Abstract
The fast and accurate identification of nerve tracts is critical for successful nerve anastomosis. Taking advantage of differences in acetylcholinesterase content between the spinal ventral and dorsal roots, we developed a novel quartz crystal microbalance method to distinguish between these nerves based on acetylcholinesterase antibody reactivity. The acetylcholinesterase antibody was immobilized on the electrode surface of a quartz crystal microbalance and reacted with the acetylcholinesterase in sample solution. The formed antigen and antibody complexes added to the mass of the electrode inducing a change in frequency of the electrode. The spinal ventral and dorsal roots were distinguished by the change in frequency. The ventral and dorsal roots were cut into 1 to 2-mm long segments and then soaked in 250 μL PBS. Acetylcholinesterase antibody was immobilized on the quartz crystal microbalance gold electrode surface. The results revealed that in 10 minutes, both spinal ventral and dorsal roots induced a frequency change; however, the frequency change induced by the ventral roots was notably higher than that induced by the dorsal roots. No change was induced by bovine serum albumin or PBS. These results clearly demonstrate that a quartz crystal microbalance sensor can be used as a rapid, highly sensitive and accurate detection tool for the quick identification of spinal nerve roots intraoperatively.
Keywords: neural regeneration, peripheral nerve injury, basic research, quartz crystal microbalance, biosensor, spinal nerve, nerve tract, acetylcholinesterase, antigen-antibody reaction, identification, anastomosis, nerve repair, grants-supported paper, neuroregeneration
Research Highlights
(1) An acetylcholinesterase antibody-based quartz crystal microbalance was used for the rapid identification of spinal ventral and dorsal roots.
(2) Using the quartz crystal microbalance, spinal ventral and dorsal roots can be identified in 10 minutes. The frequency change induced by ventral roots was notably higher than that induced by dorsal roots. This novel method permits the rapid identification of spinal ventral and dorsal roots, and provides a basis for the correct anastomosis and repair of nerve roots.
INTRODUCTION
Peripheral nerve injury is very common, both in wartime and peace. End-to-end neurorrhaphy is the treatment of choice to repair neurotmesis in the clinic. However, nerve regeneration and functional recovery are not satisfactory in most cases. The spinal nerve roots connect peripheral nerves with the spinal cord, and can be divided into two types: a ventral root that mainly consists of motor tracts and a dorsal root that mainly consists of sensory tracts. If the motor and sensory tracts in the spinal nerve roots are not coapted accurately during surgical operation, the regenerated motor fibers do not grow onto the corresponding terminals and the sensory fibers could fail to grow into the spinal cord, leading to the loss of sensory and motor functions. Thus, the fast and accurate identification of spinal ventral and dorsal roots is critical during neuroanastomosis.
Identifying nerve tracts with conventional procedures can take several days. A variety of methods have been proposed to identify and screen nerve tracts, including anatomic[1], thiocholine-based[2], electrophysiological[3], radioisotopic[4], histochemical[5] and immunohistochemical[6] approaches. However, these methods are time consuming (requiring at least 2 hours or more) and are not rapid enough to identify the nerve tracts intraoperatively. Recently, several methods have been developed to quickly identify nerve fascicles, including electrochemistry[7], near-infrared diffuse reflectance spectroscopy[8] and Raman spectroscopy[9]. Though these methods allow for rapid identification, their sensitivity is not high enough to permit accurate identification of nerve roots.
The quartz crystal microbalance technique is a fast and highly sensitive method that permits accurate identification in a few minutes. In recent years, biosensing systems have been developed for trace detection of biologically active substances[10]. Biosensors are devices that detect biological or chemical interactions in the form of antigen-antibody, nucleic acid, enzyme-substrate or receptor-ligand complexes. Interest in using biosensors in clinical medicine is on the rise[11,12,13]. Immunohistochemistry is a powerful molecular tool for monitoring and detecting specific antigens in cells or tissues[14,15]. In this method, the detection of peptides or proteins requires an appropriate cognate antibody labeled with fluorescein or an enzyme, such as horseradish peroxidase or alkaline phosphatase[14,15]. Piezoelectric immunosensors based on quartz crystal microbalance have recently undergone rapid development. They can perform label-free detection of ligands, proteins and nucleic acids[16]. Moreover, they are useful for on-site monitoring, and are easily arrayed for rapid real-time multi-sample analysis[17,18].
