Abstract
Inhibition of neurite growth, which is in large part mediated by the Nogo-66 receptor, affects neural regeneration following bone marrow mesenchymal stem cell transplantation. The tissue engineering scaffold poly(D,L-lactide-co-glycolic acid) has good histocompatibility and can promote the growth of regenerating nerve fibers. The present study used small interfering RNA to silence Nogo-66 receptor gene expression in bone marrow mesenchymal stem cells and Schwann cells, which were subsequently transplanted with poly(D,L-lactide-co-glycolic acid) into the spinal cord lesion regions in rats. Simultaneously, rats treated with scaffold only were taken as the control group. Hematoxylin-eosin staining and immunohistochemistry revealed that at 4 weeks after transplantation, rats had good motor function of the hind limb after treatment with Nogo-66 receptor gene-silenced cells plus the poly(D,L-lactide-co-glycolic acid) scaffold compared with rats treated with scaffold only, and the number of bone marrow mesenchymal stem cells and neuron-like cells was also increased. At 8 weeks after transplantation, horseradish peroxidase tracing and transmission electron microscopy showed a large number of unmyelinated and myelinated nerve fibers, as well as intact regenerating axonal myelin sheath following spinal cord hemisection injury. These experimental findings indicate that transplantation of Nogo-66 receptor gene-silenced bone marrow mesenchymal stem cells and Schwann cells plus a poly(D,L-lactide-co-glycolic acid) scaffold can significantly enhance axonal regeneration of spinal cord neurons and improve motor function of the extremities in rats following spinal cord injury.
Keywords: neural regeneration, spinal cord injury, bone marrow mesenchymal stem cells, Schwann cells, poly(D, L-lactide-co-glycolic acid), Nogo-66 receptor gene, rats, gene silencing, grants-supported paper, photographs-containing paper, neuroregeneration
Research Highlights
(1) Transplantation of Nogo-66 receptor gene-silenced bone marrow mesenchymal stem cells and Schwann cells, combined with a poly(D,L-lactide-co-glycolic acid) scaffold, can significantly enhance axonal regeneration of spinal cord neurons and improve motor function of the extremities in rats following spinal cord injury.
(2) Combined transplantation of bone marrow mesenchymal stem cells and Schwann cells, with Nogo-66 receptor gene silencing, can reduce glial scar formation and promote axonal growth of nerve cells, and rapidly repair the injured nerves.
INTRODUCTION
Spinal cord injury is damage that results in complete or partial loss of sensation and/or mobility, and affects the quality of life of patients[1,2,3,4,5]. Because of its high morbidity, spinal cord injury is considered a major threat to human health. In recent years, stem cell therapy has shown considerable therapeutic potential in spinal cord injury[6,7,8]. However, bone marrow mesenchymal stem cell transplantation alone is not sufficient for spinal cord repair because the majority of bone marrow mesenchymal stem cells engrafted into the spinal cord differentiate along glial lineages and rarely survive at adequate quantity. The microenvironment of the injured spinal cord is believed to play a crucial role in driving the differentiation and survival of the engrafted bone marrow mesenchymal stem cells[9,10]. Schwann cells can secrete various neurotrophic factors, as well as extracellular matrix and cell adhesion molecules, which promote nerve repair and neurite outgrowth in the central nervous system[11]. Poly(D,L-lactide-co-glycolic acid) has good histocompatibility and promotes the orderly growth of regenerating nerve fibers[12]. The neurite growth inhibition mediated by the Nogo-66 receptoris a major factor impeding repair following bone marrow mesenchymal stem cell transplantation[13].
In this study, we subjected bone marrow mesenchymal stem cells and Schwann cells to Nogo-66 receptor gene silencing using small interfering RNA. These cells were then transplanted along with a poly(D,L-lactide-co-glycolic acid) scaffold into the spinal cord lesion in rats to assess the therapeutic potential of this treatment strategy for spinal cord injury.
RESULTS
Quantitative analysis of experimental animals
A total of 72 healthy adult female Wistar rats were randomly divided into simple poly(D,L-lactide-co-glycolic acid) group, bone marrow mesenchymal stem cells + Schwann cells + poly(D,L-lactide-co-glycolic acid) group, and Nogo-66 receptor gene-silenced cells + poly(D,L-lactide-co-glycolic acid) group, with 24 animals in each group. As a model of spinal cord injury, hemisection of the T9 spinal cord was performed on rats in each group.
Morphology of PKH26-labeled bone marrow mesenchymal stem cells
The proliferation of bone marrow mesenchymal stem cells at passage 3 was assessed using flow cytometry. Results showed that these cells were positive for CD29, CD44 and CD105, and negative for CD34, CD80 and CD86. Purity was high, with the bone marrow mesenchymal stem cells representing greater than 97% of the total cell population. PKH26-labeled bone marrow mesenchymal stem cells exhibited a red fluorescence under the fluorescence microscope (PKH26 is a lipophilic fluorescent dye that irreversibly binds the lipid membrane bilayer, allowing the contour of the cells to be observed with fluorescence microscopy; Figure 1).
Figure 1.
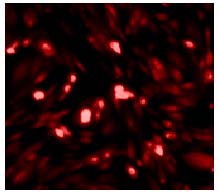
PKH26-labeled bone marrow mesenchymal stem cells (fluorescence microscopy, × 200).
