < 0.01, vs. control group; bP < 0.05, vs. model group; cP < 0.01, vs. the previous time point.
Electroacupuncture at He-Mu points diminished P2X4 receptor immunoreactivity in the colon of rats with chronic visceral hypersensitivity
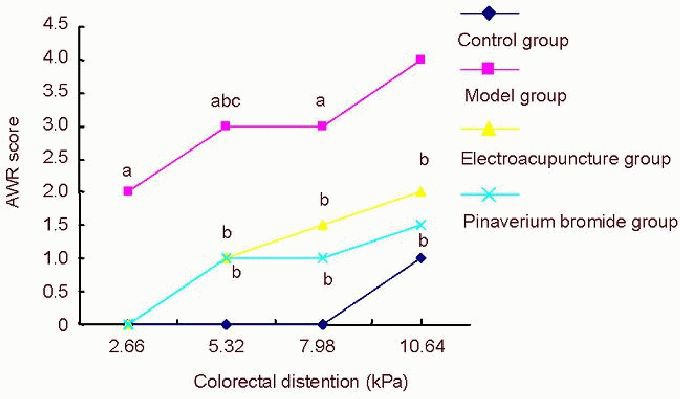
Immunohistochemistry revealed that P2X4 receptor immunoreactivity was increased in the colon of rats with irritable bowel syndrome (P < 0.01). Compared with the model group, P2X4 receptor immunoreactivity was significantly lower in the electroacupuncture and pinaverium bromide groups (P < 0.01; Figure 2).
Figure 2.
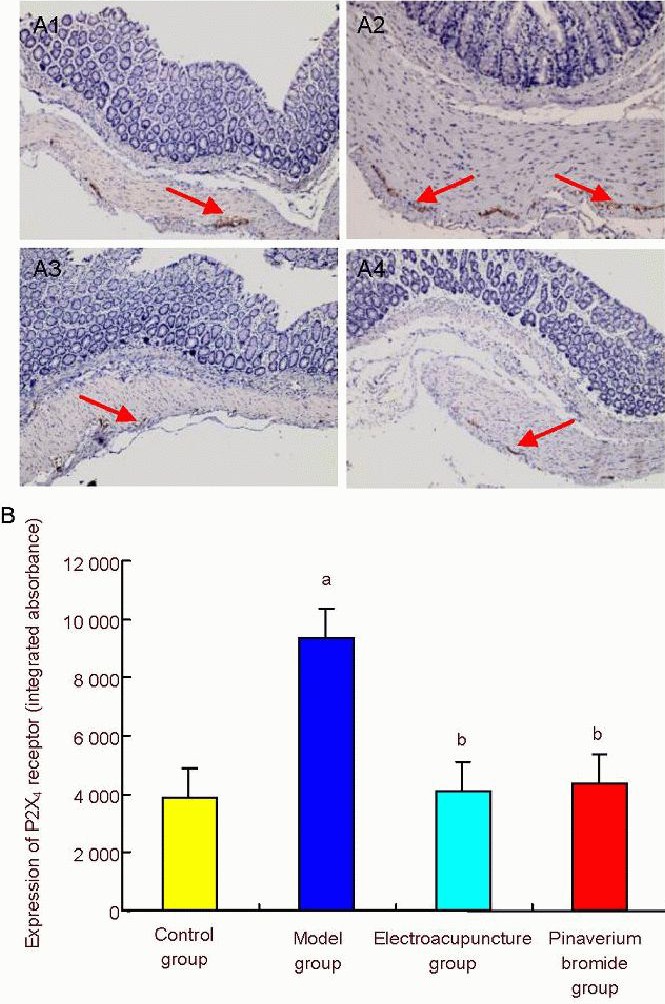
The effect of electroacupuncture at He-Mu points on P2X4 receptor immunoreactivity in the colon of rats with chronic visceral hypersensitivity.
(A) P2X4 receptor immunoreactivity in the rat colon (× 400). (A1-4) Control, model, electroacupuncture and pinaverium bromide groups, respectively. P2X4 receptor immunoreactivity increased in the model group, but significantly reduced in the electroacupuncture and pinaverium bromide groups. Arrows show P2X4 receptor immunoreactivity. (B) Semiquantitative analysis of P2X4 receptor immunoreactivity. Data are expressed as mean ± SD. Intergroup comparison was performed using Dunnett's t-test. aP < 0.01, vs. control group; bP < 0.01, vs. model group.
Electroacupuncture at He-Mu points diminished P2X4 receptor immunoreactivity in spinal cord tissue of rats with chronic visceral hypersensitivity
Immunohistochemistry revealed that P2X4 receptor immunoreactivity was increased in the spinal cord of rats with irritable bowel syndrome (P < 0.01). Compared with the model group, P2X4 receptor immunoreactivity was significantly lower in the electroacupuncture and pinaverium bromide groups (P < 0.01; Figure 3).
