Abstract
This review hopes to clearly explain the following viewpoints: (1) Neuronal synchronization underlies brain functioning, and it seems possible that blocking excessive synchronization in an epileptic neural network could reduce or even control seizures. (2) Local field potential coupling is a very common phenomenon during synchronization in networks. Removal of neurons or neuronal networks that are coupled can significantly alter the extracellular field potential. Interventions of coupling mediated by local field potentials could result in desynchronization of epileptic seizures. (3) The synchronized electrical activity generated by neurons is sensitive to changes in the size of the extracellular space, which affects the efficiency of field potential transmission and the threshold of cell excitability. (4) Manipulations of the field potential fluctuations could help block synchronization at seizure onset.
Keywords: neural regeneration, reviews, epilepsy, neurons, synchronized discharge, neural network, extracellular space, local potential coupling, field potentials, cell excitation threshold value, grants-supported paper, neuroregeneration
Research Highlights
(1) Previous studies on epileptic pathogenesis have mainly focused on synaptic transmission and action potential generation. Conventional and novel antiepileptic drugs control epileptic seizures by inhibiting action potentials. Regulatory effects of the extracellular fluid on electric fields and long-range electrical interactions between neurons can explain neuronal hypersynchrony and epileptic activities.
(2) This study review evidence of field potential coupling and synchronization of neuronal networks. First, we propose that local field potential coupling plays an important role in synchronization at seizure onset, and suggest that interventions can reduce field potential fluctuations and block early synchronization. Then, we outline the development of a new anti-epileptic treatment based on decoupling of field potentials by electrostimulation.
INTRODUCTION
A previous study on the basic mechanisms of epilepsy and the design of new antiepileptic drugs focused on synaptic transmission and action potential generation[1]. However, numerous studies have suggested that nonsynaptic mechanisms, such as electric field interactions in the extracellular space, might also explain neuronal hypersynchrony and epileptogenicity[2,3,4,5,6]. It has been hypothesized that changes in the extracellular space may regulate neuronal synchrony by affecting nonsynaptic mechanisms such as gap junctions, brain tissue electrical resistance, extracellular ion concentrations, and remote electric field effects[1]. A large number of clinical and basic experimental studies have suggested that modulation of extracellular osmolarity adjusts the volume fraction of the extracellular space by directly affecting cell volume, and that this can significantly affect epileptic activity[3,4,5,7,8,9,10]. Epileptic hypersynchrony also relies on electric-field effects and ion concentration changes in the extracellular space[5]. In vivo studies in rats demonstrated that systemically injected hyperosmotic solutions increased the electroshock seizure threshold and prevented the development of kainic acid-induced seizures[11,12]. In vitro studies on the role of nonsynaptic mechanisms in epilepsy have shown that if the calcium concentration of the bathing medium is reduced (eliminating chemical synaptic transmission), synchronous discharges occur in hippocampal slices[5,13,14,15,16]. These observations suggest that nonsynaptic mechanisms may play an important role in the regulation of epileptic activity in the human brain. Massive neuronal hypersynchrony is a defining feature of the electrical activity in epileptic neural networks and neuronal synchronization is the basis of many brain functions.
The significance and role of synchrony are likely to depend on the nature and extent of the interconnections of neurons. Therefore, at least in theory, it is possible that blocking the excessive synchronization in an epileptic neural network can reduce or even control seizures. Studies have shown that the mechanisms of synchronization in a neural network may include: a) classic chemical synaptic transmission, b) electrical coupling mediated by gap junctions, c) transmission mediated by extracellular field potentials and ion concentrations, and d) intracellular mechanisms contributing to neuronal hyperexcitability[2,3,5,17,18]. Seizures are believed to result from mechanisms involving classical synaptic transmission and intrinsic neuronal hyperexcitability. Drugs acting on ion channels, which are widely used as antiepileptic drugs, exert their effects by reducing synaptic transmission and membrane excitability[19]. Quinine, a blocking compound of the gap junction protein connexin 36, has shown antiepileptic activity in experimental animal models[17,20]. This suggests that blockade of connexin 36-mediated epileptic synchronization could contribute to antiepileptic treatment. However, at present, no ideal intervention technique exists that can slow nonsynaptic synchronization and achieve the goal of controlling seizures. This may in part explain why more than 20% of epileptic patients are refractory to treatment. We hypothesize that technological interventions applied externally could be used to “clamp” the extracellular local field potential of epileptogenic tissue to a suitable level and thereby prevent epileptic oscillations. Ideally, we hope to prevent hypersynchronization of neural networks, which will help to reduce or control seizures. In this article, we review the roles and mechanisms of field potential effects in epileptic network synchronization.
LOCAL FIELD POTENTIAL COUPLING IS COMMON DURING NETWORK SYNCHRONIZATION
Neurons are embedded in an electrically conducting extracellular fluid, which allows the extracellular activity of one cell to be perceived by neighboring cells[21,22,23,24,25,26,27,28,29]. The membrane potential of individual neurons can be influenced by extracellular fields, and conversely the transmembrane current of individual neurons can influence the extracellular field[30]. The electric fields are generated by neurons and glia in a cooperative manner.
Local field potential coupling is a very common mechanism of synchronization in neural networks (Figure 1)[31,32]. Ephaptic coupling occurs between axons. Since extracellular fields have the strongest effects in subthreshold and perithreshold voltage ranges, ephaptic effects may not be able to initiate spikes in a membrane at rest. Even during spiking they will not have any significant effect on the membrane potential.