Acetylcholinesterase is found in many types of nervous tissue[19]. The activity of acetylcholinesterase in motor fibers is markedly higher than in sensory fibers[20]. Thus, acetylcholinesterase levels in spinal ventral roots are notably greater than in dorsal roots. Currently, some acetylcholinesterase-based quartz crystal microbalance devices are capable of detecting organophosphorus in agricultural products[21]. However, an antibody-based biosensing system for the fast detection of acetylcholinesterase has yet to be fully developed.
The present study sought to develop an acetylcholinesterase antibody-based piezoelectric biosensor, quartz crystal microbalance, for the rapid and sensitive real-time detection of acetylcholinesterase. The spinal ventral and dorsal roots were excised from beagles and were investigated in this study. We aimed to establish a fast, label-free, accurate and inexpensive identification method to distinguish the two nerve roots using the antibody-based quartz crystal microbalance device.
RESULTS
Quantitative analysis of experimental animals
A total of 10 beagles were used in the experiment. The beagles were anesthetized and were sacrificed using an intravenous injection of air. The spinal canal was exposed via median incision in the lumbosacral region; then both ventral and dorsal roots were excised for the experiment (L1–7). All ventral and dorsal roots were included in the final analysis.
Quartz crystal microbalance system and detection
The quartz crystal microbalance device comprises thin film electrodes, usually gold (Au), deposited on each face of a crystal. Voltage is applied across these electrodes to deform the crystal plate, producing relative motion between the two parallel crystal surfaces. The crystal is induced to oscillate at a specific resonant frequency. The frequency decreases gradually with addition of mass, such as glutaraldehyde, immobilization of the acetylcholinesterase antibody or reaction of the acetylcholinesterase antibody and acetylcholinesterase protein on the gold surface of the quartz crystal microbalance. In the quartz crystal microbalance system, the real-time frequency shift was recorded. Figure 1 shows an example of the real-time detection by the quartz crystal microbalance system performed in our study. There was a 138 Hz decrease in series resonant frequency induced by the immobilization of glutaraldehyde. There was a decrease in series resonant frequency of approximately 131 Hz when the acetylcholinesterase antibody was introduced and immobilized onto the glutaraldehyde. A 20 Hz decrease was induced when the acetylcholinesterase protein hybridized with the antibody. In our quartz crystal microbalance system, the PBS stream removed the non-specific binding, and the curve rose immediately after the trough.
Figure 1.
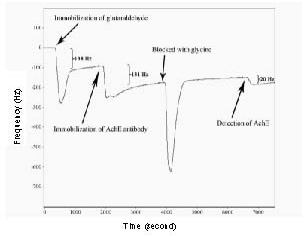
An example of the detection of acetylcholinesterase (AChE) with the quartz crystal microbalance (QCM) system.
The whole process included the immobilization of glutaraldehyde and AChE antibody, glycine blocking and the detection of AChE. First, the glutaraldehyde was injected into the QCM device to activate the surface of the gold electrode; then the AChE antibody was injected into the device to be immobilized onto the electrode surface via the aldehyde group of the glutaraldehyde. After glycine blocking, samples were added into the QCM device for detection.
Optimal concentration of acetylcholinesterase antibody immobilized on the electrode surface
In these experiments, different concentrations of anti-acetylcholinesterase antibody (25, 50, 100 and 150 μg/mL) were evaluated for the efficiency of immobilization onto the electrode surface. The results showed that frequency changes increased almost linearly with increasing antibody concentration, with a peak at 100 and 150 μg/mL. Antibody concentrations of 100 and 150 μg/mL produced the strongest covalent attachment to the glutaraldehyde, compared with other concentrations (P < 0.05) (Figure 2). There was no significant difference between frequency changes at concentrations of 100 and 150 μg/mL (P > 0.05), indicating saturation of the immobilization sites on the glutaraldehyde of the quartz crystal microbalance device. Therefore, an antibody concentration of 100 μg/mL was selected for the following experiments.
