Abstract
Impairment of dopamine function, which is known to have major effects on behaviors and cognition, is one of the main problems associated with cerebral ischemia. Tadalafil, a long-acting phosphodiesterase type-5 inhibitor, is known to ameliorate neurologic impairment induced by brain injury, but not in dopaminergic regions. We investigated the neuroprotective effects of treatment with tadalafil on cyclic guanosine monophosphate level and dopamine function following cerebral ischemia. Forty adult Mongolian gerbils were randomly and evenly divided into five groups (n = 8 in each group): Sham-operation group, cerebral ischemia-induced and 0, 0.1, 1, and 10 mg/kg tadalafil-treated groups, respectively. Tadalafil dissolved in distilled water was administered orally for 7 consecutive days, starting 1 day after surgery. Cyclic guanosine monophosphate assay and immunohistochemistry were performed for thyrosine hydroxylase expression and western blot analysis for dopamine D2 receptor expression. A decrease in cyclic guanosine monophosphate level following cerebral ischemia was found with an increase in thyrosine hydroxylase activity and a decrease in dopamine D2 receptor expression in the striatum and substantia nigra region. However, treatment with tadalafil increased cyclic guanosine monophosphate expression, suppressed thyrosine hydroxylase expression and increased dopamine D2 receptor expression in the striatum and substantia nigra region in a dose-dependent manner. Tadalafil might ameliorate cerebral ischemia-induced dopaminergic neuron injury. Therefore, tadalafil has the potential as a new neuroprotective treatment strategy for cerebral ischemic injury.
Keywords: neural regeneration, brain injury, cerebral ischemia, Tadalafil, phosphodiesterase type-5 inhibitor, dopamine, dopamine D2 receptor, cyclic guanosine monophosphate, grants-supported paper, photographs-containing paper, neuroregneration
Research Highlights
(1) A phosphodiesterase type-5 inhibitor tadalafil significantly increased cyclic guanosine monophosphate level, suppressed thyrosine hydroxylase expression and increased dopamine D2 receptor expression in the striatum and substantia nigra region in a dose-dependent manner.
(2) Tadalafil has the potential as a new neuroprotective treatment strategy for cerebral ischemic injury.
INTRODUCTION
Ischemic brain damage shows many manifestations, from neonatal asphyxia to adult stroke. Cerebral ischemia due to neonatal asphyxia is a common cause of functional and neurological damage in the developing brain of neonates. On the other hand, adult stroke is the second most common cause of death in industrialized countries and it has a deeply negative social and economic impact on those who survived, 80% of which is composed of ischemic injury after occlusion of a major cerebral artery or its branches[1,2,3]. Tissue damage following cerebral ischemia is caused by complex pathophysiological processes, such as membrane depolarization, inflammation, glutamate excitotoxicity, and overexpression of dopamine[4]. Ischemic episodes induced injury to multiple brain regions, including substantia nigra, basal ganglia, striatum, and hippocampus results in cognitive impairments and neurological damage[5]. Dopamine, a major catecholamine in the central nervous system, contributes to control of motor activity, behavior, and cognition, and its dysfunctions are involved in a variety of neurological disorders, such as Parkinson's disease[6]. The main determinant of dopamine pathway is the dopamine receptors. Dopamine receptors are divided into two classes, D1-like (D1, D2) and D2-like (D2, D3, D4) dopamine receptors. It has been shown that dopamine D2-like receptor is closely related to various neurodegenerative disorders such as Alzheimer's disease, Parkinson's disease and stroke[7,8]. The nigro-striatal pathway, one of the major dopamine pathways, is associated with the expression of tyrosine hydroxylase-positive cells and it is closely influenced after focal ischemia[9]. Thyrosin hydroxylase is the rate-limiting enzyme in the synthesis of catecholamine neurotransmitters, such as dopamine, epinephrine, and norepinephrine. More specifically, it converts L-tyrosine to L-dihydroxyphenylalanine, which is the rate-limiting step in the synthesis of dopamine[10]. Therefore, thyrosin hydroxylase activity shows a progressive change following the alteration of dopaminergic neurons in the substantia nigra of patients[11]. Immunohistochemistry for detection of thyrosin hydroxylase activity has been used as an important method for detection of injury or death of dopaminergic fibers and cell bodies[12]. Tadalafil, a long-acting phosphodiesterase type-5 inhibitor, is a highly specific key modulator of intracellular cyclic guanosine monophosphate signaling pathways[13,14]. Cyclic guanosine monophosphate also plays critical roles in modulation of brain functions, including neurogenesis, synaptic plasticity, and physiological modulation of neurotransmitters[15,16,17,18]. Cyclic guanosine monophosphate is down-regulated in both striatum and nucleus accumbens by dopamine loss after brain injury[18], otherwise, elevated production of cyclic guanosine monophosphate inhibits apoptosis and repairs damage by stimulating neurotransmitters[19]. Phosphodiesterase type-5 inhibitors have also been reported to regulate neurotransmitters, such as dopamine and 5-hydroxytryptamine, after hippocampal neurodegeneration by multiple mechanisms[20,21].