Figure 3.
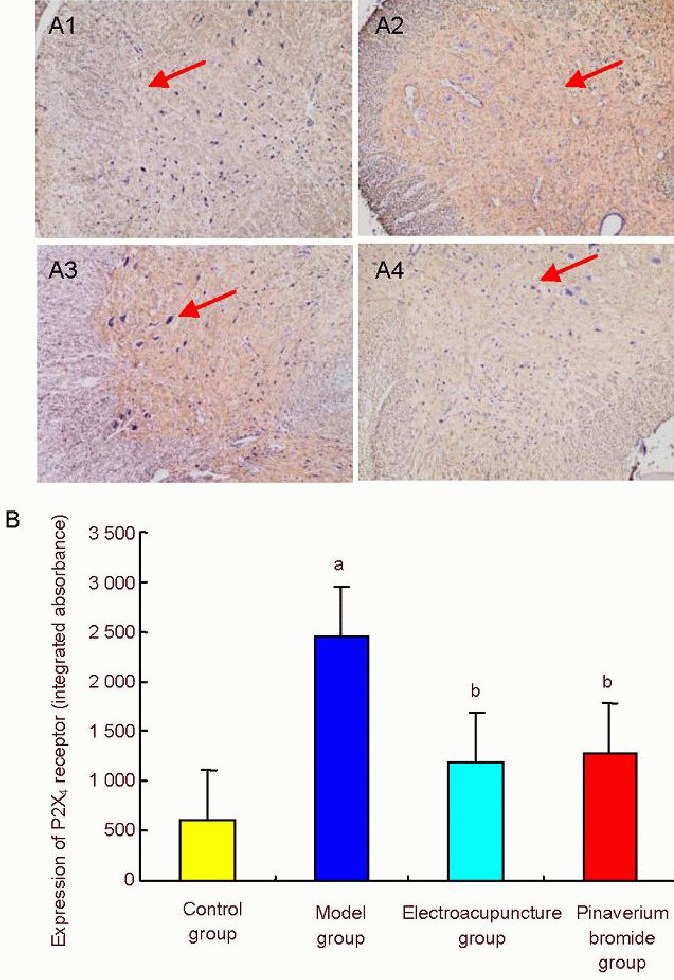
The effect of electroacupuncture at He-Mu points on P2X4 receptor immunoreactivity in the spinal cord of rats with chronic visceral hypersensitivity.
(A) Immunohistochemistry for P2X4 receptor immunoreactivity in the rat spinal cord (× 400). (A1-4) Control, model, electroacupuncture and pinaverium bromide groups, respectively. P2X4 receptor immunoreactivity was increased in the model group, but significantly reduced in the electroacupuncture and pinaverium bromide groups. Arrows show P2X4 receptor immunoreactivity. (B) Semiquantitative analysis of P2X4 receptor immunoreactivity. Data are expressed as mean ± SD. Intergroup comparison was performed using the least significant difference t-test. aP < 0.01, vs. control group; bP < 0.01, vs. model group.
DISCUSSION
Hypotonic pressure, electrostimulation and mechanical pressure are sensitive signals of abrupt release of adenosine triphosphate from cells[39]. Adenosine triphosphate transfers information via binding to membrane P2 purinergic receptors (P2X and P2Y) to produce extensive biological effects[40]. The P2X and P2Y receptor subtypes are determined according to their pharmacological properties. P2X receptors are ligand gated, nonselective, cation channels that contain seven subtypes (P2X1–7). After activation by adenosine triphosphate, these receptors are permeable to Na+, K+; and Ca2+. P2Y receptors are G protein coupled, and nine subtypes have been cloned from human tissues, including P2Y1–2, 4, 6, 11–15.
The P2X receptor is extensively expressed in cells of nerve tissue, smooth muscle, intestine, skin, bladder and myocardium of mammals[41,42]. Burnstock and Wood[43] proposed that adenosine triphosphate secreted from various cell types exerts effects on pain sensation by activating corresponding receptors on sensory nerve endings. In that study, epithelial cells of the ureter, bladder and intestine were confirmed to release adenosine triphosphate after distention stimulation. After activation, P2X receptors become permeable to Na+, K+ and Ca2+ ions, resulting in a rapid increase in intracellular Ca2+ concentration and activation of down-stream signaling[44]. Extracellular adenosine triphosphate binds to and activates the P2X4 receptor, resulting in afferent pain signals[45]. P2X receptors were also suggested to play a role in formation of visceral hypersensitivity, as P2X receptors, in particular P2X2 and P2X3 receptors, are involved in conduction and modulation of nociceptive information in both the peripheral and central nervous systems, and as P2X4 receptor expression in spinal cord microglia is associated with abnormal pain[46,47]. However, there are few studies examining the relationship of the P2X4 receptor with visceral pain. Wang and colleagues[48] verified that the P2X4 receptor is widely distributed in the rat nervous system. Ma et al[49] also reported that the P2X2 and P2X4 receptors were expressed on neurons and fibers extensively distributed in the rat medulla, suggesting a binding site for the regulatory effects of adenosine triphosphate on cardiovascular activity, respiratory activity and sensation of pain. Furthermore, Kanazawa et al[4] found that P2X4 expression was increased in the spinal cord posterior horn in patients with irritable bowel syndrome. Low-grade inflammation in the intestinal tract with irritable bowel syndrome can activate spinal cord microglia, resulting in upregulated P2X4 receptor expression and cell activation. Simultaneously, activation of the P2X4 receptor by adenosine triphosphate released from sensory neurons produced an inward current and an increase in intracellular Na+ and Ca2+ concentrations[50]; this signal was transmitted to down-stream signaling molecules by activating P38MAPK, resulting in visceral hypersensitivity. Finally, low-dose ketamine was reported to inhibit microglial activation, reduce P2X4 receptor expression and diminish visceral hypersensitivity[51].