Figure 2.
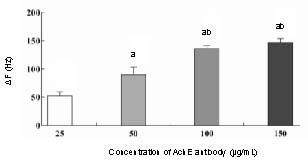
Immobilization efficiency of acetylcholinesterase (AChE) antibody on the gold surface of the quartz crystal microbalance (QCM) device.
Frequency change (ΔF) (Hz) after immobilization was measured and calculated. The data are expressed as mean ± SD, n = 3. aP < 0.05, vs. 25 μg/mL AChE antibody group; bP < 0.05, vs. 50 μg/mL AChE antibody group using a two-tailed Student's t-test.
Detection of acetylcholinesterase in spinal ventral and dorsal roots
The formation of immuno-complexes was monitored in real time based on frequency changes. As shown in Figures 3A and B, the acetylcholinesterase protein, both in ventral and dorsal roots, was detectable by the quartz crystal microbalance system. The detection process lasted for 10 minutes. The acetylcholinesterase antibody was able to specifically bind the complementary antigen. The dorsal root caused a 10.4 ± 0.3 Hz shift in frequency, while the ventral root induced a 25.3 ± 2.1 Hz shift in frequency. The frequency changes induced by the ventral roots were markedly greater (approximately 2.5-fold of the dorsal root value), indicating that the acetylcholinesterase content in ventral roots was significantly higher than that in dorsal roots (P < 0.05; Figure 3C).
Figure 3.
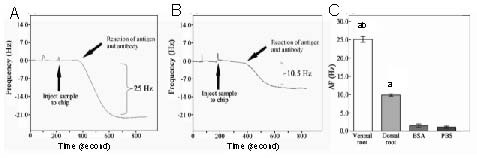
Detection of acetylcholinesterase (AChE) protein in ventral and dorsal roots of beagles with the quartz crystal microbalance (QCM) system.
(A) The detection process for the ventral root solution. There was a 22 Hz decrease in frequency induced by the AChE in the ventral root. (B) The detection process for the dorsal root solution. There was a 10 Hz shift in frequency induced by the AChE in the dorsal root. (C) Frequency change (ΔF) after antigen-antibody reaction was measured and calculated. The data are expressed as mean ± SD; n = 3. aP < 0.05, vs. bovine serum albumin and PBS; bP < 0.05, vs. dorsal root using a two-tailed Student's t-test.
Specificity and reusability of the quartz crystal microbalance system
PBS and bovine serum albumin were assayed as well, revealing that the assay was specific for acetylcholinesterase. As shown in Figure 3C, the frequency changes resulting from PBS and bovine serum albumin were 1.1 ± 0.2 and 1.3 ± 0.9 Hz, respectively. This result indicates that the detection of acetylcholinesterase can be distinguished from the buffer and other proteins. For the same crystal, a regeneration solution of NaOH (10 mM) was applied for 10 minutes; however, about 50% loss of activity was observed (data not shown). Therefore, samples were tested using a new crystal after the original crystal was saturated in this study.
DISCUSSION
The rapid identification of spinal nerve roots has long been a major challenge for researchers and clinicians. Generally, the ventral root contains motor fibers and the dorsal root contains sensory fibers. The main difference between the motor and sensory fibers is that acetylcholine is the neurotransmitter of motor fibers, while substance P is the primary neurotransmitter of sensory fibers[22]. The acetylcholinesterase content is notably greater in the ventral root than in the dorsal root[20]. In recent years, many methods have been developed to distinguish peripheral nerves. Acetylcholinesterase staining can successfully distinguish between ventral and dorsal roots, but this method requires at least 1 hour for correct identification[2,23]. The radioisotopic method requires 3 hours or more to identify motor/sensory nerves. While this method may be more sensitive, it is more expensive. Bright blue-labeled monoclonal Blue-SAB specifically reacts against the cell body and may be used to identify sensory fibers of nerve trunks by immunohistochemistry. Unfortunately, these sophisticated methods are costly in both time and money. Other methods, such as electrochemistry, near-infrared diffuse reflectance spectroscopy and Raman spectroscopy, permit rapid identification; however, their sensitivity is not high enough and the accurate identification of nerve roots by these methods is controversial. Building on previous work, we hoped to establish a novel rapid and accurate method to identify nerve roots based on differences in acetylcholinesterase content. In the present study, we show that the quartz crystal microbalance method successfully performed a rapid and accurate identification of spinal nerve roots based on levels of acetylcholinesterase content using a pre-modified chip in 10 minutes. To our knowledge, this is the first report on the rapid and accurate identification of nerve tracts using an acetylcholinesterase antibody-based quartz crystal microbalance device.