Although the functional role of tadalafil has been dramatically revealed in various brain diseases, there have been no reports on its efficacy in alleviation of the effects related to dopamine and dopaminergic receptor after cerebral ischemic injury. Therefore, in the current study, we investigated the neuroprotective effects of tadalafil on dopaminergic alteration in the striatum and substantia nigra following cerebral ischemic injury.
RESULTS
Quantitative analysis of experimental animals
Forty adult male Mongolian gerbils were randomly divided into five groups (n = 8 in each group): sham-operation, cerebral ischemia-induced, 0.1, 1, 10 mg/kg tadalafil-treated groups, and gerbils in the latter three groups were orally administered tadalafil dissolved in distilled water following cerebral ischemia. All 40 gerbils were included in the final analysis. The study timeline is presented in Figure 1.
Figure 1.
Study timeline for cerebral ischemia induction, sham-operation, tadalafil treatment and tissue preparation.
Effect of tadalafil on cyclic guanosine monophosphate level in the striatum and substantia nigra
Cyclic guanosine monophosphate level in the striatum and substantia nigra of gerbils was detected in each group (Table 1, Figure 2).
Table 1.
Effects of consecutive tadalafil treatment on cyclic guanosine monophosphate (cGMP) level, thyrosine hydroxylase (TH) activity and dopamine D2 receptor expression in the stratum and substantia nigra following cerebral ischemia
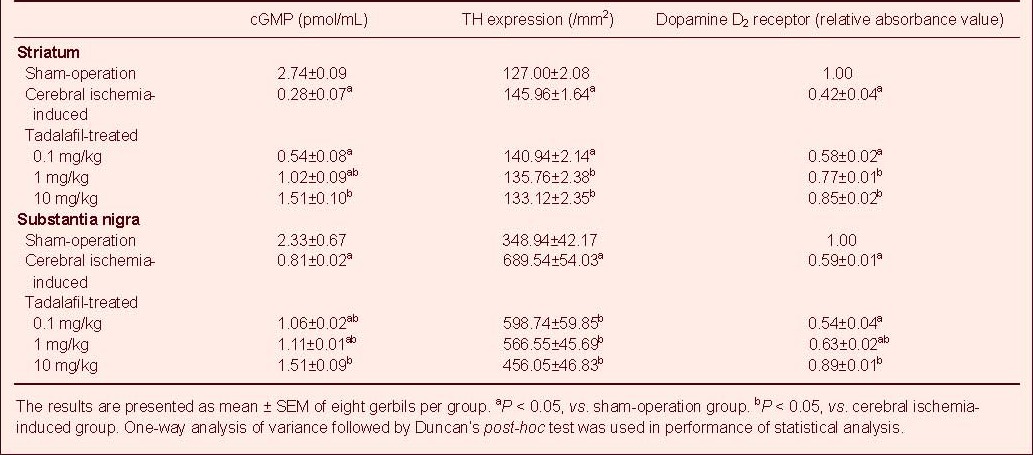
Figure 2.
Effects of treatment with tadalafil on cyclic guanosine monophosphate (cGMP) level in the striatum (A) and substantia nigra (B) following cerebral ischemia.
I: Sham-operation group; II: cerebral ischemia-induced group; III–V: 0.1, 1, and 10 mg/kg tadalafil-treated groups, respectively. One-way analysis of variance followed by Duncan's post-hoc test was used in performance of statistical analysis, and the results are presented as mean ± SEM of eight gerbils per group. Each experiment was performed in triplicate. aP < 0.05, vs. sham-operation group; bP < 0.05, vs. cerebral ischemia-induced group.
Cerebral ischemic injury led to a decrease in cyclic guanosine monophosphate level in the striatum and substantia nigra, whereas treatment with tadalafil significantly suppressed the decrease in cyclic guanosine monophosphate level in a dose-dependent manner (P < 0.05).
Effect of tadalafil on thyrosin hydroxylase expression in the striatum and substantia nigra
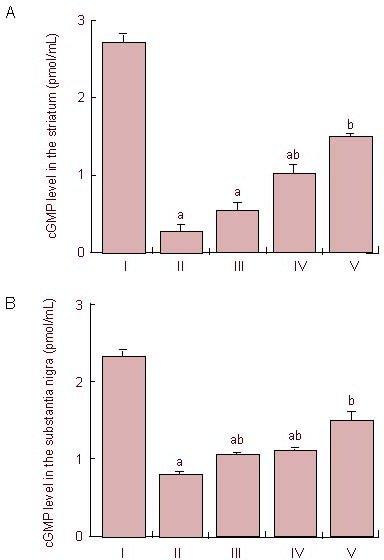
The thyrosin hydroxylase expression in the striatum and substantia nigra was analyzed in each group (Table 1, Figure 3). Cerebral ischemic injury led to an increase of thyrosin hydroxylase expression in the striatum and substantia nigra, whereas treatment with tadalafil significantly suppressed the increase in thyrosin hydroxylase expression in a dose-dependent manner (P < 0.05).
Figure 3.
Effects of treatment with tadalafil on thyroxine hydroxylase (TH) expression in the striatum (A) and substantia nigra (B) following cerebral ischemia.