Figure 1.
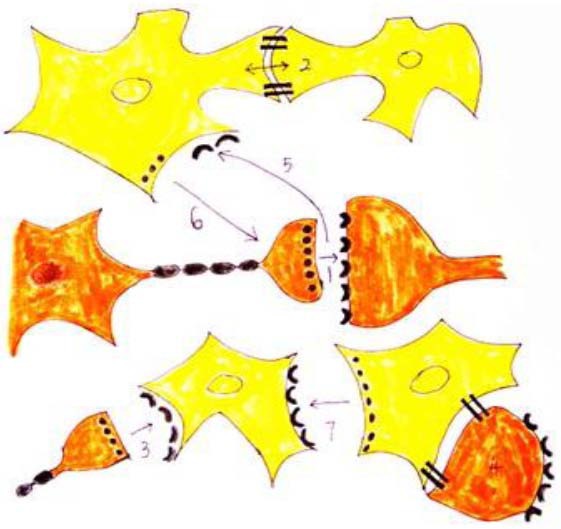
Functional interactions in the neuron-glia signaling network.
Neurons are shown in orange and glial cells in yellow. Rapid communication involves homocellular signaling, such as chemical synaptic transmission between nerve cells (1) and electrotonic coupling through gap junctions between glial cells (2). However, chemical synapses also exist between presynaptic neurons and postsynaptic glial cells (3), and gap junctions may directly couple glial cells to neurons (4). Other forms of heterocellular neuron-glia signaling have been shown. Synaptic neurotransmission may lead to the activation of perisynaptic glial cells. Neurotransmitters spill over from the cleft at a concentration sufficient to stimulate receptors located on adjacent glial cell plasma membranes (5). Glial cells can also actively respond to stimulation by releasing neuroactive transmitters, and can thereby modulate the function of adjacent neurons (6). Glial cells can also release transmitters onto surrounding glial cells to extend their range of signaling (7). It is highly likely that brain activity involves a combination of many, if not all, of the above forms of communication. Thus, we propose that the brain functions as an integrated signaling network of both neurons and glial cells.
A study of the mouse barrel cortex has reported that during strongly synchronized spiking activity, such as strong evoked responses or epileptic discharges, spiking could be effectively induced by the large and localized extracellular currents generated by the population spike in subthreshold neurons or axonal terminals nearby[33]. Seizure initiation is thought to be driven by the discharge of a single neuron, but the process of amplification and synchronization cannot occur without the evolution and spread of discharges among neurons within susceptible networks[34]. The coupling between neurons or neuronal networks seems to be the most important mechanism. There is evidence that increasing coupling between interneurons or between pyramidal cells may increase synchrony and promote seizures[35]. Synchronized inhibitory postsynaptic potentials will phasically reduce or block neuronal firing, which results in pyramidal cell action potential firing coupled to the synchronized inhibitory input[36].
COUPLING IN EXTRACELLULAR SPACE CONTRIBUTES TO EPILEPTIC HYPERSYNCHRONY
In addition to traditional synaptic interactions, neurons may communicate with each other through the extracellular environment, gap junctions, and local neuromodulator release (Figure 1)[37,38]. Among these factors, coupling through extracellular space is most strongly associated with epileptic hypersynchrony[39,40,41,42]. Classic physiology studies have shown that synaptic transmission is accompanied by a synaptic delay, which does not support the initial formation of synchronized electrical activity. Furosemide and mannitol have been found to inhibit seizure discharges in vitro and in vivo by interfering with action potential synchronization without affecting synaptic activity[1,43,44,45]. The role of gap junctions in seizure initiation is still controversial. It was previously thought that communication through gap junctions was dominant during synchronized epileptic activity, and that connexin 36 was primarily involved[46]. However, a more recent report showed that connexin 36 knock-out mice displayed an increased sensitivity to pentylenetetrazol-induced seizure-like behaviors[47]. Thus, further study is needed to identify the gap junction proteins responsible for synchronization at seizure onset.
Synchronization by direct coupling of the extracellular field potential may be involved in seizure initiation. Both fast-spiking activity and slower fluctuations can be seen in the extracellular field potential. In a given brain structure, the latter, also called local field potentials, provide experimental access to the spatiotemporal activity of afferent, local and associational processes, and reflect the summed electrical activity of neurons and associated glial cells[48]. Liu et al[49] successfully recorded local field potentials of the anterior nucleus of the thalamus of rats with acute temporal lobe epilepsy induced by intra-hippocampal kainic acid. Local field potentials were long thought simply to reflect an epiphenomenon of neuronal signaling. Local field potentials span across larger brain regions, even though they are relatively small in amplitude[50]. Additionally, since the local field potentials have relatively slow time characteristics (> 5 ms), the low-pass filtering of the membrane affects them much less[51]. Laminar morphology (neuronal alignment) of brain regions such as the hippocampus gives rise to a large increase in extracellular potential fluctuations. Thus, it has been speculated that local field potentials may be helpful in those regions.
Previous data have shown that local field potentials and electrocorticogram show synchronized fluctuations during seizures[49]. It has been shown that the synchronization of neuronal populations was largely created by the extracellular field potential even in the absence of synaptic exchange[52,53].