Quartz crystal microbalance is a new type of high-accuracy resonance-type metering device. The core of the quartz crystal microbalance is a specifically manufactured quartz plate with a fundamental resonance frequency in the range of 5–30 MHz. The crystal is excited to resonance, and the effect of molecular absorption is monitored[24]. Changes in the mass of the material on the surface will alter the resonance frequency of the crystal[16]. A linear relationship exists between deposited mass and frequency response for quartz crystals. The resonance frequency decreases linearly with increasing mass on the quartz crystal microbalance electrode at the nanogram level or less. This characteristic of quartz crystal microbalance can be exploited to develop bioanalytical tools on a 10–10 to 10–12 g scale[17]. Quartz crystal microbalance-based immunoassay has been designed and applied in several different areas[25,26]. Recently, quartz crystal microbalance has been successfully developed as a dynamic and sensitive tool to monitor antigen or antibody attachment to the quartz surface and antibody-antigen specific recognition[27].
In our study, the ventral and dorsal roots were cut into 1 to 2-mm long segments for the detection. We immobilized various concentrations of acetylcholinesterase antibody on the electrode surface of the quartz crystal microbalance device. The optimal concentration (100 μg/mL) was found according to the saturation concentration of antibody on the surface of the quartz crystal microbalance device. The segments of ventral and dorsal roots were dissolved in the PBS. The acetylcholinesterase antibody specifically bound the acetylcholinesterase protein (but not bovine serum albumin), leading to an increase in frequency shift. We observed that both ventral and dorsal roots contained acetylcholinesterase and could induce a frequency shift. This observation is consistent with previous study[5]. The frequency changes induced by ventral root solution was notably more than that induced by dorsal root solution. Therefore, we identified the nerve root based on the difference in acetylcholinesterase content between the ventral and dorsal roots. After the antibody was immobilized on the sensor chip, we only needed 10 minutes for the detection. Bovine serum albumin and PBS did not bind onto the surface of the quartz crystal microbalance device and the frequency remained stable. The specificity of the quartz crystal microbalance immunosensor is mainly dependent upon the antibodies immobilized on the crystal surface. Our result also demonstrates that the monoclonal acetylcholinesterase antibody developed in this study works well for the specific detection of acetylcholinesterase protein.
The preparation of the chip, including activation, immobilization and blocking, required approximately 1.5 hours, and the chip modified with the acetylcholinesterase antibody could be stored in PBS at 4°C for 1 month. The detection of acetylcholinesterase can be performed immediately with the pre-modified chip. Acetylcholinesterase is also found on erythrocyte membranes, where it constitutes the Yt blood group antigen[28]. Therefore, it is necessary to wash the blood out of the nerve samples before detection.
In conclusion, a new quartz crystal microbalance method was developed for the fast identification of spinal nerve roots. Current data clearly demonstrate that the acetylcholinesterase antibody-based quartz crystal microbalance sensor might be used as a fast (10 minutes), label-free, sensitive, convenient and inexpensive detection tool for the identification of spinal nerve roots intraoperatively. The application of the present sensor for the rapid identification of all branches of major peripheral nerves, such as the ulnar nerve, radial nerve and peroneal nerve, will be performed in the future. Ultimately, we hope to accomplish the rapid and precise identification of all peripheral nerves intraoperatively in a non-invasive manner to aid the accurate anastomosis of nerve stumps and improve nerve regeneration and functional recovery.