Morphology of 4’,6-diamidino-2-phenylindole-labeled Schwann cells
At 5–6 days, cells covered the bottom of the culture dish. The majority were Schwann cells, and a small number of fibroblasts were observed. Schwann cell purity was greater than 95%. These cells exhibited a fusiform or constricted shape, and possessed small nuclei. Immunofluorescence staining revealed that the Schwann cell bodies and neurites were positive for myelin basic protein. Under the fluorescence microscope, Schwann cell and fibroblast nuclei were stained blue by 4’,6-diamidino-2-phenylindole, while the cytoplasm was not stained. These staining results indicate that the majority of cells were Schwann cells (Figure 2).
Figure 2.
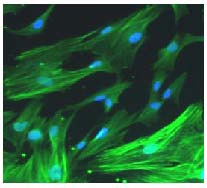
Immunohistochemistry for myelin basic protein (green) of 4’,6-diamidino-2-phenylindole-labeled (blue) Schwann cells (fluorescence microscopy, × 200).
Purified Schwann cells were up to 95% or more pure, with a fusiform or constricted shape, and with small nuclei. Myelin basic protein immunofluorescence revealed the Schwann cell bodies.
Morphology of Nogo-66 receptor gene-silenced bone marrow mesenchymal stem cells and Schwann cells
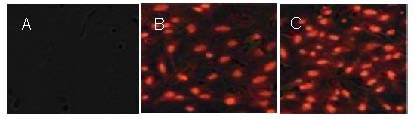
At 4 days after transplantation, a large number of Nogo-66 receptor gene-silenced bone marrow mesenchymal stem cells and Schwann cells were visible in the serum-impregnated poly(D,L-lactide-co-glycolic acid) under the fluorescence microscope. The cells adhered to and grew along the scaffold, and only a small number of cells adhered to the adjacent area where they grew in a disordered manner. A large number of implanted cells were found to adhere and grow in the Nogo-66 receptor gene-silenced group compared with cells not subjected to gene silencing (Figure 3).
Figure 3.
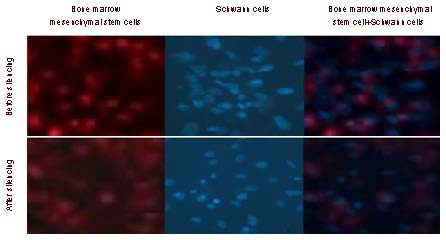
Morphology of bone marrow mesenchymal stem cells with PKH26 (red) labeling and that of Schwann cells with 4’6-diamidino-2-phenylindole (blue) staining before and after Nogo-66 receptor gene silencing (fluorescence microscope, × 200).
A large number of implanted cells were found to adhere and grow in the Nogo-66 receptor gene-silenced cells + poly(D,L-lactide-co-glycolic acid) group compared with cells not subjected to silencing.
Lower extremity motor function after transplantation in rats with spinal cord injury
Spinal cord functions were evaluated using the inclined plate test and the modified Basso-Beattie-Bresnahan scoring system 4–8 weeks after transplantation (Tables 1 and 2). Lower extremity motor function in the Nogo-66 receptor gene-silenced + Schwann cells + poly(D,L-lactide-co-glycolic acid) group was better than in the simple poly(D,L-lactide-co-glycolic acid) group (P < 0.01), and also better than in the bone marrow mesenchymal stem cells + Schwann cells + poly(D,L-lactide-co-glycolic acid) group (P < 0.05).
Table 1.
Effect of Nogo-66 receptor gene-silenced BMSCs + Schwann cells + PLGA scaffold transplantation on spinal cord function following spinal cord injury
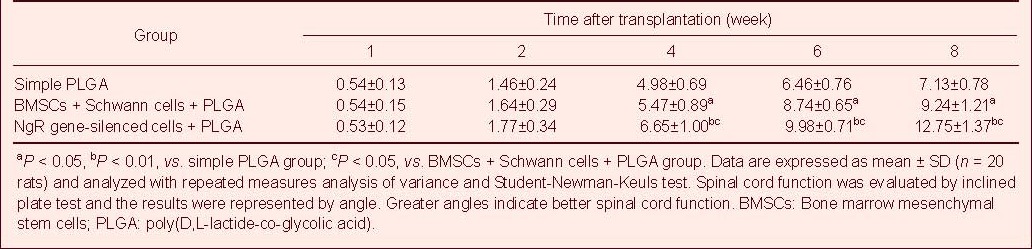
Table 2.
Effect of Nogo-66 receptor gene-silenced BMSCs + Schwann cells + PLGA scaffold transplantation on Basso-Beattie-Bresnahan scale scores after spinal cord injury
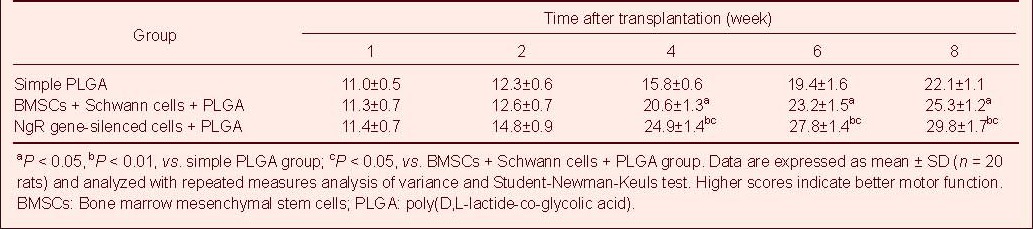
Spinal cord morphology after transplantation
At 4 weeks after transplantation, hematoxylin-eosin staining showed that spinal cord tissue rupturing, scar formation, structural defects and obvious cavity formation were visible in the simple poly(D,L-lactide-co-glycolic acid) group (Figure 4A). In the bone marrow mesenchymal stem cells + Schwann cells + poly(D,L-lactide-co-glycolic acid) group, typical nerve cell-like morphological changes were observed, and the tissue cavity was smaller than in the simple poly(D,L-lactide-co-glycolic acid) group, although it was larger than in the Nogo-66 receptor gene-silenced cells + poly(D,L-lactide-co-glycolic acid) group (Figure 4B). In the Nogo-66 receptor gene-silenced cells + poly(D,L-lactide-co-glycolic acid) group, typical neuron-like morphological changes were observed, and a cavity was not present (Figure 4C).