Upper: Photomicrographs of TH expression in the striatum and substantia nigra. WB: Whole brain. (A1, B1) Sham-operation group; (A2, B2) cerebral ischemia-induced group; (A3–A5, B3–B5) 0.1, 1, and 10 mg/kg tadalafil-treated groups. The sections were stained for TH immunoreactivity (brown). Scale bars: 200 μm. Lower: Quantification of TH expression in each group. One-way analysis of variance followed by Duncan's post-hoc test was used in performance of statistical analysis, and the results are presented as mean ± SEM of eight gerbils per group. Each experiment was performed in triplicate. aP < 0.05,vs. sham-operation group; bP < 0.05, vs. cerebral ischemia-induced group.
Effect of tadalafil on dopamine D2 receptor expression in the striatum and substantia nigra as confirmed by western blot analysis
Mature dopamine D2 receptor level was detected after the level of mature dopamine D2 receptor (48–51 kDa) expression in the striatum and substantia nigra of gerbils from the sham-operation group was designated as 1.00 (Table 1, Figure 4).
Figure 4.
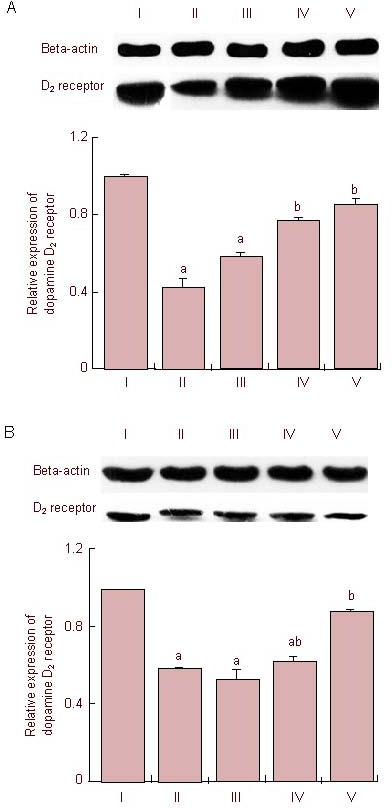
Effects of treatment with tadalafil on dopamine D2 receptor expression in the striatum (A) and substantia nigra (B) following cerebral ischemia.
I: Sham-operation group; II: cerebral ischemia-induced group; III–V: 0.1, 1, and 10 mg/kg tadalafil-treated group, respectively.
One-way analysis of variance followed by Duncan's post-hoc test was used in performance of statistical analysis, and the results are presented as mean ± SEM of eight gerbils per group. The results are expressed as relative absorbance value of dopamine D2 receptor/beta-actin normalized to sham-operation group. Each experiment was performed in triplicate. aP < 0.05, vs. sham-operation group; bP < 0.05, vs. cerebral ischemia- induced group.
Cerebral ischemic injury led to a decrease in expression of dopamine D2 receptor in the striatum and substantia nigra, whereas treatment with tadalafil significantly suppressed the decrease in dopamine D2 receptor in a dose-dependent manner (P < 0.05).
DISCUSSION
Dopamine appears to play an important role in mediation of cerebral ischemic brain injury[22,23,24]. Occurrence of ischemic episodes, such as those encountered in cerebral ischemia or anoxia, induces depletion of ATP level, leading to neuronal loss through mechanisms involving massive release of neurotransmitters, mainly dopamine and glutamate in the striatum[22,23,24]. Accordingly, increased extracellular dopamine concentration in cerebral ischemia was demonstrated using in vivo brain microdialysis[25,26]. It has also been demonstrated that ischemia-induced cell damage is accelerated by dopamine[27] and extracellular levels of dopamine in the brain are related to the severity of cerebral ischemic injury[28]. The loss of terminal dopaminergic fibers in the striatum appears to be more profoundly affected than the dopaminergic neuronal loss in the pars compacta of substantia nigra[29]. In rodent models, thyrosin hydroxylase activity showed a progressive increase following the gain of dopaminergic neurons in the striatum and substantia nigra after cerebral ischemia[30]. In this study, a significant increase in thyrosin hydroxylase-positive cells was also observed in the striatum and substantia nigra following induction of cerebral ischemia in gerbils.
Dopamine might enhance the striatal damage via dopamine D2 receptor on ischemic neuronal cell[27]. Dopamine D2 receptor is a family of G protein-coupled receptors; its activation inhibits synaptogenesis in dopaminergic neurons through translational regulation of protein synthesis required for synapse formation[31].
A decrease in expression of dopamine D2 receptor was reported in the dorsal striatum, substantia nigra, and ventral tegmental area in an animal model of cerebral ischemia[32]. Dopamine D2 receptor also appears to be a key modulator of dopamine stimulation. The enhancement of dopamine D2 receptor function, induced by increased expression of Gi-α protein, accounts for the decrease in release of dopamine in the striatum of rats with cerebral ischemia[33]. Several studies have reported that dopamine D2 receptor stimulation may protect mesencephalic dopaminergic neurons from glutamate-and oxidative stress-induced cytotoxicity[34] and cultured cortical neurons from glutamate-induced cytotoxicity[35]. Dopamine D2 receptor agonists are known to protect against ischemia-induced hippocampal neurodegeneration in global cerebral ischemia[32,33,34,35,36]. On the other hand, chronic oral administration of a specific antagonist of dopamine D2 receptor to rats and mice triggers decrement of the size of the axonal arbor of substantia nigra, pars compacta dopaminergic neurons in the striatum[37]. Results of our study also showed significantly decreased expression of dopamine D2 receptor in the striatum and substantia nigra following cerebral ischemia, indicating that cerebral ischemia suppressed dopamine D2 receptor expression in the striatum and substantia nigra.