Recent studies have confirmed that acupuncture exhibits therapeutic effects on ulcerative colitis[52,53,54,55], Crohn disease[56,57,58] and irritable bowel syndrome[23] 59]. Tianshu, the Front-Mu point of the large intestine, and Shangjuxu, the Lower He-Sea point of the large intestine, have been widely used in the clinic[60,61]. Different manipulations or quantity of stimulus have positive regulatory effects on gastrointestinal functions, and show good therapeutic effects on diarrhea or constipation[60,61]. Previous studies demonstrated that acupuncture at Tianshu and Shangjuxu produces strong effects on irritable bowel syndrome, and can regulate immune function of the patients[62,63]. Experimentally, acupuncture at Tianshu and Shangjuxu effectively diminished abdominal withdrawal reflex scores in rats with visceral hypersensitivity in irritable bowel syndrome, elevated pain threshold and regulated expression of multiple cytokines[64].
In summary, we found that visceral hypersensitivity was increased in rats with irritable bowel syndrome, with concurrent elevation in P2X4 receptor expression in the colon and spinal cord. The effects of acupuncture at Tianshu and Shangjuxu on visceral hypersensitivity were comparable to those of Western medicine, as both acupuncture and Western medicine effectively improved the clinical symptoms in this model. Acupuncture at He-Mu points also effectively reduced P2X4 receptor expression in the colon and spinal cord of rats with visceral hypersensitivity, suggesting a potential mechanism of action of treatment of visceral hypersensitivity in irritable bowel syndrome.
MATERIALS AND METHODS
Design
A completely randomized controlled animal study.
Time and setting
Model preparation and treatment were conducted in 2011 at the Experimental Animal Center of Shanghai Medical College of Fudan University in China. Sample collection and index detection were performed at the Third-Level Laboratory of Acupuncture and Immunity, State Administration of Traditional Chinese Medicine of the China in 2011.
Materials
Experimental animals
A total of 32 male specific pathogen-free Sprague-Dawley neonatal rats aged 8 days were provided by the Experimental Animal Center of Shanghai Medical College of Fudan University in China. Groups of eight neonatal rats were housed with a lactating rat in a quiet laboratory at 22 ± 2°C and humidity of 60 ± 5% with a 14 hour light/10 hour dark cycle, avoiding strong light. Lactating rats were allowed free access to food and water. All protocols were in accordance with Guidance Suggestions for the Care and Use of Laboratory Animals, for-mulated by the Ministry of Science and Technology of China[65].
Drugs
Pinaverium bromide tablets were purchased from Solvay (Shanghai) Company, Ltd., China, approval No. H20080414 (50 mg/tablet, lot No. 613173). Pinaverium bromide was suspended in double distilled water, and made into 5 mg/mL pinaverium bromide suspension.
Methods
Establishment of rat models of visceral hypersensitivity in irritable bowel syndrome
A rat model for visceral hypersensitivity in irritable bowel syndrome was induced by performing colorectal distension daily in 8–21 day-old rats according to a previously published method[66]. An in-house made saccule of 20.0 mm length and 3.0 mm diameter was slowly inserted into the descending colon. Saccule distension (0.2 mL) was performed for 1 minute. Air was exhausted and the saccule was pulled out. One hour later, the same stimulus was repeated. After stimulation, the rats were housed for 2–3 weeks. Stool character was observed and abdominal withdrawal reflex was scored to confirm the success of model induction.