Figure 4.
Morphology of injured spinal cord tissue after cell transplantation (hematoxylin-eosin staining, × 40).
(A) Spinal cord tissue fracturing, scar formation, structural disorder, and obvious cavity formation were visible in the simple PLGA group.
(B) In the BMSCs + Schwann cells + PLGA group, typical neuron-like morphological changes were observed, and the tissue cavity was smaller than in the simple PLGA group, but larger than in the Nogo-66 receptor gene-silenced cells + PLGA group.
(C) In the Nogo-66 receptor gene-silenced cells + PLGA group, typical neuron-like morphological changes were observed, and a cavity was not present.
BMSCs: Bone marrow mesenchymal stem cells; PLGA: Poly(D,L-lactide-co-glycolic acid).
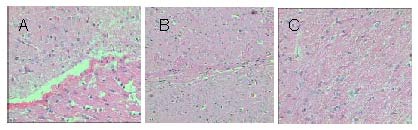
PKH26-positive cells (red fluorescence) were scattered in all sections; 0 cells/high-power field in the simple poly(D,L-lactide-co-glycolic acid) group (Figure 5A), 32.64 ± 10.83 cells/high-power field in the bone marrow mesenchymal stem cells + Schwann cells + poly(D,L-lactide-co-glycolic acid) group (Figure 5B), and 72.64 ± 8.54 cells/high-power field in the Nogo-66 receptor gene-silenced cells + poly(D,L-lactide-co-glycolic acid) group (Figure 5C). Analysis of variance and Dunnett's t-test showed significant differences among the groups (P < 0.01).
Figure 5.
Morphology of PKH26-labeled cells (red fluorescence) in the injured spinal cord tissue after cell transplantation (× 200).
(A) In the simple PLGA group, no PKH26-labeled cells were visible in frozen sections under the microscope.
(B) In the BMSCs + Schwann cells + PLGA group, the number PKH26-labeled cells in frozen sections was lower than that in the Nogo-66 receptor gene-silenced cells + PLGA group, but higher than that in the simple PLGA group.
(C) In the Nogo-66 receptor gene-silenced cells + PLGA group, a large number of PKH26-labeled cells were visible in frozen sections.
BMSCs: Bone marrow mesenchymal stem cells; PLGA: poly(D,L-lactide-co-glycolic acid).
Expression of Nogo-66 receptor mRNA in spinal cord tissue after silencing of the Nogo-66 receptor gene, as determined by reverse transcription-PCR
At 4 weeks after transplantation, Nogo-66 receptor mRNA expression (absorbance) in the Nogo-66 receptor gene-silenced cells + poly(D,L-lactide-co-glycolic acid) group (0.47 ± 0.12) was significantly lower than that in the simple poly(D,L-lactide-co-glycolic acid) group (0.47 ± 0.12) or the bone marrow mesenchymal stem cells + Schwann cells + poly(D,L-lactide-co-glycolic acid) group (0.46 ± 0.09, P < 0.05; Figure 6.
Figure 6.
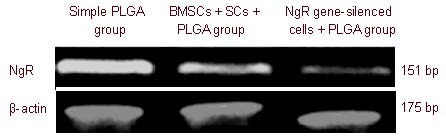
NgR mRNA expression in rat spinal cord tissue 4 weeks after cell transplantation.
NgR gene expression in the NgR gene-silenced cells + PLGA group was significantly lower than in the simple PLGA group or the BMSCs + SCs + PLGA group.
BMSCs: Bone marrow mesenchymal stem cells; SCs: Schwann cells; PLGA: poly(D,L-lactide-co-glycolic acid); NgR: Nogo-66 receptor.
Visualization of nerve fibers in the spinal cord by horseradish peroxidase retrograde tracing
After the 3,3’-diaminobenzidine color reaction, an area in which the central part was deeply stained and the surrounding was progressively lighter, was visible at the injection site. In the simple poly(D,L-lactide-co-glycolic acid) group, 2 days after the rats were injected with horseradish peroxidase (which is retrogradely transported) via the lumbar enlargement, only a small number of horseradish peroxidase-labeled nerve fibers were visible above the T8 segment (Figure 7A). In the bone marrow mesenchymal stem cells + Schwann cells + poly(D,L-lactide-co-glycolic acid) group, the number of horseradish peroxidase-positive nerve fibers was significantly lower than that in the Nogo-66 receptor gene-silenced cells + poly(D,L-lactide-co-glycolic acid) group, but higher than that in the simple poly(D,L-lactide-co-glycolic acid) group (Figure 7B).
Figure 7.
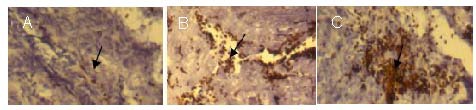
Horseradish peroxidase-labeled nerve fibers in the spinal cord visualized by horseradish peroxidase retrograde tracing in each group at 8 weeks after cell transplantation (3,3’-diaminobenzidine, × 200).