Therefore, under physiological conditions, coherent spiking activity is not necessarily implied by proximity and coherent membrane potential fluctuations. Because the extracellular field has the strongest effect in subthreshold and perithreshold voltage ranges, ephaptic coupling also affects axons[54]. Experimental evidence has reported that the cooperative action of brain cells in generating local electric fields can influence the timing of neural activity[30]. The amplitude of the field potentials recorded extracellularly not only reflected, but also directly quantified, the degree of epileptic hypersynchrony[55].
DECOUPLING DECREASES SYNCHRONIZATION IN NETWORKS
Synchrony represents the simultaneous firing of a huge population of neurons on the millisecond time scale, so that their action potentials can summate into a large field potential[3]. Neuronal excitability could be altered by both endogenous and applied electric fields over a few millivolts per millimeter[15]. The first and second statistical moments of the degree distribution play a more important role in the equation obtained for the critical coupling than the network average degree, which has been verified[56]. Different synchronization intervals have distinct influences on the synchronization period and amplitude[57]. Shen and Cao[58] showed that pinning control can achieve finite-time synchronization. Interference with short-range synchrony may help to terminate seizures. Long-range synchrony plays an important role in terminating seizures. It also has effects on large areas of the cortex and distant subcortical structures[59,60]. Thus, removal of coupling between neurons and neuronal networks can significantly alter the extracellular field potential, depress network synchronization, and reduce or even terminate seizures.
INTERVENTION OF COUPLING MEDIATED BY LOCAL FIELD POTENTIALS COULD CAUSE DESYNCHRONIZATION
Many experiments have indicated that the synchronized electrical activity generated by neurons is sensitive to changes in the size of the extracellular space, which affects the efficiency of field potential transmission and the threshold of cell excitability[3,4,6,7,8,9,12,15,22,24,40,41,42,61,62]. Under hypotonic conditions, the extracellular space shrinks, shortening the distance between adjacent cells and helping to directly transmit epileptic electrical activity[61,62]. The extracellular fluid is electrically neutral and under normal conditions the field potential of the extracellular fluid is zero. Simulation experiments have confirmed that the potential changes in the extracellular space are low-pass filtered, with severe attenuation over distance of fast currents (sodium-mediated action potentials), while slow currents (potassium currents) can spread farther[26]. As illustrated in Figure 2, when cell A is activated there is a large influx of Na+ ions accompanied by a transient decrease in positive charge and an increase in negative charge in the extracellular fluid. This reduces the local field potential, resulting in a reduction of the transmembrane potential and depolarization of the adjacent cell B. If the distance between cell A and cell B is short, the likelihood of interactions between the cells increases. An increasing number of studies indicate that extracellular field potential transmission plays a prominent role in synchronization at seizure onset[63]. Interference with the field potential fluctuations would be expected to block epileptic synchronization.
Figure 2.
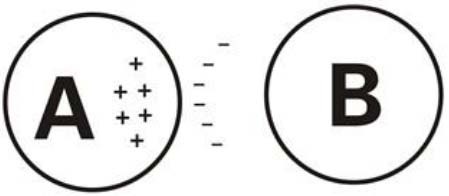
Electric potential transfer between cells through the extracellular space.
A is an excitatory cell. The large transient influx of sodium ions leads to a relative increase of negative ions in the extracellular space, reducing the transmembrane potential of A compared with B.
APPLICATION OF EXTERNAL INTERVENTION TECHNOLOGY IN CLINICAL PRACTICE
Because seizures are a result of excessive neuronal synchronization, intervention methods involving external electrical stimulation have become the research focus at home and abroad[32,64,65,66,67,68,69]. This type of research is now called neural engineering. Over the past 20 years, three types of technologies have been used in clinical studies and animal models of epilepsy[70]. The first technique is called vagus nerve stimulation. The vagus nerve stimulation system (Cyberonics, Houston, TX, USA; Figure 3) was approved by the US Food and Drug Administration in 1997 and has been confirmed to be an effective auxiliary treatment for partial seizures[71].
Figure 3.

Vagus nerve stimulator.
In 1999, the American Academy of Neurology considered the effectiveness and safety evidence of vagus nerve stimulation as grade I clinical evidence. The estimated number of patients treated by vagus nerve stimulation was more than 50 000 worldwide by 2010[72]. The treatment can reduce the frequency of epileptic seizures on average by 30–40%, and completely controls seizures in about 10% of patients[73]. The second technique, which is called Kinetra nerve stimulation (Medtronic; New York, NY, USA; Figure 4), controls the seizures through stimulating the anterior nucleus of the thalamus. It is very similar to deep brain stimulation used to treat Parkinson's disease. The nerve stimulator is implanted in the anterior nucleus of the thalamus using stereotactic methods[74,75,76,77]. However, the method for stimulating the anterior nucleus of the thalamus is slightly different from the method for treating Parkinson's disease or tremor[74,75], and uses intermittent stimulation instead of persistent stimulation[76,77]. By implanting the deep brain stimulation electrodes into the bilateral anterior nucleus of the thalamus of three epileptic patients, Molnar et al[78] found that the seizure frequencies decreased prominently. In 2010, deep brain stimulation of the anterior nucleus of the thalamus received Conformité Européenne approval as an epilepsy therapy in Europe. A multicenter randomized controlled trial reported a reduction in seizure frequency by 40.4% compared with 14.5% in controls[79].
Figure 4.

Kinetra bilateral stimulator.