Electroacupuncture treatment
After model establishment, in the electroacupuncture group, electroacupuncture was performed at Tianshu (0.2 cm lateral to the intersection of upper 8/13 and lower 5/13 from xiphoid to symphysis) and Shangjuxu (intersection of upper 6/16 and lower 10/16 of lateral condyle of tibia and lateral malleolus, 0.1 cm lateral to crista anterior tibiae) at equal pace[67]. Needling depth was 5 mm. The disposable aseptic needles (performance standard No. GB2024-94, 0.25 mm diameter × 25 mm length) were purchased from Cloud & Dragon Medical Device Co., Ltd. (Wujiang, Jiangsu Province, China). A Han's acupoint nerve stimulator (model HANS100A; Nanjing Gensun Technology, Nanjing, Jiangsu Province, China) was used with a frequency of sparse and dense waves of 2/100 Hz, a current of 2 mA, for 20 minutes, once a day, for 7 consecutive days.
Abdominal withdrawal reflex scores
Colorectal distension was conducted within 60 minutes after the last electroacupuncture. The self-made saccule was connected to a T-valve. One end was connected to a 10 mL syringe and the other end was connected to a blood pressure monitor (model XJ11D; Shanghai Medical Instruments Co., Ltd., Shanghai, China). Stimuli at 2.66, 5.32, 7.98 or 10.64 kPa (20, 40, 60 or 80 mmHg, respectively) were given after the saccule was inserted. Each stimulus lasted for approximately 20 seconds with a five-minute interval. Each stimulus was repeated three times, and an average value (integer) was calculated as the final score. Reactions were scored in accordance with a previously published method[66]: 0 = no behavior reaction; 1 = movement standstill and transient head movement; 2 = contraction of abdominal muscles during stimuli; 3 = raised abdomen; 4 = body raised, elevation of pelvic cavity and scrotum.
Sample collection
The rats were anesthetized with 10% chloral hydrate (0.3 mL/100 g). The chest was opened, and 100 mL of saline was rapidly perfused into the ascending aorta through left ventricle intubation, followed by perfusion with PBS (pH 7.4) containing 4% paraformaldehyde. A total of 2–3 cm colon 5 cm above the anus and L6–S3 segment of the spinal cord were fixed in PBS (pH 7.4) supplemented with 4% paraformaldehyde, followed by paraffin imbedding and slicing (4 μm thick).
Immunohistochemistry for P2X4 receptor expression in rat colon and spinal cord
After dewaxing and hydrating, tissue was incubated in rabbit anti-rat P2X4 polyclonal antibody (1:400; Alomone Labs, Israel) at 37°C for 2 hours, and then washed in 0.01 mol/L PBS (pH 7.2–7.6) five times, for 5 minutes each. Using a goat anti-rabbit/rat immunohistochemistry kit (Gene Tech Co., Ltd., Shanghai, China), tissues were incubated with the A liquid for 30 minutes at room temperature in a wet box, washed in 0.01 mol/L PBS three times at 5 minutes each, followed by visualization in 3,3’-diaminobenzidine/H2O2. Staining time was controlled under a light microscope (Olympus, Tokyo, Japan). Staining was terminated by PBS 0.01 mol/L or distilled water. A wash with 0.01 mol/L PBS (pH 7.4) was performed between each step. Sections were mounted on gel-coated slides, dehydrated through a graded alcohol series, permeabilized with xylene, and then mounted. Images were collected from three fields randomly selected under a 400 × light microscope. The average value of integral absorbance was calculated using Motic Med 6.0 image analysis (Beijing Maikeaodi Image Technique Co., Ltd., Beijing, China).
Statistical analysis
All data were analyzed using SPSS 18.0 software (SPSS, Chicago, IL, USA). Normally distributed measurement data were presented as mean ± SD. Non-normally distributed data were presented as median values. One-way analysis of variance was employed for intergroup comparison if normally distributed and with homogenous variance. Non-parametric tests were used if heterogeneity of variance was present. Least significant difference t-test or Dunnett's t-test were utilized for paired comparison among multiple means. A value of P < 0.05 was considered statistically significant.
Research background: Numerous clinical studies have demonstrated that electroacupuncture at Shangjuxu (ST37) and Tianshu (ST25) improve irritable bowel syndrome and mitigate visceral hypersensitivity. It is assumed that acupuncture can signal the affected receptor of the local Shu point, which is transmitted to the center via cell signaling pathways to regulate the internal environment of the body.
Research frontiers: We hypothesized that the analgesic effect of acupuncture relates to adenosine triphosphate signaling via a purinergic receptor on sensory nerve endings of the skin, and subsequent activation of a signal conduction pathway resulting in adjustment of pain sensation in the cerebral cortex.
Clinical significance: Our investigation addressing the effects of electroacupuncture at He-Mu points on P2X4 receptor expression in the colon and spinal cord of rats with chronic visceral hypersensitivity verified that electroacupuncture could adjust purinergic receptor expression, providing theoretical evidence for electroacupuncture as a treatment strategy for irritable bowel syndrome and acupuncture analgesia.
Academic terminology: Abdominal withdrawal reflex score is a scoring method that measures visceral pain after colorectal distension with a saccule. The score includes five grades (0–4), with a higher score indicating higher sensitivity.