(A–C) simple PLGA group, BMSCs + Schwann cells + PLGA group, NgR gene-silenced cells + PLGA group, respectively. At 8 weeks after transplantation, the number of horseradish peroxidase-positive nerve fibers (arrows) was lower in the simple PLGA group than that in the BMSCs + Schwann cells + PLGA group, and it was lower in the BMSCs + Schwann cells + PLGA group than that in the NgR gene-silenced cells + PLGA group.
BMSCs: Bone marrow mesenchymal stem cells; PLGA: poly(D,L-lactide-co-glycolic acid); NgR: Nogo-66 receptor.
In the Nogo-66 receptor gene-silenced cells + poly(D,L-lactide-co-glycolic acid) group, there was a large number of horseradish peroxidase-labeled nerve fibers in the spinal cord (Figure 7C). There were significant differences in the number of horseradish peroxidase-positive nerve fibers among the three groups (P < 0.01). In the simple poly(D,L-lactide-co-glycolic acid) group, 17.6 ± 4.68 cells/high-power field were visible; in the bone marrow mesenchymal stem cells + Schwann cells + poly(D,L-lactide-co-glycolic acid) group, 45.87 ± 12.33 cells/high-power field were observed; and in the Nogo-66 receptor gene-silenced cells + poly(D,L-lactide-co-glycolic acid) group, 97.34 ± 13.67 cells/high-power field were visible.
Transmission electron microscopy observation of myelinated nerve fibers
At 8 weeks after transplantation, transmission electron microscopy revealed that a glial scar, a small number of myelinated nerve fibers, phagocytic degeneration, and necrotic myelinated nerve fibers were present in the simple poly(D,L-lactide-co-glycolic acid) group (Figure 8A).
Figure 8.
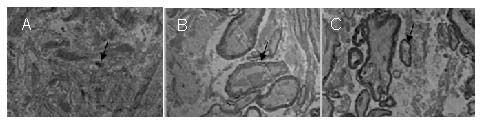
Axonal regeneration in the injured spinal cord in rats 8 weeks after cell transplantation (transmission electron microscopy, × 8 000).
(A) In the simple PLGA group, glial scarring and a small amount of myelinated nerve fibers were visible (arrow).
(B) In the BMSCs + Schwann cells + PLGA group, there were many myelinated and unmyelinated nerve fibers (arrow).
(C) In the Nogo-66 receptor gene-silenced cells + PLGA group, a large number of myelinated and unmyelinated nerve fibers (arrow), axons, as well as intact regenerating axonal myelin were observed.
BMSCs: Bone marrow mesenchymal stem cells; PLGA: poly(D,L-lactide-co-glycolic acid).
In the bone marrow mesenchymal stem cells + Schwann cells + poly(D,L-lactide-co-glycolic acid) group, there were many myelinated and unmyelinated nerve fibers, with the number greater than in the simple poly(D,L-lactide-co-glycolic acid) group (Figure 8B). In the Nogo-66 receptor gene-silenced cells + poly(D,L-lactide-co-glycolic acid) group, a large number of myelinated and unmyelinated nerve fibers, axons, as well as intact regenerating axonal myelin were observed (Figure 8C).
DISCUSSION
The hemisection approach used in this study causes light injury, reduces animal mortality, and has advantages over other methods[14], such as spinal cord contusion and transection. The experimental rats were found to recover right lower limb functions, based on the Basso-Beattie-Bresnahan scale and the inclined plate test, 4 weeks after transplantation of cells plus scaffold. Hematoxylin-eosin staining revealed that the cavity in the injured spinal cord was smaller at 4 weeks, and the horseradish peroxidase retrograde tracing experiment showed that the number of horseradish peroxidase-positive nerve fiber bundles in the spinal cord lesion was significantly increased. Following spinal cord injury, Nogo protein expression increases in response to tissue injury, thereby forming a vicious cycle[15,16,17,18]. Nogo-66 receptor gene silencing inhibits Nogo protein expression and promotes axonal growth, which is the major novel finding of the present study.
We found that the Nogo-66 receptor gene in bone marrow mesenchymal stem cells and Schwann cells was silenced with the RNA interference technique. These cells were then seeded into poly(D,L-lactide-co-glycolic acid) scaffolds for treatment of spinal cord hemisection injury. Results showed that bone marrow mesenchymal stem cells differentiated into neuron-like cells, and the number was significantly higher than in the other cell transplantation groups. The number of regenerating axons was also increased significantly at the spinal cord transplantation site. In addition, the Basso-Beattie-Bresnahan score was increased, indicating that spinal nerve functioning had recovered. Bone marrow mesenchymal stem cells can be obtained in abundance, especially as autologous mesenchymal stem cells. They have the potential for auto-proliferation and can differentiate into multiple lineages. Furthermore, isolation and culture techniques are quite advanced, allowing for autotransplantation. Following spinal cord injury, the transplanted mesenchymal stem cells survive and migrate, and differentiate into neurons and glial cells in the injured spinal cord. They connect with nerve processes located at both ends of the injury to form synaptic connections, and promote neural regeneration and functional recovery[19,20]. Recent studies have demonstrated that, in vitro, Schwann cells can induce the differentiation of bone marrow mesenchymal stem cells into neurons[21], which was also confirmed by the present study. Therefore, co-transplantation of bone marrow mesenchymal stem cells and Schwann cells is advantageous. This results in a synergistic effect, with the Schwann cells promoting the differentiation of bone marrow mesenchymal stem cells into neurons.