MATERIALS AND METHODS
Design
A difference detection in vitro study.
Time and setting
This study was performed at the Laboratory of Dalian Institute of Chemical Physics, Chinese Academy of Sciences, China between January and July 2011.
Materials
Animals
A total of 10 adult beagles, 12 months old, of equal gender, weighing 7–12 kg (8.4 ± 1.5 kg), were provided by the animal experiment center of Nanjing Medical University (SCXK (Su) 2002-0031). All experimental procedures were performed in accordance with the Guidance Suggestions for the Care and Use of Laboratory Animals, formulated by the Ministry of Science and Technology of China[29].
Device
A 9 MHz AT cut piezoelectric quartz crystal slab (ANT Technology Co., Ltd., Taipei, Taiwan, China), attached to a layer of a gold electrode on each side (0.091 cm2 in area on each side; the detection limit of the quartz crystal microbalance instrument in liquid = 0.1 Hz), was used as the transducer in our experiment. The size of the quartz crystal microbalance device was 45 × 45 × 40 cm3. The flow injection and continuous frequency variation recordings were performed using an Affinity Detection System (ANT Technology Co., Ltd.). The system has five main components, including an electronic oscillation circuit, a frequency counter, a piezoelectric quartz of fixed biosensor molecule (p-chip), a single loop flow system and a computer to record and analyze the curve of frequency changes in real time (Figure 4). The sensor unit had the following features: resolution, 0.1 Hz; sampling period, 1 second; frequency range, 2–16 MHz; temperature range, 4–60°C; voltage, 220 V; alternating current frequency, 50–60 Hz. The reaction cell was one sensor signal channel with 30 μL of reaction cell volume. The single loop flow system consisted of a temperature controller, sample tubes, pipelines and a tubing pump with a flow rate of 10–200 μL/min and a sample loop volume of 200 μL. The gold electrode surface of the quartz crystal microbalance was pre-incubated with amidogen (ANT Technology Co., Ltd.) by the manufacturer.
Figure 4.
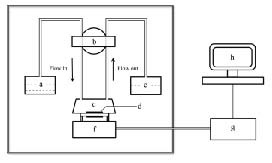
Schematic diagram of the apparatus for the quartz crystal microbalance (QCM) system.
a: Sample cell; b: pump; c: reaction cell; d: gold surface of QCM device; e: disposal bottle; f: oscillation circuit; g: frequency counter; h: personal computer and operating software.
Methods
Surgical procedures
Ketamine 15 mg/kg, droperidol 5 mg and atropine 0.5 mg were used for the induction of anesthesia, and animals were sacrificed using an intravenous injection of air. The spinal canal was exposed via a median incision in the lumbosacral region with the dog lying prone; then both ventral and dorsal roots were excised for the experiment (L1–7). These specimens were immediately frozen with liquid nitrogen for preservation.
Acetylcholinesterase antibody immobilization
The acetylcholinesterase antibody was immobilized on the gold electrode surface of the quartz crystal microbalance device as previously described, with minor modifications[28]. In brief, the quartz crystal microbalance system was washed with 1 M NaOH for 30 minutes, 1 N HCl for 30 minutes and then deionized water (18.2 MΩ × cm; Millipore-Milli-Q system, Bedford, MA, USA) for 60 minutes. Then, 0.01 M phosphate buffer (0.03 M NaCl, 1 M KH2PO4 and 1 M K2HPO4, pH 7.4) was flushed through the system at a speed of 60 μL/min. With the frequency of the chip steady at 500 seconds, frequency changes within ± 5 Hz, the solution containing 2.5% glutaraldehyde (total volume: 200 μL; Sigma, St. Louis, MO, USA) was injected into the system to activate the surface for antibody immobilization. After immobilization of the glutaraldehyde, the pipeline was cleaned by flowing through the PBS (0.01 M, pH 7.4, containing 0.154 M NaCl) for 15 minutes to remove unbound glutaraldehyde on the gold surface and glutaraldehyde residues in the pipeline. Then, various concentrations (25, 50, 100 and 150 μg/mL) of acetylcholinesterase antibody (Monoclonal antibody to acetylcholinesterase; Abgent, San Diego, CA, USA) were added to the sample tube and immobilized onto the glutaraldehyde by covalent binding. The pipeline was cleaned by flowing through the PBS for 15 minutes to remove unbound antibody. Afterward, the remaining active aldehyde groups were blocked by injecting 1 M glycine (Sigma) solution into the system. Finally, the acetylcholinesterase antibody-modified sensor chip was prepared for use. The chip was stored in PBS at 4°C for 1 month.