Tadalafil, phosphodiesterase type-5 inhibitor, has been widely used in treatment of erectile dysfunction, and has also been used therapeutically for men with erectile dysfunction in various brain disorders[38]. Furthermore, it has been reported to be effective in treatment of an increasing number of various diseases, such as chronic obstructive pulmonary disease, hypertension, and coronary heart disease[39]. For example, sildenafil citrate, a potent phosphodiesterase type-5 inhibitor, protected against ischemia/reperfusion-induced cardiac injury in adult rabbits[40]. Production of cyclic guanosine monophosphate occurs in response to nitric oxide and natriuretic peptides. Cyclic guanosine monophosphate is a key regulator of cell proliferation, differentiation, and apoptosis, and it plays an important role in many pathophysiological processes, including synaptic plasticity, angiogenesis, inflammation, and cardiac hypertrophy[19]. A possible neuroprotective effect is also suggested. The neuroprotective effect of phosphodiesterase type-5 inhibitors has been attributed to a decrease of cerebral metabolic rate, the retardation of the rate of high-energy phosphate depletion, and the promotion of post-ischemic metabolic recovery[41,42]. In addition, although it is still obscure, phosphodiesterase type-5 inhibitors modulate neurotransmitters such as glutamate, dopamine, and serotonin after cerebral injury[14]. Tadalafil can cross the blood-brain barrier and induce significant improvement of learning/memory through modulation of the glutamate-nitric oxide-cyclic guanosine monophosphate signal transduction pathway. In these effects, signaling has been shown to play an important role in modulation of ion channels, synaptic transmission, and neurotransmitters[43,44,45]. Elevated cyclic guanosine monophosphate modulates excessive neurotransmitters, and promotes blood overflowing in the brain. Nitric oxide enhances angiogenesis via synthesis of vascular endothelial growth factor and cyclic guanosine monophosphate after stroke in the rat. Sildenafil and an analog of cyclic guanosine monophosphate also induced formation of capillary-like tubes and these findings suggest that exogenous nitric oxide enhances angiogenesis in ischemic brain, which is mediated by the nitric oxide-cyclic guanosine monophosphate pathway[14]. In this study, using enzyme immunoassay, we evaluated the effect of tadalafil on cerebral ischemia-induced cyclic guanosine monophosphate alteration, and observed a decrease in cyclic guanosine monophosphate level after transient cerebral ischemia.
We demonstrated that treatment with tadalafil significantly increased cyclic guanosine monophosphate level, suppressed thyrosin hydroxylase expression, and increased cerebral ischemia-induced expression of dopamine D2 receptor in the striatum and substantia nigra in a dose-dependent manner.
Results from this study suggest that tadalafil treatment can suppress cerebral ischemia-induced excessive dopamine expression in the striatum and substantia nigra. These results also indicate that treatment with phosphodiesterase type-5 inhibitors promotes neurological function recovery of embolic stroke[46].
Taken together, a phosphodiesterase type-5 inhibitor, tadalafil, can ameliorate cerebral ischemia-induced dopaminergic changes, thus, it might facilitate recovery after cerebral ischemic injury.
MATERIALS AND METHODS
Design
Randomized, controlled, animal experiments.
Time and setting
This study was performed in Gachon University of Medicine and Science and College of Medicine, Kyung Hee University, Republic of Korea from March 2010 to September 2011.
Materials
Forty adult male Mongolian gerbils, weighing 70 ± 5 g, aged 15 weeks, obtained from a commercial breeder (Orient Co, Seoul, Korea), were used for the experiments. Each animal was housed under controlled temperature (22 ± 2°C) and lighting (08:00–20:00) conditions with food and water made available ad libitum during the entire period of study. All experimental procedures were performed in accordance with the animal care guidelines of the National Institutes of Health (NIH) and the Korean Academy of Medical Sciences (http://www.kams.or.kr/).
Methods
Induction of transient global ischemia and tadalafil treatment
A previously described surgical procedure was used for induction of transient global ischemic injury of the cerebrum[21]. In brief, gerbils were anesthetized with Zoletil 50® (10 mg/kg, intraperitoneal; Vibac Laboratories, Carros, France) and bilateral neck incisions were made. After neck dissection, both common carotid arteries were exposed and occluded with aneurysm clips for 5 minutes; the clips were then removed in order to restore cerebral blood flow. Body and rectal temperature was maintained at 36 ± 0.5°C during surgery using a Homeothermic Blanket Control Unit (Harvard Apparatus, Massachusetts, MA, USA), and the gerbils were monitored for 2 hours to prevent hypothermia after the operation. For the sham-operation group, the same procedures, except for the common carotid artery occlusions, were performed according to the same timeline. Tadalafil-treated groups received tadalafil (Cialis®: Eli Lilly Co, Indianapolis, IN, USA) orally in distilled water once a day for 7 consecutive days, starting 1 day after surgery. The other groups received the same dose of distilled water only instead of tadalafil administration for the same period. The study timeline is presented in Figure 1.