Teng et al[22] found that 70 days after a poly(D,L-lactide-co-glycolic acid) scaffold incubated with bone marrow mesenchymal stem cells was transplanted into the hemisected spinal cord, cells survived and promoted the recovery of spinal cord function. Some researchers demonstrated that after poly(D,L-lactide-co-glycolic acid) scaffolds incubated with Schwann cells were transplanted into the transected spinal cord, the cavity was decreased at the affected site, with the transplanted poly(D,L-lactide-co-glycolic acid)-cell complex filling the cystic cavity caused by secondary injury[23]. The cell-scaffold complex transplanted into the injured spinal cord of the paraplegic rats significantly improved the Basso-Beattie-Bresnahan score, compared with the simple poly(D,L-lactide-co-glycolic acid) group or the bone marrow mesenchymal stem cells + Schwann cells + poly(D,L-lactide-co-glycolic acid) group. Hematoxylin-eosin staining showed that at 4 weeks, cells in the Nogo-66 receptor gene-silenced cells + poly(D,L-lactide-co-glycolic acid) group had undergone typical neuroblast-like morphological changes, and tissue voids were not present. This improvement was better than in the simple poly(D,L-lactide-co-glycolic acid) group or the bone marrow mesenchymal stem cells + Schwann cells + poly(D,L-lactide-co-glycolic acid) group. Transmission electron microscopy revealed that in the Nogo-66 receptor gene-silenced cells + poly(D,L-lactide-co-glycolic acid) group, there was a large number of myelinated and unmyelinated nerve fibers. The number of axons was greater in this group compared with the simple poly(D,L-lactide-co-glycolic acid) group or the bone marrow mesenchymal stem cells + Schwann cells + poly(D,L-lactide-co-glycolic acid) group. Immunohistochemistry performed after horseradish peroxidase was given at the injection site showed a deeply stained center and a lightly stained surrounding area, suggesting that the number of PKH26-positive cells and horseradish peroxidase-labeled nerve fibers in the Nogo-66 receptor gene-silenced cells + poly(D,L-lactide-co-glycolic acid) group was significantly higher than in the bone marrow mesenchymal stem cells + Schwann cells + poly(D,L-lactide-co-glycolic acid) group, and higher in the bone marrow mesenchymal stem cells + Schwann cells + poly(D,L-lactide-co-glycolic acid) group than in the simple poly(D,L-lactide-co-glycolic acid) group; all comparisons had statistically significant differences. The Basso-Beattie-Bresnahan score and the inclined plate test showed that rats in the Nogo-66 receptor gene-silenced cells + poly(D,L-lactide-co- glycolic acid) group performed better than rats in the bone marrow mesenchymal stem cells + Schwann cells + poly(D,L-lactide-co-glycolic acid) group, compared with the simple poly(D,L-lactide-co-glycolic acid) group, at 4 weeks with significant differences (P < 0.05).
In summary, Nogo-66 receptor gene-silenced bone marrow mesenchymal stem cells and Schwann cells within poly(D,L-lactide-co-glycolic acid) scaffolds are considered an effective treatment strategy for spinal cord injury[24]. Poly(D,L-lactide-co-glycolic acid) can be used as a bridge to fill the cavity in spinal cord injury, while co-transplanted bone marrow mesenchymal stem cells and Schwann cells improve the microenvironment at the lesion[25,26]. This promotes the survival of bone marrow mesenchymal stem cells and their differentiation into neurons and oligodendrocytes. It also improves host nerve fiber regeneration and myelination, and encourages the repair of damaged neural pathways, resulting in the functional recovery of the injured spinal cord[15,27]. Taken together, our results indicate that the transplantation of Nogo-66 receptor gene-silenced cells within a poly(D,L-lactide-co-glycolic acid) scaffold is an effective treatment strategy for spinal cord injury.
MATERIALS AND METHODS
Design
A randomized, controlled animal experiment.
Time and setting
The experiment was performed between May 2009 and May 2012 at the Laboratory Animal Center of Tianjin Medical University in China.
Materials
A total of 72 healthy adult female Wistar rats, weighing 250–300 g, were provided by the Laboratory Animal Center of the Chinese Academy of Medical Sciences, China (license No. SCXK20060008). Experimental procedures were conducted in accordance with the Guidance Suggestions for the Care and Use of Laboratory Animals, formulated by the Ministry of Science and Technology of China[28].
Methods
Culture, transfection and identification of bone marrow mesenchymal stem cells and Schwann cells
PKH26 cell surface labeling (PKH26 kit; Sigma, St. Louis, MO, USA) was used to identify rat bone marrow mesenchymal stem cells using flow cytometry (Beckman Coulter, Miami, FL, USA). Schwann cells were identified using immunofluorescence for myelin basic protein (Sigma). Bone marrow mesenchymal stem cells and Schwann cells were isolated, purified, labeled, identified, amplified, and transfected with small interfering RNA to silence Nogo-66 receptor gene expression. Levels of Nogo-66 receptor mRNA expression in bone marrow mesenchymal stem cells and Schwann cells before and after transfection were assessed with reverse transcription-PCR, to verify the success of Nogo-66 receptor gene silencing.