Peer review: Recent studies have reported that P2X4 receptor expression is increased in the colon and spinal cord after visceral noxious stimulation, indicating that P2X4 may be an important factor for enhanced visceral sensitivity. Our study found a potential pathogenetic mechanism of visceral hypersensitivity, and provided a target for clinical treatment strategies.
Acknowledgments
We thank Liu HR from the Shanghai Research Institute of Acupuncture and Meridian for help in manuscript writing.
Footnotes
Funding: This study was supported by the National Natural Science Foundation of China, No. 30973783; the Open Research Fund of Zhejiang First-foremost Key Subject--Acupuncture & Moxibustion, No. ZTK2010A01; and the scientific research grants of Shanghai Health Bureau, No. 2009209.
Conflicts of interest: None declared.
Ethical approval: This study was approved by the Animal Ethics Committee, Third Affiliated Hospital of Zhejiang Chinese Medical University, China.
(Reviewed by Dean J, Hindle A, Zhang L, Lan L)
(Edited by Yu J, Qiu Y, Li CH, Song LP, Liu WJ, Zhao M)
REFERENCES
- [1].Saito YA, Schoenfeld P, Locke GR., 3rd The epidemiology of irritable bowel syndrome in North America: a systematic review. Am J Gastroenterol. 2002;97(8):1910–1915. doi: 10.1111/j.1572-0241.2002.05913.x. [DOI] [PubMed] [Google Scholar]
- [2].Zhang SS, Li QG, Wei W, et al. Consensus on standard management of irritable bowel syndrome in TCM. Zhonghua Zhongyiyao Zazhi. 2010;25(7):1062–1065. [Google Scholar]
- [3].Kanazawa M, Hongo M, Fukudo S. Visceral hypersensitivity in irritable bowel syndrome. J Gastroenterol Hepatol. 2011;26(Suppl 3):119–121. doi: 10.1111/j.1440-1746.2011.06640.x. [DOI] [PubMed] [Google Scholar]
- [4].Keszthelyi D, Troost FJ, Masclee AA. Irritable bowel syndrome: methods, mechanisms, and pathophysiology. Methods to assess visceral hypersensitivity in irritable bowel syndrome. Am J Physiol Gastrointest Liver Physiol. 2012;303(2):G141–154. doi: 10.1152/ajpgi.00060.2012. [DOI] [PubMed] [Google Scholar]
- [5].Mathew P, Bhatia SJ. Pathogenesis and management of irritable bowel syndrome. Trop Gastroenterol. 2009;30(1):19–25. [PubMed] [Google Scholar]
- [6].Kang MX, Jia H. Progress in mechanisms of visceral hypersensitivity in irritable bowel syndrome. Shijie Huaren Xiaohua Zazhi. 2008;16(14):1554–1558. [Google Scholar]
- [7].Elsenbruch S. Abdominal pain in Irritable Bowel Syndrome: a review of putative psychological, neural and neuro-immune mechanisms. Brain Behav Immun. 2011;25(3):386–394. doi: 10.1016/j.bbi.2010.11.010. [DOI] [PubMed] [Google Scholar]
- [8].Liu B, Hu YM, Tenner SM. A randomized controlled trial of acupuncture for irritable bowel syndrome. Am J Gastroenterol. 2000;95(9):2498. [Google Scholar]
- [9].Chan J, Carr I, Mayberry JF. The role of acupuncture in the treatment of irritable bowel syndrome: a pilot study. Hepatogastroenterology. 1997;44(17):1328–1330. [PubMed] [Google Scholar]
- [10].Xu GY, Shenoy M, Winston JH, et al. P2X receptor-mediated visceral hyperalgesia in a rat model of chronic visceral hypersensitivity. Gut. 2008;57(9):1230–1237. doi: 10.1136/gut.2007.134221. [DOI] [PubMed] [Google Scholar]
- [11].Burnstock G. Purines and sensory nerves. Handb Exp Pharmacol. 2009;194:333–392. doi: 10.1007/978-3-540-79090-7_10. [DOI] [PubMed] [Google Scholar]
- [12].Burnstock G. Introductory overview of purinergic signalling. Front Biosci (Elite Ed) 2011;3:896–900. doi: 10.2741/e298. [DOI] [PubMed] [Google Scholar]
- [13].Burnstock G. The past, present and future of purine nucleotides as signalling molecules. Neuropharmacology. 1997;36(9):1127–1139. doi: 10.1016/s0028-3908(97)00125-1. [DOI] [PubMed] [Google Scholar]
- [14].Burnstock G. Puncturing the Myth: purinergic signaling, not mystical energy, may explain how acupuncture works. Scientist. 2011 Sep 01; [Google Scholar]
- [15].Bleehen T, Keele CA. Observations on the algogenic actions of adenosine compounds on the human blister base preparation. Pain. 1977;3(4):367–377. doi: 10.1016/0304-3959(77)90066-5. [DOI] [PubMed] [Google Scholar]