Tissue preparation
The gerbils were sacrificed 1 day after the last administration of tadalafil or distilled water (8 days after the operation). The animals were anesthetized using Zoletil 50® (10 mg/kg, intraperitoneal; Vibac Laboratories), followed by transcardial perfusion with 50 mM PBS, and fixed with a freshly prepared solution consisting of 4% paraformaldehyde in 100 mM phosphate buffer (pH 7.4). Brains were harvested, post-fixed in the same fixative overnight, and transferred to 30% sucrose for cryoprotection. Coronal sections (40 μm thick) were made using a freezing microtome (Leica, Nussloch, Germany). Ten consecutive sections on average in the striatum and substantia nigra were collected from each gerbil, and 40–50 sections were used.
Measurement of cyclic guanosine monophosphate production
In order to determine the effect of tadalafil on cyclic guanosine monophosphate production, cyclic guanosine monophosphate assay[47] was performed according to the manufacturer's instructions using a commercially available cyclic guanosine monophosphate competitive enzyme immunoassay kit (Sapphire Bioscience Pty. Ltd., Redfern, Australia).
Thyrosin hydroxylase immunohistochemistry
Following incubation of free-floating tissue sections overnight with mouse anti-thyrosin hydroxylase antibody (1:1 000, Santa Cruz Biotechnology, Santa Cruz, CA, USA) at 4°C, the sections were incubated with biotinylated anti-mouse secondary antibody (1:200, Vector Laboratories, Burlingame, CA, USA) at 4°C for 1 hour. The sections were subsequently incubated with avidin-biotin-peroxidase complex (Vector Laboratories) for 1 hour at room temperature. Immunoreactivity was visualized by incubating the sections in a solution consisting of 0.05% 3,3-diaminobenzidine and 0.01% H2O2 in 50 mM Tris-buffer (pH 7.6) for approximately 3 minutes. The sections were then washed three times with PBS and mounted onto gelatin-coated slides. The slides were air-dried overnight at room temperature, and cover slips were mounted using Permount® (Fisher Scientific, Hampton, NH, USA).
Western blot analysis for dopamine D2 receptor expression
Striatum and substantia nigra tissues were collected, and were immediately frozen at –70°C. These tissues were homogenized on ice, and lysed in a lysis buffer containing 50 mM hydroxyethyl piperazine ethanesulfonic acid (pH 7.5), 150 mM NaCl, 10% glycerol, 1% Triton X-100, 1 mM phenylmethyl sulfonylfluoride, 1 mM ethylene glycol tetraacetate, 1.5 mM MgCl2·6H2O, 1 mM sodium orthovanadate, and 100 mM sodium flouride. A Bio-Rad colorimetric protein assay kit (Bio-Rad Laboratories, Hercules, CA, USA) was used for measurement of protein content. Protein (30 μg) was separated on sodium dodecyl sulphate-polyacrylamide gels and transferred onto a nitrocellulose membrane. Mouse anti-actin antibody (1:500; Santa Cruz Biotechnology) and mouse dopamine D2 receptor antibody (1:1 000; Santa Cruz Biotechnology) were used as the primary antibodies for 24 hours at 4°C. Horseradish peroxidase-conjugated anti-mouse antibody for dopamine D2 receptor (1:2 000; Vector Laboratories) was used as the secondary antibody. All experimental procedures were performed in normal laboratory conditions and at room temperature; however, membrane transfers were performed at 4°C using a cold pack and pre-chilled buffer. Band detection was performed using an enhanced chemiluminescence detection kit (Santa Cruz Biotechnology).
Data analysis
For comparison of the relative expression of proteins, the detected bands were calculated densitometrically using Molecular Analyst™, version 1.4.1 (Bio-Rad Laboratories). The numbers of thyrosin hydroxylase-positive cells in the substantia nigra were counted. The area of the striatum and substantia nigra on each section was measured using an Image-Pro® Plus computer-assisted image analysis system (Media Cyberbetics, Silver Spring, MD, USA) attached to a light microscope (Olympus, Tokyo, Japan). The numbers of thyrosin hydroxylase-positive cells in the substantia nigra were counted hemilaterally through a light microscope (Olympus). The density of thyrosin hydroxylase-immunoreactive fibers in the striatum was measured in 100 μm × 100 μm square images of the striatum using an image analyzer (Multiscan, Fullerton, CA, USA). To estimate the thyrosin hydroxylase staining density, the optical density was corrected for the nonspecific background density, which was measured in the completely denervated parts of the striatum. The density ratio of thyrosin hydroxylase-positive fibers in the striatum was calculated as follows: the absorbance in the lesion side divided by the absorbance in the intake side[48].
All results were statistically analyzed using SPSS for Window, version 20.0 (SPSS, Chicago, IL, USA). One-way analysis of variance followed by Duncan's post-hoc test was used in performance of statistical analysis and the results are expressed as mean ± SEM. Significance was set as P < 0.05.
Footnotes
Funding: This study was supported by the Research Fund of Gachon University Gil Medical Center in 2011 and the National Research Foundation of Korea funded by the Korean Government, No. 2012R1A1A1013173.
Conflicts of interest: None declared.
Ethical approval: The experimental procedures were approved by the Institutional Animal Care and Use Committee of Gachon University of Medicine and Science and Kyung Hee University, Republic of Korea.
