Abstract
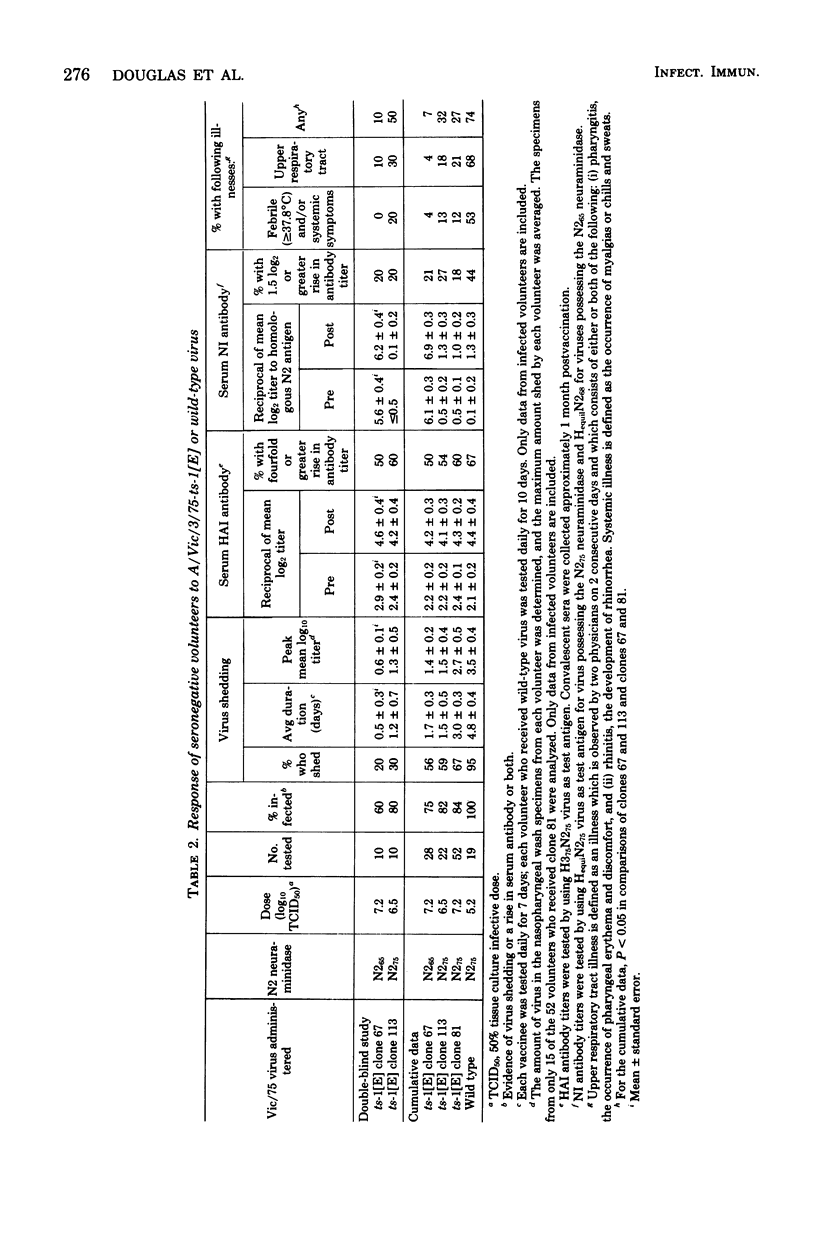
To explore the relationship between neuraminidase immunity and the degree of attenuatíon of live influenza A virus vaccines, a comparative evaluation of three Victoria/75-ts-1[E] (Vic/75-ts-1[E]) recombinant viruses in serum hemagglutination-inhibiting-negative (titer, ≤1:8) adult volunteers was performed. These three ts-1[E] viruses had a similar restriction of replication at 38°C in vitro, and each possessed the two attenuating genes of the ts-1[E] donor strain (13). However, Vic/75-ts-1[E] recombinants 81 and 113 possessed both Vic/75 hemagglutinin (H375) and Vic/75 neuraminidase (N275), whereas Vic/75-ts-1[E] recombinant 67 had Vic/75 hemagglutinin but the N265 neuraminidase. Vic/75-ts-1[E] recombinant 67 was significantly more attenuated than Vic/75-ts-1[E] recombinants 81 and 113 in that fewer local and systemic signs and symptoms of illness were observed in those volunteers who received clone 67. These findings were consistent with our previous observations which suggested that the following two factors contribute to the attenuation of ts-1[E] vaccine strains in adults: (i) the attenuating effect of the two ts-1[E] genes and (ii) the neuraminidase immunity of the host. Vic/75-ts-1[E] recombinant clone 67 vaccinees developed an immunological response to the H375 hemagglutinin in the absence of a response to the N275 neuraminidase. To assess the role that anti-hemagglutinin immunity induced by an attenuated live virus vaccine plays in resistance to influenza A virus, vaccinees who received recombinant 67 were challenged with Vic/75 wild-type virus, and their responses were compared with those of Vic/75-ts-1[E] vaccinees who received recombinant 81 or 113. Each of the three groups of ts-1[E] vaccinees was significantly protected against illness induced by wild-type virus infection, although resistance was not complete. However, the clone 67 vaccinees were protected less against infection. The infection-permissive resistance induced by clone 67 resembled that previously described for inactivated neuraminidase-specific vaccines. These results suggested that a ts-1[E] recombinant that possessed the hemagglutinin of a new pandemic variant, the neuraminidase of the preceding subtype, and the two ts-1[E] ts genes would be satisfactorily attenuated for children and adults with neuraminidase immunity and could induce resistance to illness caused by the new pandemic wild-type influenza A virus.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allan W. H., Madeley C. R., Kendal A. P. Studies with avian influenza A viruses: cross protection experiments in chickens. J Gen Virol. 1971 Aug;12(2):79–84. doi: 10.1099/0022-1317-12-2-79. [DOI] [PubMed] [Google Scholar]
