Abstract
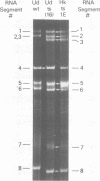
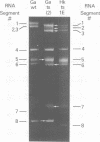
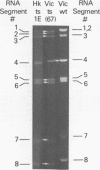
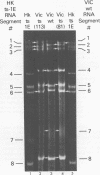
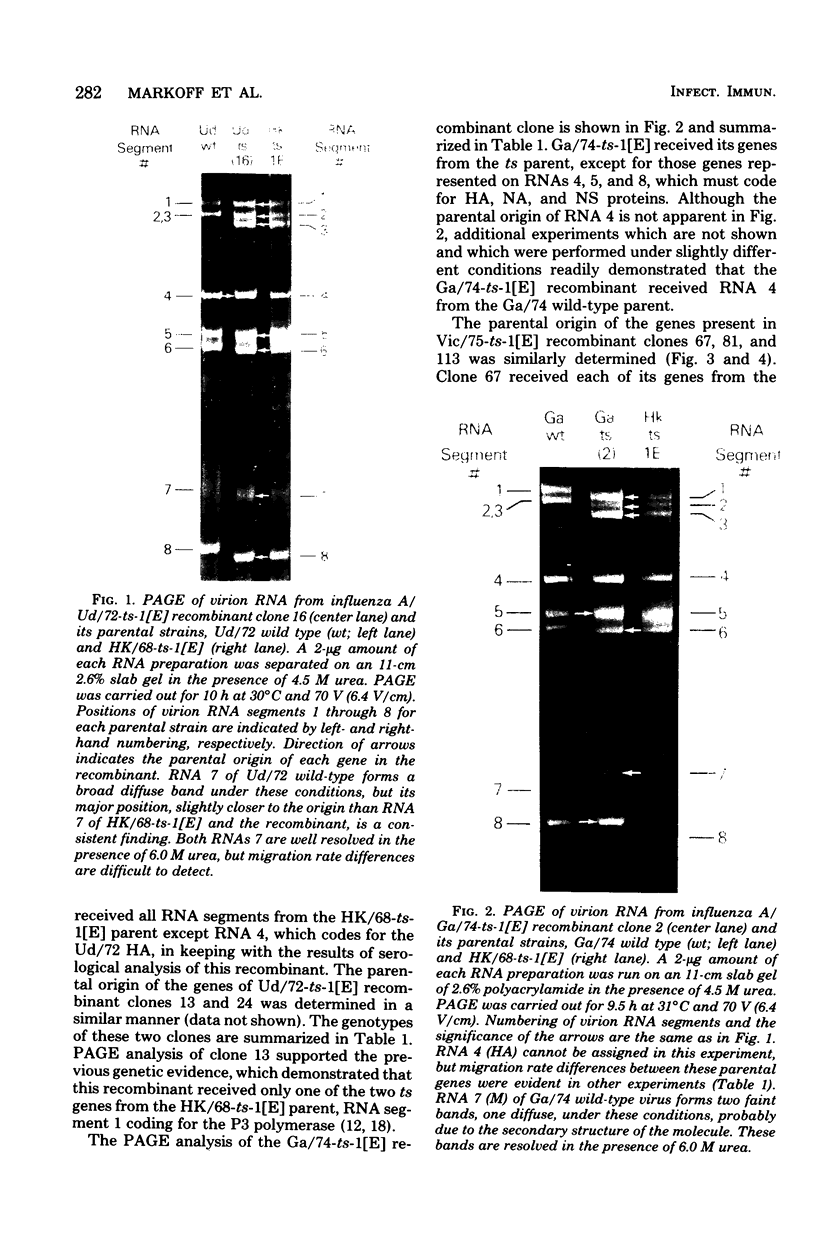
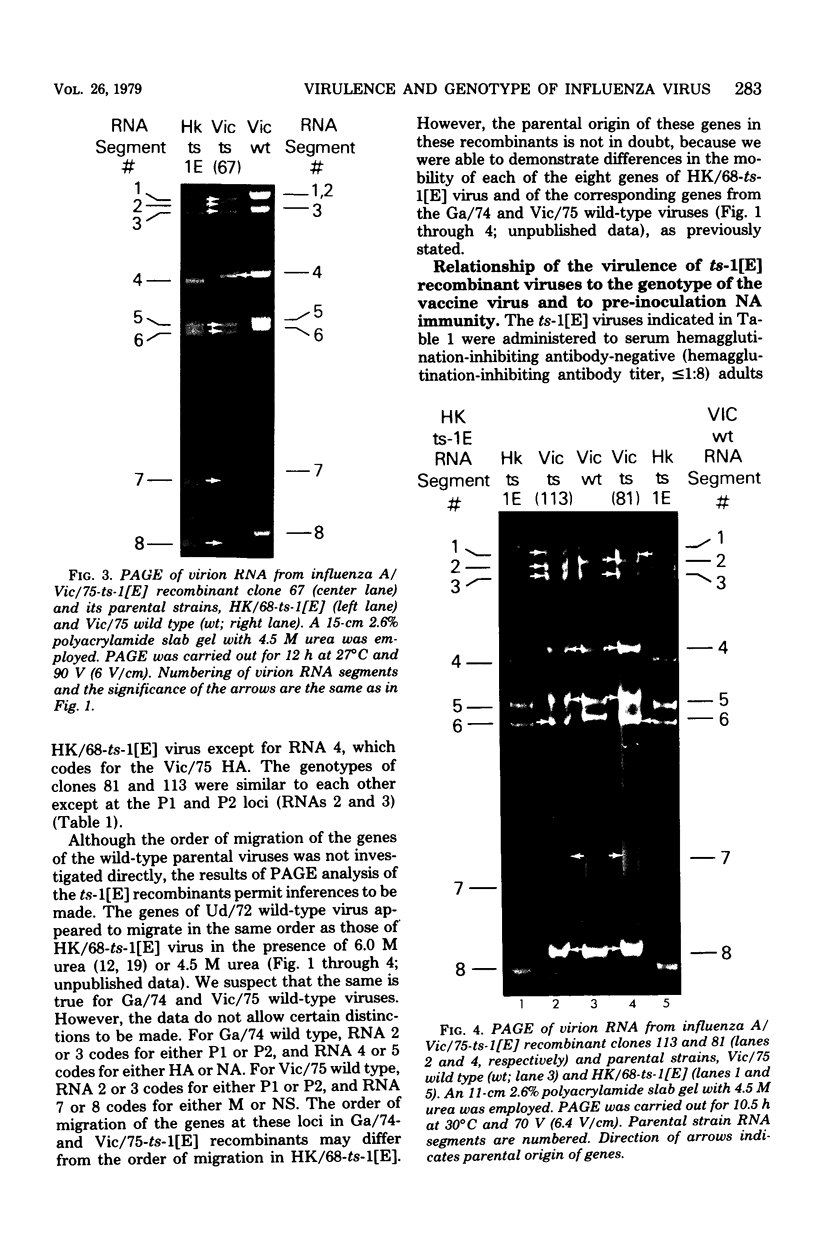
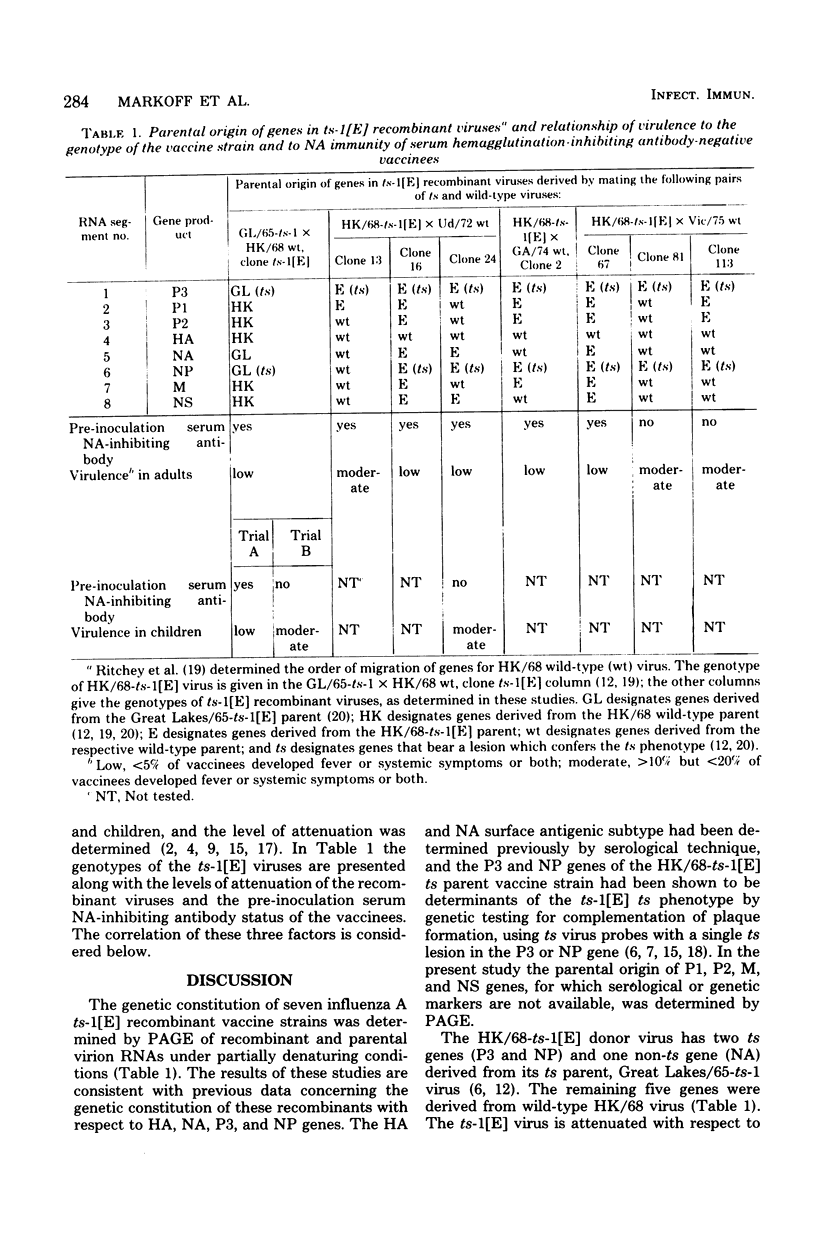
Influenza A/Hong Kong/68-ts-1[E] virus is a temperature-sensitive mutant developed for use as a live virus vaccine (B. R. Murphy, E. G. Chalhub, S. R. Nusinoff, J. Kasel, and R. M. Chanock, J. Infect. Dis. 128:479--487, 1973). This virus and temperature-sensitive recombinants derived by mating it with A/Udorn/72, A/Georgia/74, or A/Victoria/75 wild-type virus have been administered to volunteers in clinical trials on the assumption that the ts-1[E] temperature-sensitive genetic lesions on a polymerase gene (P3) and on the nucleoprotein gene (NP) would determine a satisfactory and reproducible level of attentuation regardless of the genetic constitution of ts-1[E] recombinants at other loci (B. R. Murphy, D. D. Richman, S. B. Spring, and R. M. Chanock, Postgrad. Med. 52:381--388, 1976). In this paper, the parental origin of genes in the ts-1[E] recombinants was determined by using the technique of polyacrylamide gel electrophoresis of virion ribonucleic acid segments in the presence of a denaturing agent (urea). When tested in individuals who lacked immunity to hemagglutinin antigen, attenuation of the ts-1[E] recombinants appeared to correlate with inheritance of the ts-1[E] temperature-sensitive genes at the P3 and NP loci and with the level of preinfection neuraminidase immunity. There was no evidence that other genes from the ts-1[E] donor virus played a role in attenuation.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Desselberger U., Palese P. Molecular weights of RNA segments of influenza A and B viruses. Virology. 1978 Jul 15;88(2):394–399. doi: 10.1016/0042-6822(78)90297-0. [DOI] [PubMed] [Google Scholar]