(Edited by Song LP)
REFERENCES
- [1].Green AR. Pharmacological approaches to acute ischaemic stroke: reperfusion certainly, neuroprotection possibly. Br J Pharmacol. 2008;153(Suppl 1):S325–338. doi: 10.1038/sj.bjp.0707594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Wardlaw JM, Murray V, Berge E, et al. Recombinant tissue plasminogen activator for acute ischaemic stroke: an updated systematic review and meta-analysis. Lancet. 2012;379(9834):2364–2372. doi: 10.1016/S0140-6736(12)60738-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Davis SM, Donnan GA. Clinical practice. Secondary prevention after ischemic stroke or transient ischemic attack. N Engl J Med. 2012;366(20):1914–1922. doi: 10.1056/NEJMcp1107281. [DOI] [PubMed] [Google Scholar]
- [4].Dirnagl U, Iadecola C, Moskowitz MA. Pathobiology of ischaemic stroke: an integrated view. Trends Neurosci. 1999;22(9):391–397. doi: 10.1016/s0166-2236(99)01401-0. [DOI] [PubMed] [Google Scholar]
- [5].Fan LW, Lin S, Pang Y, et al. Hypoxia-ischemia induced neurological dysfunction and brain injury in the neonatal rat. Behav Brain Res. 2005;165(1):80–90. doi: 10.1016/j.bbr.2005.06.033. [DOI] [PubMed] [Google Scholar]
- [6].Bosboom JL, Stoffers D, Wolters E. Cognitive dysfunction and dementia in Parkinson's disease. J Neural Transm. 2004;111(10-11):1303–1315. doi: 10.1007/s00702-004-0168-1. [DOI] [PubMed] [Google Scholar]
- [7].Piggott MA, Owens J, O’Brien J, et al. Muscarinic receptors in basal ganglia in dementia with Lewy bodies, Parkinson's disease and Alzheimer's disease. J Chem Neuroanat. 2003;25(3):161–173. doi: 10.1016/s0891-0618(03)00002-4. [DOI] [PubMed] [Google Scholar]
- [8].Cantagrel S, Gressens P, Bodard S, et al. mRNA D(2) dopaminergic receptor expression after hypoxia-ischemia in rat immature brain. Biol Neonate. 2001;80(1):68–73. doi: 10.1159/000047123. [DOI] [PubMed] [Google Scholar]
- [9].Yanagisawa D, Qi M, Kim DH, et al. Improvement of focal ischemia-induced rat dopaminergic dysfunction by striatal transplantation of mouse embryonic stem cells. Neurosci Lett. 2006;407(1):74–79. doi: 10.1016/j.neulet.2006.08.007. [DOI] [PubMed] [Google Scholar]
- [10].Asanuma M, Miyazaki I, Ogawa N. Dopamine- or L-DOPA-induced neurotoxicity: the role of dopamine quinone formation and tyrosinase in a model of Parkinson's disease. Neurotox Res. 2003;5(3):165–176. doi: 10.1007/BF03033137. [DOI] [PubMed] [Google Scholar]
- [11].Haavik J, Toska K. Tyrosine hydroxylase and Parkinson's disease. Mol Neurobiol. 1998;16(3):285–309. doi: 10.1007/BF02741387. [DOI] [PubMed] [Google Scholar]
- [12].Hurley MJ, Gerard DJ, Jauniaux E, et al. Cultured human foetal cerebral cortex, transfected with tyrosine hydroxylase cDNA, as a source of neural transplant material. J Neural Transm. 2001;108(7):781–792. doi: 10.1007/s007020170028. [DOI] [PubMed] [Google Scholar]
- [13].Rybalkin SD, Rybalkina IG, Feil R, et al. Regulation of cGMP-specific phosphodiesterase (PDE5) phosphorylation in smooth muscle cells. J Biol Chem. 2002;277(5):3310–3317. doi: 10.1074/jbc.M106562200. [DOI] [PubMed] [Google Scholar]
- [14].Zhang R, Wang L, Zhang L, et al. Nitric oxide enhances angiogenesis via the synthesis of vascular endothelial growth factor and cGMP after stroke in the rat. Circ Res. 2003;92(3):308–313. doi: 10.1161/01.res.0000056757.93432.8c. [DOI] [PubMed] [Google Scholar]
- [15].Prickaerts J, Steinbusch HW, Smits JF, et al. Possible role of nitric oxide-cyclic GMP pathway in object recognition memory: effects of 7-nitroindazole and zaprinast. Eur J Pharmacol. 1997;337(2-3):125–136. doi: 10.1016/s0014-2999(97)01301-0. [DOI] [PubMed] [Google Scholar]
- [16].Prickaerts J, Sik A, van Staveren WC, et al. Phosphodiesterase type 5 inhibition improves early memory consolidation of object information. Neurochem Int. 2004;45(6):915–928. doi: 10.1016/j.neuint.2004.03.022. [DOI] [PubMed] [Google Scholar]
- [17].Lin CS, Lin G, Xin ZC, et al. Expression, distribution and regulation of phosphodiesterase 5. Curr Pharm Des. 2006;12(27):3439–3457. doi: 10.2174/138161206778343064. [DOI] [PubMed] [Google Scholar]