- [16].Zhang Q, Zhao Y, Guo Y, et al. Activation and sensitization of C and Adelta afferent fibers mediated by P2X receptors in rat dorsal skin. Brain Res. 2006;1102(1):78–85. doi: 10.1016/j.brainres.2006.05.040. [DOI] [PubMed] [Google Scholar]
- [17].Vial C, Roberts JA, Evans RJ. Molecular properties of ATP-gated P2X receptor ion channels. Trends Pharmacol Sci. 2004;25(9):487–493. doi: 10.1016/j.tips.2004.07.008. [DOI] [PubMed] [Google Scholar]
- [18].Burnstock G. Acupuncture: a novel hypothesis for the involvement of purinergic signalling. Med Hypotheses. 2009;73(4):470–472. doi: 10.1016/j.mehy.2009.05.031. [DOI] [PubMed] [Google Scholar]
- [19].Zhao ZQ. Neural mechanism underlying acupuncture analgesia. Prog Neurobiol. 2008;85(4):355–375. doi: 10.1016/j.pneurobio.2008.05.004. [DOI] [PubMed] [Google Scholar]
- [20].Yu XJ, Ding GH, Yao W, et al. The role of collagen fiber in “Zusanli” (ST 36) in acupuncture analgesia in the rat. Zhongguo Zhen Jiu. 2008;28(3):207–213. [PubMed] [Google Scholar]
- [21].Qin M, Wang JJ, Duan L, et al. The changes of p2x4 receptor expression in nucleus of tractus solitarius and the dorsal horn of thoracic cord after visceral pain induced by acetic acid. Shenjing Jiepou Xue Zazhi. 2006;22(5):551–554. [Google Scholar]
- [22].Wang JJ, Wang SZ, Xia DY, et al. Effects of expressions of P2X4 receptor in nerve center induced by the stimulation of colorectal distention in rats with irritable bowel syndrome. Weichang Bing Xue yu Gan Bing Xue Zazhi. 2008;17(10):813–818. [Google Scholar]
- [23].Wang W, Bai L, Gao ZX, et al. Acupuncture at Tianshu, Shangjuxu point in diarrhea-predominant irritable bowel syndrome. Zhongguo Wuzhen Xue Zazhi. 2008;26(8):6335–6336. [Google Scholar]
- [24].Zhang HS, Wang FC. Application of combination of he-mu points and combination of shu-yuan in syndrome differentiation of zang- and fu-organs. Zhongguo Zhen Jiu. 2006;26(5):378–380. [PubMed] [Google Scholar]
- [25].Yang B, Yan XK. Nano-2D-LC analysis of proteomic alterations in rats with gastric stress ulcer after acupuncture at He-Sea point and Front-Mu point of the stomach meridian. Shijie Huaren Xiaohua Zazhi. 2012;18(22):2355–2358. [Google Scholar]
- [26].Gao ZX, Wang W, Lv EJ, et al. Influence of electro-acupuncture on Shang-ju-xu on 5-HT and AWR in rats with visceralgia. Shanxi Zhongyi. 2010;26(4):53–55. [Google Scholar]
- [27].Zhou EH, Liu HR, Wu HG, et al. Suspended moxibustion relieves chronic visceral hyperalgesia via serotonin pathway in the colon. Neurosci Lett. 2009;451(2):144–147. doi: 10.1016/j.neulet.2008.12.026. [DOI] [PubMed] [Google Scholar]
- [28].Liu HR, Wang XM, Zhou EH, et al. Acupuncture at both ST25 and ST37 improves the pain threshold of chronic visceral hypersensitivity rats. Neurochem Res. 2009;34(11):1914–1918. doi: 10.1007/s11064-009-9972-1. [DOI] [PubMed] [Google Scholar]
- [29].Zhou EH, Liu HR, Wu HG, et al. Herb-partition moxibustion relieves chronic visceral hyperalgesia and 5-HT concentration in colon mucosa of rats. Neurol Res. 2009;31(7):734–737. doi: 10.1179/174313209X382313. [DOI] [PubMed] [Google Scholar]
- [30].Wang XM, Liu HR, Ding GH, et al. Effects of electroacupuncture on c-Fos expression in the spinal cord and brain of rats with chronic visceral hypersensitivity. Neural Regen Res. 2009;4(5):339–343. [Google Scholar]
- [31].Wu HG, Liu HR, Zhang ZA, et al. Electro-acupuncture relieves visceral sensitivity and decreases hypothalamic corticotropin-releasing hormone levels in a rat model of irritable bowel syndrome. Neurosci Lett. 2009;465(3):235–237. doi: 10.1016/j.neulet.2009.09.018. [DOI] [PubMed] [Google Scholar]
- [32].Wu HG, Jiang B, Zhou EH, et al. Regulatory mechanism of electroacupuncture in irritable bowel syndrome: preventing MC activation and decreasing SP VIP secretion. Dig Dis Sci. 2008;53(6):1644–1651. doi: 10.1007/s10620-007-0062-4. [DOI] [PubMed] [Google Scholar]