- Douglas R. G., Jr, Markoff L. J., Murphy B. R., Chanock R. M., Betts R. F., Hayden F. G., Levine M. M., Van Blerk G. A., Sotman S. B., Nalin D. R. Live Victoria/75-ts-1[E] influenza A virus vaccines in adult volunteers: role of hemagglutinin immunity in protection against illness and infection caused by influenza A virus. Infect Immun. 1979 Oct;26(1):274–279. doi: 10.1128/iai.26.1.274-279.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floyd R. W., Stone M. P., Joklik W. K. Separation of single-stranded ribonucleic acids by acrylamide-agarose-urea gel electrophoresis. Anal Biochem. 1974 Jun;59(2):599–609. doi: 10.1016/0003-2697(74)90313-3. [DOI] [PubMed] [Google Scholar]
- Kim H. W., Arrobio J. O., Brandt C. D., Parrott R. H., Murphy B. R., Richman D. D., Chanock R. M. Temperature-sensitive mutants of influenza A virus: response of children to the influenza A/Hong Kong/68-ts-1(E) (H3N2) and influenza A/Udorn/72-ts-1(E) (H3N2) candidate vaccine viruses and significance of immunity to neuraminidase antigen. Pediatr Res. 1976 Apr;10(4):238–242. doi: 10.1203/00006450-197604000-00008. [DOI] [PubMed] [Google Scholar]
- Murphy B. R., Chalhub E. G., Nusinoff S. R., Chanock R. M. Temperature-sensitive mutants of influenza virus. II. Attenuation of ts recombinants for man. J Infect Dis. 1972 Aug;126(2):170–178. doi: 10.1093/infdis/126.2.170. [DOI] [PubMed] [Google Scholar]
- Murphy B. R., Chalhub E. G., Nusinoff S. R., Kasel J., Chanock R. M. Temperature-sensitive mutants of influenza virus. 3. Further characterization of the ts-1(E) influenza A recombinant (H3N2) virus in man. J Infect Dis. 1973 Oct;128(4):479–487. doi: 10.1093/infdis/128.4.479. [DOI] [PubMed] [Google Scholar]