The third technique is a closed-loop feedback system[80,81], which can record in real-time and monitor electroencephalogram signals, and then switch to intervention mode when detecting evidence of an epilepsy aura (Figure 5)[82]. At present, testing of the feedback nerve stimulator has reached the clinical trial phase. The company (Neuropace, Mountain View, CA, USA) sought Food and Drug Administration approval for use in patients with refractory epilepsy in 2010[64]. Responsive neurostimulation is the first generation of closed-loop feedback devices. It has an intracranial electrode, and can record, calculate, and analyze changes in the intracranial electrical signal. When a seizure breaks out or is about to begin, the closed loop feedback system will start to deliver as many as five local electrical stimulation sequences to prevent or stop the seizure. An important characteristic of this technology is that it uses a personalized “training period”. The instrument begins to record seizures after implantation and will adapt to the characteristics of the patient's seizures. Nelson et al[65] found that a closed-loop neural electrical stimulation system could control seizures induced by high-frequency electrical stimulation in rat models of absence epilepsy. Pineda et al[64] significantly altered epileptic seizure frequency using a closed-loop stimulation system in a zebrafish epilepsy model. At the end of 2011, the research was still at the preclinical stage, but the preliminary results show promise for its application in humans in the future.
Figure 5.
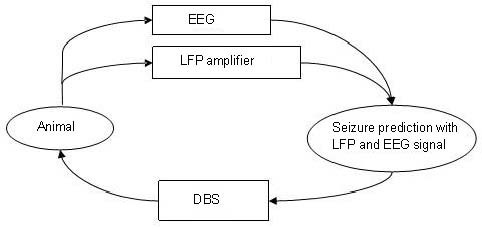
Schematic of close-loop DBS for seizure detection and prevention.
DBS: Deep brain stimulation; EEG: electroencephalogram; LFP: local field potential.
The above three engineering technologies are obviously not sufficient. First, until now, there have been no worldwide standards for stimulation parameters or time limits for operation, and the effective rate of vagus nerve stimulation is too low[73]. Second, because the technology was invented to treat Parkinson's disease and involves identifying a specific kernel to stimulate, the stimulator is not ideal for treating the origin of epileptic seizures. It is still unclear whether stimulation of a certain nuclear group has any definite clinical effects. The mechanism underlying the anti-epileptic effects provided by electrical stimulation of the anterior thalamic nucleus is still unclear. It remains controversial whether the mechanism involves thalamic injury caused by the implanted electrodes, stimulation of the nucleus, or both[69]. No randomized controlled trials with large sample sizes have been done. Finally, although the responsive neurostimulation technique in theory is in good agreement with the mechanisms of epilepsy, a big problem lies in that the expression of epilepsy clinically and pathologically appears to be nonuniform. For example, intractable epilepsy can have many pathological causes, including genetic factors, trauma, infection, brain malformation (such as cortical dysplasia), and drug factors[70]. Therefore, we cannot develop a standard for treating epilepsy such as is done for treating arrhythmia[70]. Also, the technology is still not fully adequate. There is a long way to go and many obstacles have to be overcome before this technique can be widely used in the clinic.
CONCLUSION AND PERSPECTIVE
We are convinced that the mechanism of synchronization by extracellular fields is one of the most important mechanisms in seizure initiation. We hypothesize that it is possible to develop a system that achieves desynchronization of neuronal networks through clamping of the extracellular field potential. This system (Figure 6) will detect the extracellular field potential with an exploratory electrode implanted in the epileptic focus, and use computers to analyze and process the electrical signal. The extracellular field potential will be manipulated through the electrode to achieve desynchronization of the neural network. Our hypothesis is in line with the present understanding of the pathogenesis of epilepsy, is more scientific and reliable than previous technologies, and aims to bring about a breakthrough in the treatment of epilepsy. Nevertheless, whether this new approach can be successful depends on the degree of extracellular field potential fluctuations at seizure onset, which is the most critical technical parameter. It is possible to obtain the parameters in vivo and in vitro through recording the time course of neural network synchronization with a microelectrode. Our hypothesis might provide a new explanation of the pathophysiology of epilepsy, provide insights into a novel pathophysiological mechanism of seizures, and potentially offer new therapeutic opportunities in the future.
Figure 6.
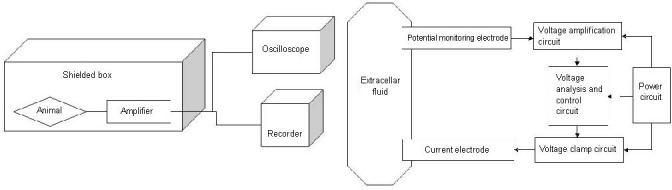
A clamp system for detecting the extracellular field potential.
Footnotes
Funding: This work was supported by grants from the National Natural Science Foundation of China, No. 30971534 and 125 Project of the Third Xiangya Hospital of Central South University, China.
Conflicts of interest: None declared.