Preparation of cell-scaffold complex
Poly(D,L-lactide-co-glycolic acid) (85:15; Jinan Tomorrow New Technology Company, Jinan, Shandong Province, China; viscosity (g/dL): 0.76–0.85) was modified with ammonia plasma, and the poly(D,L-lactide-co-glycolic acid) block was cut into 2.5 mm × 1.5 mm × 1 mm pieces and then vacuum dried and sterilized with epoxy ethane fumigation for further use. Using a micropipette, poly(D,L-lactide-co-glycolic acid) scaffolds were sequentially incubated with 10 μL Dulbecco's modified Eagle's medium for the simple poly(D,L-lactide-co-glycolic acid) group, with 10 μL of each of 2 × 1010/L bone marrow mesenchymal stem cells and Schwann cells for the bone marrow mesenchymal stem cells + Schwann cells + poly(D,L-lactide-co-glycolic acid) group, and with 10 μL of each of 2 × 1010/L Nogo-66 receptor gene-silenced bone marrow mesenchymal stem cells and Schwann cells for the combined group. All poly(D,L-lactide-co-glycolic acid) scaffolds were incubated at 37°C, 5% CO2 saturated humidity. Cellular adhesion, growth and differentiation were determined at 4 days. Adherent cells were used for transplantation.
Establishment of acute spinal cord injury models
Wistar rats were anesthetized with 2.5% ketamine (20 mg/kg; Beijing Zizhu Pharmaceutical Co., Ltd., Beijing, China) via intraperitoneal injection and fixed in a prone position. At the T9 spinous process, an incision in the skin and subcutaneous tissue, 2–3 cm long, was made along the posterior midline, then the paraspinal muscle was stripped laterally to expose the T8–9 spinous process and lamina, which were then removed using laminectomy forceps to expose the dura mater. The right spinal cord was cut off and right hindlimb paralysis was used as an indicator of modeling success. The wound was rinsed with penicillin-saline and sutured. The injured spinal cord was exposed 6 hours after hemisection in the combined group and in the bone marrow mesenchymal stem cells + Schwann cells + poly(D,L-lactide-co-glycolic acid) group, and then transplanted with the corresponding poly(D,L-lactide-co-glycolic acid)-cell complex. In the simple poly(D,L-lactide-co-glycolic acid) group, the injured spinal cord was exposed at 6 hours and then transplanted with poly(D,L-lactide-co-glycolic acid)/Dulbecco's modified Eagle's medium, followed by wound suturing. Rats were given daily postoperative abdominal massage and artificial voiding twice daily as paraplegia care.
Basso-Beattie-Bresnahan scoring and inclined plate test
The motor function of the lower extremity was assessed using the inclined plate test and the Basso-Beattie-Bresnahan scoring system before surgery, and at 1, 2, 4, 6 and 8 weeks after modeling. In the inclined plate test, the rat head was fixed on the modified Rivlin ramp, facing right. The angle of the ramp was gradually increased from the horizontal position (0°) to identify the maximum angle at which the rat could firmly stay on for at least 5 seconds. All rats were assessed three times, and the average was calculated. The Basso-Beattie-Bresnahan locomotor rating scale was used to determine motor function as previously described[29]. In brief, all rats were scored with a double blind method at 1, 2, 4, 6 and 8 weeks after hemisection, and the mean Basso-Beattie-Bresnahan scale scores were obtained.
Hematoxylin-eosin staining and fluorescence microscopic observations
At 4 weeks after injury, rats were randomly selected from each group and killed under anesthesia. Specimens were harvested for histological examination to determine the degree of recovery. The injured spinal cord tissue (1 cm × 1 cm × 1 cm) was fixed with 4% paraformaldehyde, embedded with paraffin, stained with hematoxylin-eosin and observed under a fluorescence microscope (Olympus, Tokyo, Japan). Each section was observed at high magnification (200 ×), and 10 visual fields were randomly selected to calculate the number of PKH26-positive cells and the average value for each group.
Detection of Nogo-66 receptor mRNA expression using reverse transcription-PCR
Expression of Nogo-66 receptor mRNA after silencing the Nogo-66 receptor gene was determined by reverse transcription-PCR. Six rats in each group were randomly selected and killed under anesthesia at 4 weeks post-injury. Total RNA was extracted from the affected spinal cord using Trizol reagent (Beijing Xin Xing Tang Biotechnology Co., Ltd., Beijing, China). 5 μL total RNA was synthesized into cDNA with M-MLV reverse transcriptase enzyme (Shanghai Bao Cayman Biological Technology Co., Ltd., Shanghai, China). 5 μL aliquots of the reverse-transcribed products were amplified by PCR. Primer sequences are as follows:
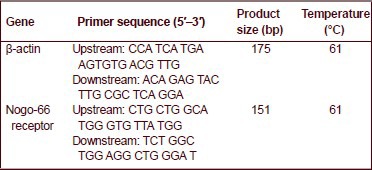
The PCR reaction was performed as follows: 35 cycles of 94°C denaturation for 1 minute, 61°C annealing for 45 seconds, 72°C extension for 1 minute, 72°C extension for 7 minutes. PCR was repeated three times. The reverse transcription-PCR product was subjected to 1.3% agarose gel electrophoresis and processed with a gel imaging system (Beijing Seclaser Technology Co., Ltd., Beijing, China), and the ratio of Nogo-66 receptor absorbance to β-actin absorbance was used as an index of Nogo-66 receptor mRNA expression level.