- Murphy B. R., Hosier N. T., Spring S. B., Mostow S. R., Chanock R. M. Temperature-sensitive mutants of influenza A virus: production and characterization of A/Victoria/3/75-ts-1[E] recombinants. Infect Immun. 1978 Jun;20(3):665–670. doi: 10.1128/iai.20.3.665-670.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy B. R., Kasel J. A., Chanock R. M. Association of serum anti-neuraminidase antibody with resistance to influenza in man. N Engl J Med. 1972 Jun 22;286(25):1329–1332. doi: 10.1056/NEJM197206222862502. [DOI] [PubMed] [Google Scholar]
- Murphy B. R., Markoff L. J., Hosier N. T., Rusten H. M., Chanock R. M., Kendal A. P., Douglas R. G., Betts R. F., Cate T. R., Jr, Couch R. B. Temperature-sensitive mutants of influenza A virus: evaluation of A/Victoria/3/75-ts-1[E] recombinant viruses in volunteers. Infect Immun. 1978 Jun;20(3):671–677. doi: 10.1128/iai.20.3.671-677.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy B. R., Richman D. D., Spring S. B., Chanock R. M. Use of temperature-sensitive mutants of influenza A virus as live virus vaccine strains. Evaluation in laboratory animals, adults and children. Postgrad Med J. 1976 Jun;52(608):381–388. doi: 10.1136/pgmj.52.608.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palese P., Ritchey M. B. Live attenuated influenza virus vaccines. Strains with temperature-sensitive defects in P3 protein and nucleoprotein. Virology. 1977 May 1;78(1):183–191. doi: 10.1016/0042-6822(77)90090-3. [DOI] [PubMed] [Google Scholar]
- Palese P., Schulman J. L. Differences in RNA patterns of influenza A viruses. J Virol. 1976 Mar;17(3):876–884. doi: 10.1128/jvi.17.3.876-884.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palese P., Schulman J. L. Mapping of the influenza virus genome: identification of the hemagglutinin and the neuraminidase genes. Proc Natl Acad Sci U S A. 1976 Jun;73(6):2142–2146. doi: 10.1073/pnas.73.6.2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palese P. The genes of influenza virus. Cell. 1977 Jan;10(1):1–10. doi: 10.1016/0092-8674(77)90133-7. [DOI] [PubMed] [Google Scholar]