(Edited by Wang JT, Shi TS/Qiu Y/Song LP)
REFERENCES
- [1].Haglund MM, Hochman DW. Furosemide and mannitol suppression of epileptic activity in the human brain. J Neurophysiol. 2005;94(2):907–918. doi: 10.1152/jn.00944.2004. [DOI] [PubMed] [Google Scholar]
- [2].Bikson M, Baraban SC, Durand DM. Conditions sufficient for nonsynaptic epileptogenesis in the CA1 region of hippocampal slices. J Neurophysiol. 2002;87(1):62–71. doi: 10.1152/jn.00196.2001. [DOI] [PubMed] [Google Scholar]
- [3].Dudek FE, Snow RW, Taylor CP. Role of electrical interactions in synchronization of epileptiform bursts. Adv Neurol. 1986;44:593–617. [PubMed] [Google Scholar]
- [4].Faber DS, Korn H. Electrical field effects: their relevance in central neural networks. Physiol Rev. 1989;69(3):821–863. doi: 10.1152/physrev.1989.69.3.821. [DOI] [PubMed] [Google Scholar]
- [5].Jefferys JG. Nonsynaptic modulation of neuronal activity in the brain: electric currents and extracellular ions. Physiol Rev. 1995;75(4):689–723. doi: 10.1152/physrev.1995.75.4.689. [DOI] [PubMed] [Google Scholar]
- [6].Andrew RD. Seizure and acute osmotic change: clinical and neurophysiological aspects. J Neurol Sci. 1991;101(1):7–18. doi: 10.1016/0022-510x(91)90013-w. [DOI] [PubMed] [Google Scholar]
- [7].Sykova E. Extrasynaptic volume transmission and diffusion parameters of the extracellular space. Neuroscience. 2004;129(4):861–876. doi: 10.1016/j.neuroscience.2004.06.077. [DOI] [PubMed] [Google Scholar]
- [8].Sykova E. The extracellular space in the CNS: Its regulation, volume and geometry in normal and pathological neuronal function. The Neuroscientist. 1997;3:28–41. [Google Scholar]
- [9].Pasantes-Morales H, Tuz K. Volume changes in neurons: hyperexcitability and neuronal death. Contrib Nephrol. 2006;152:221–240. doi: 10.1159/000096326. [DOI] [PubMed] [Google Scholar]
- [10].Pasantes-Morales H, Lezama RA, Ramos-Mandujano G, et al. Mechanisms of cell volume regulation in hypo-osmolality. Am J Med. 2006;119(7supp1):S4–11. doi: 10.1016/j.amjmed.2006.05.002. [DOI] [PubMed] [Google Scholar]
- [11].Baran H, Lassmann H, Sperk G, et al. Effect of mannitol treatment on brain neurotransmitter markers in kainic acid-induced epilepsy. Neuroscience. 1987;21(3):679–684. doi: 10.1016/0306-4522(87)90029-7. [DOI] [PubMed] [Google Scholar]
- [12].Reed DJ, Woodbury DM. The effect of hypertonic urea solution on electroshock seizure threshold and electrolyte distribution in rats. J Pharmacol Exp Ther. 1964;146:154–159. [PubMed] [Google Scholar]
- [13].Jefferys JG, Haas HL. Synchronized bursting of CA1 hippocampal pyramidal cells in the absence of synaptic transmission. Nature. 1982;300(5891):448–450. doi: 10.1038/300448a0. [DOI] [PubMed] [Google Scholar]
- [14].Taylor CP, Dudek FE. Synchronous neural afterdischarges in rat hippocampal slices without active chemical synapses. Science. 1982;218(4574):810–812. doi: 10.1126/science.7134978. [DOI] [PubMed] [Google Scholar]
- [15].Taylor CP, Dudek FE. Excitation of hippocampal pyramidal cells by an electrical field effect. J Neurophysiol. 1984;52(1):126–142. doi: 10.1152/jn.1984.52.1.126. [DOI] [PubMed] [Google Scholar]
- [16].Taylor CP, Dudek FE. Synchronization without active chemical synapses during hippocampal afterdischarges. J Neurophysiol. 1984;52(1):143–155. doi: 10.1152/jn.1984.52.1.143. [DOI] [PubMed] [Google Scholar]
- [17].Gajda Z, Szupera Z, Blazso G, et al. Quinine, a blocker of neuronal cx36 channels, suppresses seizure activity in rat neocortex in vivo. Epilepsia. 2005;46(10):1581–1591. doi: 10.1111/j.1528-1167.2005.00254.x. [DOI] [PubMed] [Google Scholar]
- [18].Margineanu DG. Epileptic hypersynchrony revisited. Neuroreport. 2010;21(15):963–967. doi: 10.1097/WNR.0b013e32833ed111. [DOI] [PubMed] [Google Scholar]
- [19].White HS, Smith MD, Wilcox KS. Mechanisms of action of antiepileptic drugs. Int Rev Neurobiol. 2007;81:85–110. doi: 10.1016/S0074-7742(06)81006-8. [DOI] [PubMed] [Google Scholar]
- [20].Bostanci MO, Bagirici F. Anticonvulsive effects of quinine on penicillin-induced epileptiform activity: an in vivo study. Seizure. 2007;16(2):166–172. doi: 10.1016/j.seizure.2006.11.007. [DOI] [PubMed] [Google Scholar]
- [21].Rall W, Shepherd GM. Theoretical reconstruction of field potentials and dendrodendritic synaptic interactions in olfactory bulb. J Neurophysiol. 1968;31(6):884–915. doi: 10.1152/jn.1968.31.6.884. [DOI] [PubMed] [Google Scholar]
- [22].Traub RD, Dudek FE, Taylor CP, et al. Simulation of hippocampal afterdischarges synchronized by electrical interactions. Neuroscience. 1985;14(4):1033–1038. doi: 10.1016/0306-4522(85)90274-x. [DOI] [PubMed] [Google Scholar]