- [18].Giorgi M, Melchiorri G, Nuccetelli V, et al. PDE10A and PDE10A-dependent cAMP catabolism are dysregulated oppositely in striatum and nucleus accumbens after lesion of midbrain dopamine neurons in rat: a key step in parkinsonism physiopathology. Neurobiol Dis. 2011;43(1):293–303. doi: 10.1016/j.nbd.2011.04.006. [DOI] [PubMed] [Google Scholar]
- [19].Pilz RB, Broderick KE. Role of cyclic GMP in gene regulation. Front Biosci. 2005;10:1239–1268. doi: 10.2741/1616. [DOI] [PubMed] [Google Scholar]
- [20].Sabayan B, Zamiri N, Farshchizarabi S. Phosphodiesterase-5 inhibitors: novel weapons against Alzheimer's disease? Int J Neurosci. 2010;120(12):746–751. doi: 10.3109/00207454.2010.520381. [DOI] [PubMed] [Google Scholar]
- [21].Sim YJ, Kim SS, Kim JY, et al. Treadmill exercise improves short-term memory by suppressing ischemia-induced apoptosis of neuronal cells in gerbils. Neurosci Lett. 2004;372(3):256–261. doi: 10.1016/j.neulet.2004.09.060. [DOI] [PubMed] [Google Scholar]
- [22].Banasiak KJ, Xia Y, Haddad GG. Mechanisms underlying hypoxia-induced neuronal apoptosis. Prog Neurobiol. 2000;62(3):215–249. doi: 10.1016/s0301-0082(00)00011-3. [DOI] [PubMed] [Google Scholar]
- [23].Tuor UI, Hudzik TJ, Malisza K, et al. Long-term deficits following cerebral hypoxia-ischemia in four-week-old rats: correspondence between behavioral, histological, and magnetic resonance imaging assessments. Exp Neurol. 2001;167(2):272–281. doi: 10.1006/exnr.2000.7565. [DOI] [PubMed] [Google Scholar]
- [24].Orset C, Parrot S, Sauvinet V, et al. Dopamine transporters are involved in the onset of hypoxia-induced dopamine efflux in striatum as revealed by in vivo microdialysis. Neurochem Int. 2005;46(8):623–633. doi: 10.1016/j.neuint.2005.02.005. [DOI] [PubMed] [Google Scholar]
- [25].Akiyama Y, Ito A, Koshimura K, et al. Effects of transient forebrain ischemia and reperfusion on function of dopaminergic neurons and dopamine reuptake in vivo in rat striatum. Brain Res. 1991;561(1):120–127. doi: 10.1016/0006-8993(91)90756-l. [DOI] [PubMed] [Google Scholar]
- [26].Parrot S, Cottet-Emard JM, Sauvinet V, et al. Effects of acute hypoxic conditions on extracellular excitatory amino acids and dopamine in the striatum of freely-moving rats. Adv Exp Med Biol. 2003;536:433–444. doi: 10.1007/978-1-4419-9280-2_55. [DOI] [PubMed] [Google Scholar]
- [27].Hashimoto N, Matsumoto T, Mabe H, et al. Dopamine has inhibitory and accelerating effects on ischemia-induced neuronal cell damage in the rat striatum. Brain Res Bull. 1994;33(3):281–288. doi: 10.1016/0361-9230(94)90195-3. [DOI] [PubMed] [Google Scholar]
- [28].Richards DA, Obrenovitch TP, Johonson-Mora A, et al. A previous potassium stimulation enhances the increases of striatal extracellular dopamine and 5-hydroxytryptamine during global ischaemia under simulated penumbral conditions. J Neurochem. 1993;61(6):2233–2238. doi: 10.1111/j.1471-4159.1993.tb07464.x. [DOI] [PubMed] [Google Scholar]
- [29].Bernheimer H, Birkmayer W, Hornykiewicz O, et al. Brain dopamine and the syndromes of Parkinson and Huntington. Clinical, morphological and neurochemical correlations. J Neurol Sci. 1973;20(4):415–455. doi: 10.1016/0022-510x(73)90175-5. [DOI] [PubMed] [Google Scholar]
- [30].Jakowec MW, Nixon K, Hogg E, et al. Tyrosine hydroxylase and dopamine transporter expression following 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced neurodegeneration of the mouse nigrostriatal pathway. J Neurosci Res. 2004;76(4):539–550. doi: 10.1002/jnr.20114. [DOI] [PubMed] [Google Scholar]
- [31].Fasano C, Poirier A, DesGroseillers L, et al. Chronic activation of the D2 dopamine autoreceptor inhibits synaptogenesis in mesencephalic dopaminergic neurons in vitro. Eur J Neurosci. 2008;28(8):1480–1490. doi: 10.1111/j.1460-9568.2008.06450.x. [DOI] [PubMed] [Google Scholar]
- [32].O’Neill MJ, Hicks CA, Ward MA, et al. Dopamine D2 receptor agonists protect against ischaemia-induced hippocampal neurodegeneration in global cerebral ischaemia. Eur J Pharmacol. 1998;352(1):37–46. doi: 10.1016/s0014-2999(98)00333-1. [DOI] [PubMed] [Google Scholar]