Procedure for acetylcholinesterase detection
The ventral and dorsal roots were cut into 1 to 2-mm-long segments, and then soaked in Eppendorf tubes with 250 μL PBS (0.01 M) for the acetylcholinesterase dissolution. The lysates were cleared by centrifugation for 5 minutes at 12 000 × g. The supernatant (200 μL) was obtained for the acetylcholinesterase detection. After the acetylcholinesterase antibody was immobilized on the quartz crystal microbalance gold electrode surface, the gold-quartz crystal microbalance device was exposed to the sample flow (total volume: 200 μL) containing lysates of nerve roots and 5 % bovine serum albumin (Sigma). Reaction of acetylcholinesterase antibody and acetylcholinesterase in the quartz crystal microbalance device was at a flow speed of 30 μL/min. The frequency shift during the reaction was recorded in real-time.
The frequency changes were monitored in real-time continuous reading and reported as the difference between the final value and the value before the antigen-antibody reaction or immobilization. The mass of immobilized glutaraldehyde as well as acetylcholinesterase antibody and acetylcholinesterase protein on the quartz crystal microbalance device was calculated using Sauerbrey's equation[30]:
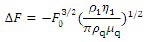
Where ΔF is the measured frequency shift, F0 is the resonant frequency of the unloaded crystal, ρ1 is the density of liquid in contact with the crystal, η1 is the viscosity of the liquid in contact with the crystal, ρq is the density of quartz (2.648 g/cm3) and μq is the shear modulus of quartz, 2.947 × 1011 g/cm/s2. A frequency change of 1 Hz corresponds to a mass change of 0.883 ng.
Statistical analysis
The experimental data were analyzed using P-Sensor software (3.0, ANT Technology Co., Ltd.) of the ADS system in real-time. Each experiment was repeated three times. All data were presented as mean ± SD. Differences between groups were evaluated using a two-tailed Student's t-test. P-values less than 0.05 were considered statistically significant.
Acknowledgments
We thank Wujun Liu, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, China, for providing technical support.
Footnotes
Funding: This work was supported by the National Natural Science Foundation of China, No. 30973058, 81171694; Jiangsu Province Natural Science Foundation, No. BE2010743; Jiangsu Graduate Student Innovation Project, No.CXZZ11_0721; the Program for Development of Innovative Research Team in the First Affiliated Hospital of Nanjing Medical University, No. IRT-015; and a Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions.
Conflicts of interest: None declared.
Ethical approval: The experiment was approved by the Animal Ethics Committee of Nanjing Medical University, China.