- [23].Tranchina D, Nicholson C. A model for the polarization of neurons by extrinsically applied electric fields. Biophys J. 1986;50(6):1139–1156. doi: 10.1016/S0006-3495(86)83558-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Holt GR, Koch C. Electrical interactions via the extracellular potential near cell bodies. J Comput Neurosci. 1999;6(2):169–184. doi: 10.1023/a:1008832702585. [DOI] [PubMed] [Google Scholar]
- [25].McIntyre CC, Grill WM. Excitation of central nervous system neurons by nonuniform electric fields. Biophys J. 1999;76(2):878–888. doi: 10.1016/S0006-3495(99)77251-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Bedard C, Kroger H, Destexhe A. Modeling extracellular field potentials and the frequency-filtering properties of extracellular space. Biophys J. 2004;86(3):1829–1842. doi: 10.1016/S0006-3495(04)74250-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Gold C, Henze DA, Koch C, et al. On the origin of the extracellular action potential waveform: A modeling study. J Neurophysiol. 2006;95(3):3113–3128. doi: 10.1152/jn.00979.2005. [DOI] [PubMed] [Google Scholar]
- [28].Logothetis NK, Kayser C, Oeltermann A. In vivo measurement of cortical impedance spectrum in monkeys: implications for signal propagation. Neuron. 2007;55(5):809–823. doi: 10.1016/j.neuron.2007.07.027. [DOI] [PubMed] [Google Scholar]
- [29].Pettersen KH, Einevoll GT. Amplitude variability and extracellular low-pass filtering of neuronal spikes. Biophys J. 2008;94(3):784–802. doi: 10.1529/biophysj.107.111179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Anastassiou CA, Montgomery SM, Barahona M, et al. The effect of spatially inhomogeneous extracellular electric fields on neurons. J Neurosci. 2010;30(5):1925–1936. doi: 10.1523/JNEUROSCI.3635-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Weiss D, Govindan RB, Rilk A, et al. Central oscillators in a patient with neuropathic tremor: evidence from intraoperative local field potential recordings. Mov Disord. 2011;26(2):323–327. doi: 10.1002/mds.23374. [DOI] [PubMed] [Google Scholar]
- [32].Denker M, Roux S, Lindén H, et al. The local field potential reflects surplus spike synchrony. Cereb Cortex. 2011;21(12):2681–2695. doi: 10.1093/cercor/bhr040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Noebels JL, Prince DA. Development of focal seizures in cerebral cortex: role of axon terminal bursting. J Neurophysiol. 1978;41(5):1267–1281. doi: 10.1152/jn.1978.41.5.1267. [DOI] [PubMed] [Google Scholar]
- [34].Cohen A, Shappir J, Yitzchaik S, et al. Reversible transition of extracellular field potential recordings to intracellular recordings of action potentials generated by neurons grown on transistors. Biosens Bioelectron. 2008;23(6):811–819. doi: 10.1016/j.bios.2007.08.027. [DOI] [PubMed] [Google Scholar]
- [35].Leung LS, Peloquin P. Cholinergic modulation differs between basal and apical dendritic excitation of hippocampal CA1 pyramidal cells. Cereb Cortex. 2010;20(8):1865–1877. doi: 10.1093/cercor/bhp251. [DOI] [PubMed] [Google Scholar]
- [36].Lado FA, Moshe SL. How do seizures stop? Epilepsia. 2008;49(10):1651–1664. doi: 10.1111/j.1528-1167.2008.01669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Bullock TH, Bennett MV, Johnston D, et al. The neuron doctrine, redux. Science. 2005;310(5749):791–793. doi: 10.1126/science.1114394. [DOI] [PubMed] [Google Scholar]
- [38].Bezzi P, Volterra A. A neuron-glia signalling network in the active brain. Curr Opin Neurobiol. 2001;11(3):387–394. doi: 10.1016/s0959-4388(00)00223-3. [DOI] [PubMed] [Google Scholar]
- [39].Agnati LF, Zoli M, Stromberg I, et al. Intercellular communication in the brain: wiring versus volume transmission. Neuroscience. 1995;69(3):711–726. doi: 10.1016/0306-4522(95)00308-6. [DOI] [PubMed] [Google Scholar]
- [40].Nicholson C, Sykova E. Extracellular space structure revealed by diffusion analysis. Trends Neurosci. 1998;21(5):207–215. doi: 10.1016/s0166-2236(98)01261-2. [DOI] [PubMed] [Google Scholar]
- [41].Zoli M, Jansson A, Sykova E, et al. Volume transmission in the CNS and its relevance for neuropsychopharmacology. Trends Pharmacol Sci. 1999;20(4):142–150. doi: 10.1016/s0165-6147(99)01343-7. [DOI] [PubMed] [Google Scholar]
- [42].Momose-Sato Y, Sato K, Mochida H, et al. Spreading depolarization waves triggered by vagal stimulation in the embryonic chick brain: optical evidence for intercellular communication in the developing central nervous system. Neuroscience. 2001;102(2):245–262. doi: 10.1016/s0306-4522(00)00477-2. [DOI] [PubMed] [Google Scholar]