- [33].Yokoyama O, Yoshiyama M, Namiki M, et al. Changes in dopaminergic and glutamatergic excitatory mechanisms of micturition reflex after middle cerebral artery occlusion in conscious rats. Exp Neurol. 2002;173(1):129–135. doi: 10.1006/exnr.2001.7833. [DOI] [PubMed] [Google Scholar]
- [34].Sawada H, Ibi M, Kihara T, et al. Dopamine D2-type agonists protect mesencephalic neurons from glutamate neurotoxicity: mechanisms of neuroprotective treatment against oxidative stress. Ann Neurol. 1998;44(1):110–119. doi: 10.1002/ana.410440117. [DOI] [PubMed] [Google Scholar]
- [35].Kihara T, Shimohama S, Sawada H, et al. Protective effect of dopamine D2 agonists in cortical neurons via the phosphatidylinositol 3 kinase cascade. J Neurosci Res. 2002;70(3):274–282. doi: 10.1002/jnr.10426. [DOI] [PubMed] [Google Scholar]
- [36].Liu XH, Kato H, Chen T, et al. Bromocriptine protects against delayed neuronal death of hippocampal neurons following cerebral ischemia in the gerbil. J Neurol Sci. 1995;129(1):9–14. doi: 10.1016/0022-510x(94)00239-k. [DOI] [PubMed] [Google Scholar]
- [37].Tripanichkul W, Stanic D, Drago J, et al. D2 Dopamine receptor blockade results in sprouting of DA axons in the intact animal but prevents sprouting following nigral lesions. Eur J Neurosci. 2003;17(5):1033–1045. doi: 10.1046/j.1460-9568.2003.02547.x. [DOI] [PubMed] [Google Scholar]
- [38].Raffaele R, Vecchio I, Giammusso B, et al. Efficacy and safety of fixed-dose oral sildenafil in the treatment of sexual dysfunction in depressed patients with idiopathic Parkinson's disease. Eur Urol. 2002;41(4):382–386. doi: 10.1016/s0302-2838(02)00054-4. [DOI] [PubMed] [Google Scholar]
- [39].van Driel MF. Phosphodiesterase inhibitors: effectiveness and new applications. Ned Tijdschr Geneeskd. 2006;150(29):1613–1616. [PubMed] [Google Scholar]
- [40].Ockaili R, Salloum F, Hawkins J, et al. Sildenafil (Viagra) induces powerful cardioprotective effect via opening of mitochondrial K(ATP) channels in rabbits. Am J Physiol Heart Circ Physiol. 2002;283(3):H1263–1269. doi: 10.1152/ajpheart.00324.2002. [DOI] [PubMed] [Google Scholar]
- [41].Arikan DC, Bakan V, Kurutas EB, et al. Protective effect of tadalafil on ischemia/reperfusion injury of rat ovary. J Pediatr Surg. 2010;45(11):2203–2209. doi: 10.1016/j.jpedsurg.2010.07.011. [DOI] [PubMed] [Google Scholar]
- [42].Ozdegirmenci O, Kucukozkan T, Akdag E, et al. Effects of sildenafil and tadalafil on ischemia/reperfusion injury in fetal rat brain. J Matern Fetal Neonatal Med. 2011;24(2):317–323. doi: 10.3109/14767058.2010.492061. [DOI] [PubMed] [Google Scholar]
- [43].White RE. Cyclic GMP and ion channel regulation. Adv Second Messenger Phosphoprotein Res. 1999;33:251–277. doi: 10.1016/s1040-7952(99)80013-5. [DOI] [PubMed] [Google Scholar]
- [44].Ahern GP, Klyachko VA, Jackson MB. cGMP and S-nitrosylation: two routes for modulation of neuronal excitability by NO. Trends Neurosci. 2002;25(10):510–517. doi: 10.1016/s0166-2236(02)02254-3. [DOI] [PubMed] [Google Scholar]
- [45].Zhang Z, Klyachko V, Jackson MB. Blockade of phosphodiesterase Type 5 enhances rat neurohypophysial excitability and electrically evoked oxytocin release. J Physiol. 2007;584(Pt 1):137–147. doi: 10.1113/jphysiol.2007.139303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Zhang L, Zhang Z, Zhang RL, et al. Tadalafil, a long-acting type 5 phosphodiesterase isoenzyme inhibitor, improves neurological functional recovery in a rat model of embolic stroke. Brain Res. 2006;1118(1):192–198. doi: 10.1016/j.brainres.2006.08.028. [DOI] [PubMed] [Google Scholar]
- [47].Ko IG, Shin MS, Kim BK, et al. Tadalafil improves short-term memory by suppressing ischemia-induced apoptosis of hippocampal neuronal cells in gerbils. Pharmacol Biochem Behav. 2009;91(4):629–635. doi: 10.1016/j.pbb.2008.10.009. [DOI] [PubMed] [Google Scholar]
- [48].Kim H, Heo HI, Kim DH, et al. Treadmill exercise and methylphenidate ameliorate symptoms of attention deficit/hyperactivity disorder through enhancing dopamine synthesis and brain-derived neurotrophic factor expression in spontaneous hypertensive rats. Neurosci Lett. 2011;504(1):35–39. doi: 10.1016/j.neulet.2011.08.052. [DOI] [PubMed] [Google Scholar]