- Couch R. B., Kasel J. A., Gerin J. L., Schulman J. L., Kilbourne E. D. Induction of partial immunity to influenza by a neuraminidase-specific vaccine. J Infect Dis. 1974 Apr;129(4):411–420. doi: 10.1093/infdis/129.4.411. [DOI] [PubMed] [Google Scholar]
- Kim H. W., Arrobio J. O., Brandt C. D., Parrott R. H., Murphy B. R., Richman D. D., Chanock R. M. Temperature-sensitive mutants of influenza A virus: response of children to the influenza A/Hong Kong/68-ts-1(E) (H3N2) and influenza A/Udorn/72-ts-1(E) (H3N2) candidate vaccine viruses and significance of immunity to neuraminidase antigen. Pediatr Res. 1976 Apr;10(4):238–242. doi: 10.1203/00006450-197604000-00008. [DOI] [PubMed] [Google Scholar]
- Magnussen C. R., Douglas R. G., Jr, Betts R. F., Roth F. K., Meagher M. P. Double-blind evaluation of oral ribavirin (Virazole) in experimental influenza A virus infection in volunteers. Antimicrob Agents Chemother. 1977 Oct;12(4):498–502. doi: 10.1128/aac.12.4.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markoff L. J., Thierry F., Murphy B. R., Chanock R. M. Relationship of genotype of recombinants of influenza A/Hong Kong/68-ts-1[E]virus used as live virus vaccines to virulence in humans. Infect Immun. 1979 Oct;26(1):280–286. doi: 10.1128/iai.26.1.280-286.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monto A. S., Kendal A. P. Effect of neuraminidase antibody on Hong Kong influenza. Lancet. 1973 Mar 24;1(7804):623–625. doi: 10.1016/s0140-6736(73)92196-x. [DOI] [PubMed] [Google Scholar]
- Murphy B. R., Chalhub E. G., Nusinoff S. R., Chanock R. M. Temperature-sensitive mutants of influenza virus. II. Attenuation of ts recombinants for man. J Infect Dis. 1972 Aug;126(2):170–178. doi: 10.1093/infdis/126.2.170. [DOI] [PubMed] [Google Scholar]
- Murphy B. R., Chalhub E. G., Nusinoff S. R., Kasel J., Chanock R. M. Temperature-sensitive mutants of influenza virus. 3. Further characterization of the ts-1(E) influenza A recombinant (H3N2) virus in man. J Infect Dis. 1973 Oct;128(4):479–487. doi: 10.1093/infdis/128.4.479. [DOI] [PubMed] [Google Scholar]