- [43].Hochman DW, Baraban SC, Owens JW, et al. Dissociation of synchronization and excitability in furosemide blockade of epileptiform activity. Science. 1995;270(5233):99–102. doi: 10.1126/science.270.5233.99. [DOI] [PubMed] [Google Scholar]
- [44].Hochman DW, Ambrosio R, Janigro D, et al. Extracellular chloride and the maintenance of spontaneous epileptiform activity in rat hippocampal slices. J Neurophsyiol. 1999;81(1):49–59. doi: 10.1152/jn.1999.81.1.49. [DOI] [PubMed] [Google Scholar]
- [45].Hochman DW, Schwartzkroin PA. Chloride-cotransport blockade desynchronizes neuronal discharge in the “epileptic” hippocampal slice. J Neurophysiol. 2000;83(1):406–417. doi: 10.1152/jn.2000.83.1.406. [DOI] [PubMed] [Google Scholar]
- [46].Peng YF, Wu JX, Yang H, et al. Expression of connexin 36 in central nervous system and its role in epileptic seizure. Chin Med J (Engl) 2012;125(13):2365–2370. [PubMed] [Google Scholar]
- [47].Jacobson GM, Voss LJ, Melin SM, et al. Connexin36 knockout mice display increased sensitivity to pentylenetetrazol-induced seizure-like behaviors. Brain Res. 2010;1360:198–204. doi: 10.1016/j.brainres.2010.09.006. [DOI] [PubMed] [Google Scholar]
- [48].Buzsaki G. Large-scale recording of neuronal ensembles. Nat Neurosci. 2004;7(5):446–451. doi: 10.1038/nn1233. [DOI] [PubMed] [Google Scholar]
- [49].Liu X, Hao H, Yang L, et al. Epileptic seizure detection with the local field potential of anterior thalamic of rats aiming at real time application. Conf Proc IEEE Eng Med Biol Soc. 2011;2011:6781–6784. doi: 10.1109/IEMBS.2011.6091672. [DOI] [PubMed] [Google Scholar]
- [50].Katzner S, Nauhaus I, Benucci A, et al. Local origin of field potentials in visual cortex. Neuron. 2009;61(1):35–41. doi: 10.1016/j.neuron.2008.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Buzsaki G, Leung LW, Vanderwolf CH. Cellular bases of hippocampal EEG in the behaving rat. Brain Res. 1983;287(2):139–171. doi: 10.1016/0165-0173(83)90037-1. [DOI] [PubMed] [Google Scholar]
- [52].Purpura DP, Malliani A. Spike generation and propagation initiated in dendrites by transhippocampal polarization. Brain Res. 1966;1(4):403–406. doi: 10.1016/0006-8993(66)90132-6. [DOI] [PubMed] [Google Scholar]
- [53].Snow RW, Dudek FE. Electrical fields directly contribute to action potential synchronization during convulsant- induced epileptiform bursts. Brain Res. 1984;323(1):114–118. doi: 10.1016/0006-8993(84)90271-3. [DOI] [PubMed] [Google Scholar]
- [54].Poulet JF, Petersen CC. Internal brain state regulates membrane potential synchrony in barrel cortex of behaving mice. Nature. 2008;454(7206):881–885. doi: 10.1038/nature07150. [DOI] [PubMed] [Google Scholar]
- [55].Margineanu DG, Klitgaard H. Differential effects of cation-chloride cotransportblocking diuretics in a rat hippocampal slice model of epilepsy. Epilepsy Res. 2006;69(2):93–99. doi: 10.1016/j.eplepsyres.2006.01.005. [DOI] [PubMed] [Google Scholar]
- [56].Peron TK, Rodrigues FA. Determination of the critical coupling of explosive synchronization transitions in scale-free networks by mean-field approximations. Phys Rev E Stat Nonlin Soft Matter Phys. 2012;86(5 Pt 2):056108. doi: 10.1103/PhysRevE.86.056108. [DOI] [PubMed] [Google Scholar]
- [57].Zhang W, Zhou X. Synchronization ability of coupled cell-cycle oscillators in changing environments. BMC Syst Biol. 2012;6(Suppl 1):S13. doi: 10.1186/1752-0509-6-S1-S13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Shen J, Cao J. Finite-time synchronization of coupled neural networks via discontinuous controllers. Cogn Neurodyn. 2011;5(4):373–385. doi: 10.1007/s11571-011-9163-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Timofeev I, Steriade M. Neocortical seizures: initiation, development and cessation. Neuroscience. 2004;123(2):299–336. doi: 10.1016/j.neuroscience.2003.08.051. [DOI] [PubMed] [Google Scholar]
- [60].Schindler K, Elger CE, Lehnertz K. Increasing synchronization may promote seizure termination: evidence from status epilepticus. Clin Neurophysiol. 2007;118(9):1955–1968. doi: 10.1016/j.clinph.2007.06.006. [DOI] [PubMed] [Google Scholar]
- [61].Kilb W, Dierkes PW, Syková E, et al. Hypoosmolar conditions reduce extracellular volume fraction and enhance epileptiform activity in the CA3 region of the immature rat hippocampus. J Neurosci Res. 2006;84(1):119–129. doi: 10.1002/jnr.20871. [DOI] [PubMed] [Google Scholar]