- [33].Yi T, Qi L, Wu HG, et al. Analgesic action of suspended moxibustion in rats with chronic visceral hyperalgesia correlates with enkephalins in the spinal cord. Neural Regen Res. 2012;7(3):219–222. doi: 10.3969/j.issn.1673-5374.2012.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Ma XP, Tan LY, Yang Y, et al. Effect of electro-acupuncture on substance P, its receptor and corticotropin-releasing hormone in rats with irritable bowel syndrome. World J Gastroenterol. 2009;15(41):5211–5217. doi: 10.3748/wjg.15.5211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Wu LY, Bao CH, Ge LB, et al. Mild moxibustion at Tianshu (ST 25) decreases expression of prokineticin-1 and prokineticin receptor-1 in colon tissue of rats with chronic visceral hyperalgesia. Neural Regen Res. 2011;6(33):2600–2604. [Google Scholar]
- [36].Zhao C, Qi L, Wu LY, et al. Suspended moxibustion at Tianshu (ST25) inhibits prokineticin 1 and prokineticin receptor 1 expression in the spinal cord of rats with chronic visceral hypersensitivity. Neural Regen Res. 2012;7(15):1145–1150. doi: 10.3969/j.issn.1673-5374.2012.15.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Liu HR, Qi L, Wang XL, et al. Electroacupuncture at Tianshu (ST 25) for diarrhea-predominant irritable bowel syndrome using positron emission tomography Changes in visceral sensation center. Neural Regen Res. 2010;5(16):1220–1225. [Google Scholar]
- [38].Zhan LX, Li ZK, Zou LW, et al. Assessment of efficacy of pinaverium bromide in treatment of irritable bowel syndrome and its effect on visceral motility and sensitivity. Zhonghua Xiaohua Zazhi. 2002;22(8):477–480. [Google Scholar]
- [39].Katsuragi T, Migita K. The mechanism of ATP release as an autocrine/paracrine molecule. Nihon Yakurigaku Zasshi. 2004;123(6):382–388. doi: 10.1254/fpj.123.382. [DOI] [PubMed] [Google Scholar]
- [40].Burnstock G. The past, present and future of purine nucleotides as signalling molecules. Neuropharmacology. 1997;36(9):1127–1139. doi: 10.1016/s0028-3908(97)00125-1. [DOI] [PubMed] [Google Scholar]
- [41].Illes P, Ribeiro JA. Neuronal P2 receptors of the central nervous system. Curr Top Med Chem. 2004;4(8):831–838. doi: 10.2174/1568026043451032. [DOI] [PubMed] [Google Scholar]
- [42].Burnstock G, Knight GE. Cellular distribution and functions of P2 receptor subtypes in different systems. Int Rev Cytol. 2004;240:31–304. doi: 10.1016/S0074-7696(04)40002-3. [DOI] [PubMed] [Google Scholar]
- [43].Burnstock G, Wood JN. Purinergic receptors: their role in nociception and primary afferent neurotransmission. Curr Opin Neurobiol. 1996;6(4):526–532. doi: 10.1016/s0959-4388(96)80060-2. [DOI] [PubMed] [Google Scholar]
- [44].Hewinson J, Mackenzie AB. P2X(7) receptor-mediated reactive oxygen and nitrogen species formation: from receptor to generators. Biochem Soc Trans. 2007;35(Pt 5):1168–1170. doi: 10.1042/BST0351168. [DOI] [PubMed] [Google Scholar]
- [45].Tsuda M, Ueno S, Inoue K. Evidence for the involvement of spinal endogenous ATP and P2X receptors in nociceptive responses caused by formalin and capsaicin in mice. Br J Pharmacol. 1999;128(7):1497–1504. doi: 10.1038/sj.bjp.0702960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Burnstock G. Purinergic P2 receptors as targets for novel analgesics. Pharmacol Ther. 2006;110(3):433–454. doi: 10.1016/j.pharmthera.2005.08.013. [DOI] [PubMed] [Google Scholar]
- [47].Burnstock G. Purine-mediated signalling in pain and visceral perception. Trends Pharmacol Sci. 2001;22(4):182–188. doi: 10.1016/s0165-6147(00)01643-6. [DOI] [PubMed] [Google Scholar]
- [48].Wang W, Wu SX, Li YQ. Localization of P2X4 receptor in the rat nervous system. Jiepou Xue Jinzhan. 2001;7(4):289–293. [Google Scholar]
- [49].Ma Y, Ning S, Lu N, et al. Expression of P2X2 and P2X4 receptors immunoreactivity in the medulla of neonatal and adult rats. Fudan Xuebao: Yixue Ban. 2006;33(5):666–670. [Google Scholar]