- Murphy B. R., Hosier N. T., Spring S. B., Mostow S. R., Chanock R. M. Temperature-sensitive mutants of influenza A virus: production and characterization of A/Victoria/3/75-ts-1[E] recombinants. Infect Immun. 1978 Jun;20(3):665–670. doi: 10.1128/iai.20.3.665-670.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy B. R., Kasel J. A., Chanock R. M. Association of serum anti-neuraminidase antibody with resistance to influenza in man. N Engl J Med. 1972 Jun 22;286(25):1329–1332. doi: 10.1056/NEJM197206222862502. [DOI] [PubMed] [Google Scholar]
- Murphy B. R., Markoff L. J., Hosier N. T., Rusten H. M., Chanock R. M., Kendal A. P., Douglas R. G., Betts R. F., Cate T. R., Jr, Couch R. B. Temperature-sensitive mutants of influenza A virus: evaluation of A/Victoria/3/75-ts-1[E] recombinant viruses in volunteers. Infect Immun. 1978 Jun;20(3):671–677. doi: 10.1128/iai.20.3.671-677.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palese P., Ritchey M. B. Live attenuated influenza virus vaccines. Strains with temperature-sensitive defects in P3 protein and nucleoprotein. Virology. 1977 May 1;78(1):183–191. doi: 10.1016/0042-6822(77)90090-3. [DOI] [PubMed] [Google Scholar]
- Palese P. The genes of influenza virus. Cell. 1977 Jan;10(1):1–10. doi: 10.1016/0092-8674(77)90133-7. [DOI] [PubMed] [Google Scholar]
- Richman D. D., Murphy B. R., Belshe R. B., Rusten H. M., Chanock R. M., Blacklow N. R., Parrino T. A., Rose F. B., Levine M. M., Caplan E. Temperature-sensitive mutants of influenza A virus. XIV. Production and evaluation of influenza A/Georgia/74-ts-1[E] recombinant viruses in human adults. J Infect Dis. 1977 Aug;136(2):256–262. doi: 10.1093/infdis/136.2.256. [DOI] [PubMed] [Google Scholar]
- Richman D. D., Murphy B. R., Chanock R. M., Gwaltney J. M., Jr, Douglas R. G., Betts R. F., Blacklow N. R., Rose F. B., Parrino T. A., Levine M. M. Temperature-sensitive mutants of influenza A virus. XII. Safety, antigenicity, transmissibility, and efficacy of influenza A/Udorn/72-ts-1[E] recombinant viruses in human adults. J Infect Dis. 1976 Dec;134(6):585–594. [PubMed] [Google Scholar]
- Schulman J. L., Khakpour M., Kilbourne E. D. Protective effects of specific immunity to viral neuraminidase on influenza virus infection of mice. J Virol. 1968 Aug;2(8):778–786. doi: 10.1128/jvi.2.8.778-786.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright P. F., Sell S. H., Shinozaki T., Thompson J., Karzon D. T. Safety and antigenicity of influenza A/Hong Kong/68-ts-1 (E) (H3N2). J Pediatr. 1975 Dec;87(6 Pt 2):1109–1116. doi: 10.1016/s0022-3476(75)80123-5. [DOI] [PubMed] [Google Scholar]