(Edited by Luo YX, He XJ/Qiu Y/Song LP)
REFERENCES
- [1].Williams HB, Jabaley ME. The importance of internal anatomy of the peripheral nerves to nerve repair in the forearm and hand. Hand Clin. 1986;2(4):689–707. [PubMed] [Google Scholar]
- [2].Karnovsky MJ, Roots L. A “Direct-Coloring” thiocholine method for cholinesterases. J Histochem Cytochem. 1964;12:219–221. doi: 10.1177/12.3.219. [DOI] [PubMed] [Google Scholar]
- [3].Deutinger M, Girsch W, Burggasser G, et al. Clinical and electroneurographic evaluation of sensory/motor-differentiated nerve repair in the hand. J Neurosurg. 1993;78(5):709–713. doi: 10.3171/jns.1993.78.5.0709. [DOI] [PubMed] [Google Scholar]
- [4].Hattori Y, Doi K. Radioisotope technique to evaluate the motor functional status of donor nerve for upper extremity reconstruction. Tech Hand Up Extrem Surg. 2004;8(3):189–192. doi: 10.1097/01.bth.0000134712.83301.0a. [DOI] [PubMed] [Google Scholar]
- [5].He YS, Zhong SZ. Acetylcholinesterase: a histochemical identification of motor and sensory fascicles in human peripheral nerve and its use during operation. Plast Reconstr Surg. 1988;82(1):125–132. [PubMed] [Google Scholar]
- [6].Shepherd AJ, Mohapatra DP. Tissue preparation and immunostaining of mouse sensory nerve fibers innervating skin and limb bones. J Vis Exp. 2012;26(59):e3485. doi: 10.3791/3485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Zhao W, Sun SX, Xu JJ, et al. Electrochemical identification of the property of peripheral nerve fiber based on a biocompatible polymer film via in situ incorporating gold nanoparticles. Anal Chem. 2008;80(10):3769–3776. doi: 10.1021/ac702395c. [DOI] [PubMed] [Google Scholar]
- [8].Xie S, Xiang B, Bu S, et al. Rapid identification of anterior and posterior root of cauda equina nerves by near-infrared diffuse reflectance spectroscopy. J Biomed Opt. 2009;14(2):024005. doi: 10.1117/1.3086611. [DOI] [PubMed] [Google Scholar]
- [9].Wang H, Ma F, Wang F, et al. Identification of motor and sensory fascicles in peripheral nerve trunk using immunohistochemistry and micro-Raman spectroscopy. J Trauma. 2011;71(5):1246–1251. doi: 10.1097/TA.0b013e31822503a7. [DOI] [PubMed] [Google Scholar]
- [10].Campbell GA, Mutharasan R. Detection of pathogen Escherichia coli O157:H7 using self-excited PZT-glass microcantilevers. Biosens Bioelectron. 2005;21(3):462–473. doi: 10.1016/j.bios.2004.11.009. [DOI] [PubMed] [Google Scholar]
- [11].Chen CY, Chang CC, Yu C, et al. Clinical application of surface plasmon resonance-based biosensors for fetal fibronectin detection. Sensors (Basel) 2012;12(4):3879–3890. doi: 10.3390/s120403879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Xu Q, Chang K, Lu W, et al. Detection of single-nucleotide polymorphisms with novel leaky surface acoustic wave biosensors, DNA ligation and enzymatic signal amplification. Biosens Bioelectron. 2012;33(1):274–278. doi: 10.1016/j.bios.2011.12.036. [DOI] [PubMed] [Google Scholar]
- [13].Gaster RS, Hall DA, Nielsen CH, et al. Matrix-insensitive protein assays push the limits of biosensors in medicine. Nat Med. 2009;15(11):1327–1332. doi: 10.1038/nm.2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Schuster C, Malinowsky K, Liebmann S, et al. Antibody validation by combining immunohistochemistry and protein extraction from formalin-fixed paraffin-embedded tissues. Histopathology. 2012;60(6B):E37–50. doi: 10.1111/j.1365-2559.2012.04184.x. [DOI] [PubMed] [Google Scholar]
- [15].Luo QH, Liu WT, Chen JY, et al. Nerve growth factor and inducible nitric oxide synthase expression in the mesencephalon and diencephalon, as well as visual- and auditory-related nervous tissues, in a macaque model of type 2 diabetes. Neural Regen Res. 2012;7(4):302–307. doi: 10.3969/j.issn.1673-5374.2012.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Tang W, Wang D, Xu Y, et al. A self-assembled DNA nanostructure-amplified quartz crystal microbalance with dissipation biosensing platform for nucleic acids. Chem Commun (Camb) 2012;48(53):6678–6680. doi: 10.1039/c2cc32747c. [DOI] [PubMed] [Google Scholar]