- [50].Cui Y, Chen Y, Zhi JL, et al. Activation of p38 mitogen-activated protein kinase in spinal microglia mediates morphine antinociceptive tolerance. Brain Res. 2006;1069(1):235–243. doi: 10.1016/j.brainres.2005.11.066. [DOI] [PubMed] [Google Scholar]
- [51].Zhou SQ, Yuan HB, Yan JQ, et al. Small-dose ketamine inhibit the expression of P2X4 receptor in cerebral cortex of the rats with chronic neuropathic pain. Linchuang Yixue Zazhi. 2009;37(4):548–551. [Google Scholar]
- [52].Wu HG, Zhou LB, Pan YY, et al. Study of the mechanisms of acupuncture and moxibustion treatment for ulcerative colitis rats in view of the gene expression of cytokines. World J Gastroenterol. 1999;5(6):515–517. doi: 10.3748/wjg.v5.i6.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Wu HG, Liu HR, Tan LY, et al. Electroacupuncture and moxibustion promote neutrophil apoptosis and improve ulcerative colitis in rats. Dig Dis Sci. 2007;52(2):379–384. doi: 10.1007/s10620-006-9561-y. [DOI] [PubMed] [Google Scholar]
- [54].Wu HG, Gong X, Yao LQ, et al. Mechanisms of acupuncture and moxibustion in regulation of epithelial cell apoptosis in rat ulcerative colitis. World J Gastroenterol. 2004;10(5):682–688. doi: 10.3748/wjg.v10.i5.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Wu HG, Zhou LB, Shi DR, et al. Morphological study on colonic pathology in ulcerative colitis treated by moxibustion. World J Gastroenterol. 2000;6(6):861–865. doi: 10.3748/wjg.v6.i6.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Shi Y, Zhou EH, Wu HG, et al. Moxibustion treatment restoring the intestinal epithelium barrier in rats with Crohn's disease by down-regulating tumor necrosis factor alpha, tumor necrosis factor receptor 1, and tumor necrosis factor receptor 2. Chin J Integr Med. 2011;17(3):212–217. doi: 10.1007/s11655-011-0669-3. [DOI] [PubMed] [Google Scholar]
- [57].Bao CH, Wu LY, Wu HG, et al. Moxibustion inhibits apoptosis and tumor necrosis factor-alpha/tumor necrosis factor receptor 1 in the colonic epithelium of Crohn's disease model rats. Dig Dis Sci. 2012;57(9):2286–2295. doi: 10.1007/s10620-012-2161-0. [DOI] [PubMed] [Google Scholar]
- [58].Bao CH, Wu LY, Shi Y, et al. Moxibustion down-regulates colonic epithelial cell apoptosis and repairs tight junctions in rats with Crohn's disease. World J Gastroenterol. 2011;17(45):4960–4970. doi: 10.3748/wjg.v17.i45.4960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Xiong JW. Clinical observation of acupuncture treatment on irritable bowel syndrome. Jiangsu Zhongyiyao. 2009;41(1):49–50. [Google Scholar]
- [60].Ma TA, Xu Z. Treatment efficacy of Tiaoqi acupuncture on habitual constipation. Zhongyuan Yikan. 2005;32(16):29–30. [Google Scholar]
- [61].Wang SL. Treatment of acupuncture at Xiahe on chronic dysentery. Henan Zhongyi. 2005;25(6):63. [Google Scholar]
- [62].Liu HR, Wu HG, Wang XL, et al. Clinical research of irritable bowel syndrome treated by electroacupuncture on Tianshu (ST 25) Zhenjiu Tuina Zazhi. 2007;5(2):91–94. [Google Scholar]
- [63].Liu HR, Hua XG, Yang Y, et al. Expression of 5-HT in colonic mucosa of diarrhea-predominant IBS and the clinical efficacy of acupuncture treatment. Liaoning Zhongyi Zazhi. 2006;33(8):953–954. [Google Scholar]
- [64].Zhou EH, Wang XM, Ding GH, et al. Suspended moxibustion relieves chronic visceral hyperalgesia and decreases hypothalamic corticotropin-releasing hormone levels. World J Gastroenterol. 2011;17(5):662–665. doi: 10.3748/wjg.v17.i5.662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].The Ministry of Science and Technology of the People's Republic of China. Guidance Suggestions for the Care and Use of Laboratory Animals. 2006 Sep 30; [Google Scholar]
- [66].Al-Chaer ED, Kawasaki M, Pasricha PJ. A new model of chronic visceral hypersensitivity in adult rats induced by colon irritation during postnatal development. Gastroenterology. 2000;119(5):1276–1285. doi: 10.1053/gast.2000.19576. [DOI] [PubMed] [Google Scholar]
- [67].Li ZR. China Press of Tradition Chinese Medicine; 2007. Experimental Acupuncture. [Google Scholar]