- Richman D. D., Murphy B. R., Belshe R. B., Rusten H. M., Chanock R. M., Blacklow N. R., Parrino T. A., Rose F. B., Levine M. M., Caplan E. Temperature-sensitive mutants of influenza A virus. XIV. Production and evaluation of influenza A/Georgia/74-ts-1[E] recombinant viruses in human adults. J Infect Dis. 1977 Aug;136(2):256–262. doi: 10.1093/infdis/136.2.256. [DOI] [PubMed] [Google Scholar]
- Richman D. D., Murphy B. R., Chanock R. M. Demonstration of a non-temperature-sensitive growth-restricting mutation in a ts mutant of influenza A virus: implications for live virus vaccine development. Virology. 1977 Dec;83(2):356–364. doi: 10.1016/0042-6822(77)90180-5. [DOI] [PubMed] [Google Scholar]
- Richman D. D., Murphy B. R., Chanock R. M., Gwaltney J. M., Jr, Douglas R. G., Betts R. F., Blacklow N. R., Rose F. B., Parrino T. A., Levine M. M. Temperature-sensitive mutants of influenza A virus. XII. Safety, antigenicity, transmissibility, and efficacy of influenza A/Udorn/72-ts-1[E] recombinant viruses in human adults. J Infect Dis. 1976 Dec;134(6):585–594. [PubMed] [Google Scholar]
- Richman D. D., Murphy B. R., Spring S. B., Coleman M. T., Chanock R. M. Temperature sensitive mutants of influenza virus. IX. Genetic and biological characterization of TS-1[E] lesions when transferred to a 1972 (H3N2) influenza A virus. Virology. 1975 Aug;66(2):551–562. doi: 10.1016/0042-6822(75)90227-5. [DOI] [PubMed] [Google Scholar]
- Ritchey M. B., Palese P., Schulman J. L. Mapping of the influenza virus genome. III. Identification of genes coding for nucleoprotein, membrane protein, and nonstructural protein. J Virol. 1976 Oct;20(1):307–313. doi: 10.1128/jvi.20.1.307-313.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spring S. B., Nusinoff S. R., Mills J., Richman D. D., Tierney E. L., Murphy B. R., Chanock R. M. Temperature-sensitive mutants of influenza virus. VI. Transfer of TS lesions from the Asian subtype of influenza A virus (H2N2) to the Hong Kong subtype (H3N2). Virology. 1975 Aug;66(2):522–532. doi: 10.1016/0042-6822(75)90224-x. [DOI] [PubMed] [Google Scholar]