- [62].Chebabo SR, Hester MA, Aitken PG, et al. Hypotonic exposure enhances synaptic transmission and triggers spreading depression in rat hippocampal tissue slices. Brain Res. 1995;695(2):203–216. doi: 10.1016/0006-8993(95)00778-o. [DOI] [PubMed] [Google Scholar]
- [63].Dudek FE, Yasumura T, Rash JE. Non-synaptic mechanisms in seizures and epileptogenesis. Cell Biol Int. 1998;22(11-12):793–805. doi: 10.1006/cbir.1999.0397. [DOI] [PubMed] [Google Scholar]
- [64].Pineda R, Beattie CE, Hall CW. Closed-loop neural stimulation for pentylenetetrazole-induced seizures in zebrafish. Dis Model Mech. 2013;6(1):64–71. doi: 10.1242/dmm.009423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Nelson TS, Suhr CL, Freestone DR, et al. Closed-loop seizure control with very high frequency electrical stimulation at seizure onset in the GAERS model of absence epilepsy. Int J Neural Syst. 2011;21(2):163–173. doi: 10.1142/S0129065711002717. [DOI] [PubMed] [Google Scholar]
- [66].Farajidavar A, Hagains CE, Peng YB, et al. A closed loop feedback system for automatic detection and inhibition of mechano-nociceptive neural activity. IEEE Trans Neural Syst Rehabil Eng. 2012;20(4):478–487. doi: 10.1109/TNSRE.2012.2197220. [DOI] [PubMed] [Google Scholar]
- [67].Young CP, Liang SF, Chang DW, et al. A portable wireless online closed-loop seizure controller in freely moving rats. IEEE Transactions on Instrumentation and Measurement. 2011;60(2):513–521. [Google Scholar]
- [68].Berényi A, Belluscio M, Mao D, et al. Closed-loop control of epilepsy by transcranial electrical stimulation. Science. 2012;337(6095):735–737. doi: 10.1126/science.1223154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Sun W, Meng XH, Mao W, et al. Research and progress of neural electrical control techniques for the treatment of intractable epilepsy. Dianxian yu Shenjing Dianshengli Xue Zazhi. 2010;19(5):303–306. [Google Scholar]
- [70].Chang PF, Li YJ. The application of neural engineering technology in the treatment of epilepsy. Zhongguo Kangfu Yixue Zazhi. 2010;25(8):812–814. [Google Scholar]
- [71].Morrell MJ. Responsive cortical stimulation for the treatment of medically intractable partial epilepsy. Neurology. 2011;77(13):1295–1304. doi: 10.1212/WNL.0b013e3182302056. [DOI] [PubMed] [Google Scholar]
- [72].Boon P, Raedt R, de Herdt V, et al. Electrical stimulation for the treatment of epilepsy. Neurotherapeutics. 2009;6(2):218–227. doi: 10.1016/j.nurt.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Imoto H, Fujii M, Uchiyama J. Use of a pehier chip with a newly devised local brain-cooling system for neocortical seizures in the rat: technical note. J Neurosurg. 2006;104(1):150–156. doi: 10.3171/jns.2006.104.1.150. [DOI] [PubMed] [Google Scholar]
- [74].Benabid AL, Krack PP, Benazzouz A, et al. Deep brain stimulation of the subthalamic nucleus for Parkinson's disease: methodologic aspects and clinical criteria. Neurology. 2000;55(12)(Suppl 6):40–44. [PubMed] [Google Scholar]
- [75].Wichmann T, DeLong MR. Deep brain stimulation for neurologic and neuropsychiatric disorders. Neuron. 2006;52(1):197–204. doi: 10.1016/j.neuron.2006.09.022. [DOI] [PubMed] [Google Scholar]
- [76].Graves NM, Fisher RS. Neurostimulation for epilepsy, including a pilot study of anterior nucleus stimulation. Clin Neurosurg. 2005;52:127–134. [PubMed] [Google Scholar]
- [77].Kerrigan JF, Litt B, Fisher RS, et al. Electrical stimulation of the anterior nucleus of the thalamus for the treatment of intractable epilepsy. Epilepsia. 2004;45(4):346–354. doi: 10.1111/j.0013-9580.2004.01304.x. [DOI] [PubMed] [Google Scholar]
- [78].Molnar GF, Sailer A, Gunraj CA, et al. Changes in motor cortex excitability with stimulation of anterior thalamus in epilepsy. Neurology. 2006;66(4):566–571. doi: 10.1212/01.wnl.0000198254.08581.6b. [DOI] [PubMed] [Google Scholar]
- [79].Moddel G, Coenen VA, Elger CE. Invasive neurostimulation as adjunct treatment for epilepsy. Nervenarzt. 2012;83(8):1001–1005. doi: 10.1007/s00115-012-3572-z. [DOI] [PubMed] [Google Scholar]
- [80].Osorio I, Frei MG, Sunderam S, et al. Automated seizure abatement in humans using electrical stimulation. Ann Neurol. 2005;57(2):258–268. doi: 10.1002/ana.20377. [DOI] [PubMed] [Google Scholar]
- [81].Lian Q, Wang J, Liu HZ, et al. Simulation study of the mechanism of the deep brain stimulation. Zhongguo Kangfu Yixue Zazhi. 2008;23(11):1037–1038. [Google Scholar]
- [82].Sun FT, Morrell MJ, Wharen RJ. Responsive cortical stimulation for the treatment of epilepsy. Neurotherapeutics. 2008;5(1):68–74. doi: 10.1016/j.nurt.2007.10.069. [DOI] [PMC free article] [PubMed] [Google Scholar]