Horseradish peroxidase retrograde tracing for labeling of spinal nerve
At 8 weeks after transplantation, horseradish peroxidase (Tianjin Xima Technology Co., Ltd., Tianjin, China) was dissolved with physiological saline. Two rats in each group were randomly selected, and the spinal cord was exposed. The needle was inserted 1 mm away from the left and right T12 medullary dorsal median vein, to a depth of 15 mm. 1 μL of 50% horseradish peroxidase was injected at a speed of 0.1 μL/10 minutes, and the needle was maintained in place for 15 minutes after injection. Rats were housed for an additional 3 days and anesthetized with chloral hydrate, and perfused with 4% (250 mL) paraformaldehyde via the heart. Spinal cord (T3–11) segments were harvested and immersed in 30% sucrose solution (4°C) for 20 hours, then specimens were cut into 5-μm frozen sections for 3,3’-diaminobenzidine staining. The number of horseradish peroxidase-positive nerve fiber bundles in transverse sections of the spinal cord was assessed under the light microscope (Olympus, Tokyo, Japan). Ten slices were randomly selected from each group for each time point.
Newborn unmyelinated and myelinated nerve fibers under the transmission electron microscope
At 8 weeks after hemisection, two rats in each group were killed with 2.5% glutaraldehyde via cardiac perfusion, and specimens were fixed with 2.5% glutaraldehyde overnight. Two spinal cord samples of 1 cm length and 1 mm width were harvested from the proximal and distal ends. Then specimens were fixed with osmium tetroxide at 4°C for 2 hours, rinsed and gradient dehydrated with acetone, stained with uranyl acetate at 4°C for 4 hours, embedded in 618 epoxy resin, and observed under the transmission electron microscope (Olympus).
Statistical analysis
Statistical analysis was performed using SPSS 11.0 software (SPSS, Chicago, IL, USA). Data were expressed as mean ± SD and analyzed with repeated measures analysis of variance and Student-Newman-Keuls test. A level of P < 0.05 was considered to indicate a significant difference between the two groups.
Acknowledgments
We express our sincere gratitude to Dr. Jingjian Ma from the Department of Neurosurgery, General Hospital of Tianjin Medical University, China for revising this manuscript.
Footnotes
Funding: This study was sponsored by the Science and Technology Foundation of Tianjin Health Bureau, No. 2010ky04 and the Application Basis and Front Technology Projects of Tianjin (Science and Technology Foundation of Tianjin), No. 12JCYBJC18000.
Conflicts of interest: None declared.
Ethical approval: This study was approved by the Scientific Review Committee and the Institutional Review Board of Tianjin Medical University in China.
(Edited by He XJ, Nan F/Yang Y/Song LP)
REFERENCES
- [1].National Spinal Cord Injury Statistical Center. Spinal cord injury facts and figures at a glance. J Spinal Cord Med. 2005;28(4):379–380. [PubMed] [Google Scholar]
- [2].Braisted JE. Netrin-1 promotes thalamic axon growth and is required for proper development of the thalamocortical projection. J Neurosci. 2000;20(15):5792–5801. doi: 10.1523/JNEUROSCI.20-15-05792.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].De Vries M, Cooper HM. Emerging roles for neogenin and its ligands in CNS development. J Neurochem. 2008;106(4):1483–1492. doi: 10.1111/j.1471-4159.2008.05485.x. [DOI] [PubMed] [Google Scholar]
- [4].Theus MH, Wei L, Cui L, et al. In vitro hypoxic preconditioning of embryonic stem cells as a strategy of promoting cell survival and functional benefits after transplantation into the ischemic rat brain. Exp Neurol. 2008;210(2):656–670. doi: 10.1016/j.expneurol.2007.12.020. [DOI] [PubMed] [Google Scholar]
- [5].Shen LH, Li Y, Gao Q, et al. Down-regulation of neurocan expression in reactive astrocytes promotes axonal regeneration and facilitates the neurorestorative effects of bone marrow stromal cells in the ischemic rat brain. Glia. 2008;56(16):1747–1754. doi: 10.1002/glia.20722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Fehlings MG, Sekhon LH. Acute interventions in spinal cord injury: what do we know, what should we do? Clin Neurosurg. 2001;26(24 Suppl):226–242. [PubMed] [Google Scholar]
- [7].Weaver FM, Burns SP, Evans CT, et al. Provider perspectives on soldiers with new spinal cord injuries returning from iraq and afghanistan. Arch Phys Med Rehabil. 2009;90(3):517–521. doi: 10.1016/j.apmr.2008.09.560. [DOI] [PubMed] [Google Scholar]
- [8].Chu D, Bakaeen FG, Shenaq SA, et al. Open-heart operations in patients with a spinal cord injury. Am J Surg. 2007;194(5):663–667. doi: 10.1016/j.amjsurg.2007.07.015. [DOI] [PubMed] [Google Scholar]
- [9].Carvalho KA, Vialle EN, Moreira GH, et al. Functional outcome of bone marrow stem cells (CD45(+)/CD34(-)) after cell therapy in chronic spinal cord injury in Wistar rats. Transplant Proc. 2008;40(3):845–846. doi: 10.1016/j.transproceed.2008.02.054. [DOI] [PubMed] [Google Scholar]