- [17].Zhou XC, Huang LQ, Li SF. Microgravimetric DNA sensor based on quartz crystal microbalance: comparison of oligonucleotide immobilization methods and the application in genetic diagnosis. Biosens Bioelectron. 2001;16(1-2):85–95. doi: 10.1016/s0956-5663(00)00136-6. [DOI] [PubMed] [Google Scholar]
- [18].Salmain M, Ghasemi M, Boujday S, et al. Piezoelectric immunosensor for direct and rapid detection of staphylococcal enterotoxin A (SEA) at the ng level. Biosens Bioelectron. 2011;29(1):140–144. doi: 10.1016/j.bios.2011.08.007. [DOI] [PubMed] [Google Scholar]
- [19].Satoh K, Armstrong DM, Fibiger HC. A comparison of the distribution of central cholinergic neurons as demonstrated by acetylcholinesterase pharmacohistochemistry and choline acetyltransferase immunohistochemistry. Brain Res Bull. 1983;11(6):693–720. doi: 10.1016/0361-9230(83)90013-8. [DOI] [PubMed] [Google Scholar]
- [20].McNeill ME, Norvell JE. Acetylcholinesterase activity of primary sensory neurons and dorsal root fibers in the cat. Anat Rec. 1978;190(1):155–159. doi: 10.1002/ar.1091900113. [DOI] [PubMed] [Google Scholar]
- [21].Du D, Chen A, Xie Y, et al. Nanoparticle-based immunosensor with apoferritin templated metallic phosphate label for quantification of phosphorylated acetylcholinesterase. Biosens Bioelectron. 2011;26(9):3857–3863. doi: 10.1016/j.bios.2011.02.047. [DOI] [PubMed] [Google Scholar]
- [22].Gruber H, Zenker W. Acetylcholinesterase: histochemical differentiation between motor and sensory nerve fibres. Brain Res. 1973;51:207–214. doi: 10.1016/0006-8993(73)90373-9. [DOI] [PubMed] [Google Scholar]
- [23].Kanaya F. Mixed nerve suture facilitated by enzymestaining techniques. Tech Hand Up Extrem Surg. 2002;6(3):140–144. doi: 10.1097/00130911-200209000-00007. [DOI] [PubMed] [Google Scholar]
- [24].Okahata Y, En-na G, Ebato H. Synthetic chemoreceptive membranes. Sensing bitter or odorous substances on a synthetic lipid multibilayer film by using quartz-crystal microbalances and electric responses. Anal Chem. 1990;62(14):1431–1438. doi: 10.1021/ac00213a017. [DOI] [PubMed] [Google Scholar]
- [25].Yan M, Wang D, Xie J, et al. Investigation of the adsorption characteristics of natural organic matter from typical Chinese surface waters onto alumina using quartz crystal microbalance with dissipation. J Hazard Mater. 2012;215-216:115–121. doi: 10.1016/j.jhazmat.2012.02.039. [DOI] [PubMed] [Google Scholar]
- [26].Bayramoglu G, Arica MY. Development of a sensitive method for selection of affinity ligand for trypsin using quartz crystal microbalance sensor. Bioprocess Biosyst Eng. 2012;35(3):423–431. doi: 10.1007/s00449-011-0581-4. [DOI] [PubMed] [Google Scholar]
- [27].Kim DM, Rahman MA, Do MH, et al. An amperometric chloramphenicol immunosensor based on cadmium sulfide nanoparticles modified-dendrimer bonded conducting polymer. Biosens Bioelectron. 2010;25(7):1781–1788. doi: 10.1016/j.bios.2009.12.024. [DOI] [PubMed] [Google Scholar]
- [28].Prall YG, Gambhir KK, Ampy FR. Acetylcholinesterase: an enzymatic marker of human red blood cell aging. Life Sci. 1998;63(3):177–184. doi: 10.1016/s0024-3205(98)00258-6. [DOI] [PubMed] [Google Scholar]
- [29].The Ministry of Science and Technology of the People s Republic of China. Guidance Suggestions for the Care and Use of Laboratory Animals. 2006-09-30 [Google Scholar]
- [30].Kanazawa KK, Gordon JG., II The oscillation frequency of a quartz resonator in contact with liquid. Analytica Chimica Acta. 1985;175:99–105. [Google Scholar]