- [10].Glazova M, Hollis S, Pak ES, et al. Embryonic stem cells inhibit expression of erythropoietin in the injured spinal cord. Neurosci Lett. 2011;488(1):55–59. doi: 10.1016/j.neulet.2010.11.002. [DOI] [PubMed] [Google Scholar]
- [11].Stenberg L, Kanje M, Dolezal K, et al. Expression of activating transcription factor 3 (ATF 3) and caspase 3 in Schwann cells and axonal outgrowth after sciatic nerve repair in diabetic BB rats. Neurosci Lett. 2012;515(1):34–38. doi: 10.1016/j.neulet.2012.03.011. [DOI] [PubMed] [Google Scholar]
- [12].Lee JY, Bashur CA, Goldstein AS, et al. Polypyrrolecoated electrospun PLGA nanofibers for neural tissue applications. Biomaterials. 2009;30(26):4325–4335. doi: 10.1016/j.biomaterials.2009.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Wang X, Chun SJ, Treloar H, et al. Localization of Nogo-A and Nogo-66 receptor proteins at sites of axon-myelin and synaptic contact. J Neurosci. 2002;22(13):5505–5515. doi: 10.1523/JNEUROSCI.22-13-05505.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Zu B, Yin ZS. Construction and evaluation of mechanical spinal cord injury models. Zhongguo Zuzhi Gongcheng Yanjiu yu Linchuang Kangfu. 2006;10(12):151–153. [Google Scholar]
- [15].Pallini R, Vitiani LR, Bez A, et al. Homologous transplantation of neural stem cells to the injured spinal cord of mice. Neurosurgery. 2005;57(5):1014–1025. doi: 10.1227/01.neu.0000180058.58372.4c. [DOI] [PubMed] [Google Scholar]
- [16].Chen G, Hu YR, Wan H, et al. Functional recovery following traumatic spinal cord injury mediated by a unique polymer scaffold seeded with neural stem cells and Schwann cells. Chin Med J (Engl) 2010;123(17):2424–2431. [PubMed] [Google Scholar]
- [17].Harel NY, Song KH, Tang X, et al. Nogo receptor deletion and multimodal exercise improve distinct aspects of recovery in cervical spinal cord injury. J Neurotrauma. 2010;27(11):2055–2066. doi: 10.1089/neu.2010.1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Fournier AE, GrandPre T, Strittmatter SM. Identification of a receptor mediating Nogo-66 inhibition of axonal regeneration. Nature. 2010;409(6818):341–346. doi: 10.1038/35053072. [DOI] [PubMed] [Google Scholar]
- [19].Parr AM, Kulbatski I, Zahir T, et al. Transplanted anted adult spinal cord-derived neural stem/progenitor cells promote erarly functional recovery after rat spinal cord injury. Neuroscience. 2008;155(3):760–770. doi: 10.1016/j.neuroscience.2008.05.042. [DOI] [PubMed] [Google Scholar]
- [20].Chi GF, Kim MR, Kim DW, et al. Schwann cells differentiated from spheroid-forming cells of rat subcutaneous fat tissue myelinate axons in the spinal cord injury. Exp Neurol. 2010;222(2):304–317. doi: 10.1016/j.expneurol.2010.01.008. [DOI] [PubMed] [Google Scholar]
- [21].Ban DX, Kong XH, Feng SQ, et al. Intraspinal cord graft of autologous activated Schwann cells efficiently promotes axonal regeneration and functional recovery after rat's spinal cord injury. Brain Res. 2009;1256:149–161. doi: 10.1016/j.brainres.2008.11.098. [DOI] [PubMed] [Google Scholar]
- [22].Teng YD, Lavik EB, Qu X, et al. Functional recovery following traumatic spinal cord injury mediated by a unique polymer scaffold seeded with neural stem cells. Proe Nail Acad Sci U S A. 2002;99(5):3024–3029. doi: 10.1073/pnas.052678899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Tuinstra HM, Aviles MO, Shin S, et al. Multiple-channel scaffolds to promote spinal cord axon regeneration. Biomaterials. 2006;27(3):419–429. doi: 10.1016/j.biomaterials.2005.07.045. [DOI] [PubMed] [Google Scholar]
- [24].Mittal G, Sahana DK, Bhardwaj V, et al. Estradiol loaded PLGA nanoparticles for oral administration: effect of polymer molecular weight and copolymer composition on release behavior in vitro and in vivo. J Control Release. 2007;119(1):77–85. doi: 10.1016/j.jconrel.2007.01.016. [DOI] [PubMed] [Google Scholar]
- [25].Berchane NS, Carson KH, Rice-Ficht AC, et al. Effect of mean diameter and polydispersity of PLG on drug release: experiment and theory. Int J Pharm. 2007;337(1-2):118–126. doi: 10.1016/j.ijpharm.2006.12.037. [DOI] [PubMed] [Google Scholar]
- [26].Yao L, Wang S, Cui W, et al. Effect of functionalized micropatterned PLGA on guided neurite growth. Acta Biomaterialia. 2009;5(2):580–588. doi: 10.1016/j.actbio.2008.09.002. [DOI] [PubMed] [Google Scholar]
- [27].Cao QL, Howard RM, Dennison JB, et al. Differentiation of engrafted neuronal-restricted precursor cells is inhibited in the traumatically injured spinal cord. Exp Neurol. 2002;177(2):349–359. doi: 10.1006/exnr.2002.7981. [DOI] [PubMed] [Google Scholar]
- [28].The Ministry of Science and Technology of the People's Republic of China. Guidance Suggestions for the Care and Use of Laboratory Animals. 2006-09-30 [Google Scholar]
- [29].Zheng Z, Yin Z, Gao W, et al. Effects of neurogenesin 1 gene on functional recovery of spinal cord injury in rats and its mechanism. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2010;24(4):410–414. [PubMed] [Google Scholar]


