Abstract
The glucose-inhibited neurons present in the lateral hypothalamic area are regarded as glucose detectors. This structure is involved in the regulation of food intake through extracellular blood glucose concentrations, and plays a crucial role in obesity onset. In the present study, obesity models established with high fat feeding were treated with electroacupuncture at Zusanli (ST36)/Inner Court (ST44) on the left side and Tianshu (ST25) bilaterally. We found that electroacupuncture could effectively reduce body weight and the fat-weight ratio, and decrease serum leptin, resistin, tumor necrosis factor alpha, and neuropeptide Y levels, while increase serum adiponectin and cholecystokinin-8 levels. This treatment altered the electrical activity of glucose-inhibited neurons in the lateral hypothalamic area, with electroacupuncture at Zusanli/Inner Court exerting an inhibitory effect, while electroacupuncture at bilateral Tianshu exerting an excitatory effect. These data suggest that electroacupuncture at the lower limbs and abdominal cavity is an effective means for regulating the activity of glucose-inhibited neurons in the lateral hypothalamic area and for improving the secretory function of adipose tissue.
Keywords: neural regeneration, acupuncture and moxibustion, electroacupuncture, acupoint, adipose tissue, hypothalamus, obesity, feeding center, acupuncture, regeneration, grants-supported paper, neuroregeneration
Research Highlights
-
(1)
Electroacupuncture at the lower limbs and abdominal cavity is an effective means for fat weight loss and improvement of secretion function of adipose tissue.
-
(2)
Glucose-inhibited neurons are present in the lateral hypothalamic area, and can be regulated by electroacupuncture treatment at the lower limbs and abdominal cavity. This regulatory action may be one of the mechanisms underlying acupuncture treatment for simple obesity.
-
(3)
Electroacupuncture at different acupoints exerts various effects on the glucose-inhibited neurons in the lateral hypothalamic area.
INTRODUCTION
Simple obesity is a high-risk factor for hypertension, coronary heart disease, and type 2 diabetes mellitus. As such, the prevention and treatment of obesity has become a serious public health challenge. With the identification of numerous adipocyte factors including leptin, adipose-derived tumor necrosis factor, adiponectin, and resistin, adipose tissue is now well established as a highly active metabolic and endocrine organ[1,2]. Adipocyte factor secreted by adipose tissue also acts on the hypothalamic energy regulation center, and generates a connection between adipose tissue and the hypothalamus[3], providing feedback regulation on the in vivo energy balance. Under normal conditions, the body fat content is stable. The body fat signal is present in the circulation, and when the body fat content changes, the signal stimulates the energy regulation center in the hypothalamus to adjust the body energy balance. Leptin is a key body fat signaling molecule[4] that is secreted by adipose tissue, and can directly regulate in vivo energy balance and fat storage through the binding with the hypothalamic leptin receptor. Glucose-inhibited neurons act as a glucose detector to regulate feeding and maintain energy balance via alterations of neuronal discharge rate[5]. Glucose-inhibited neurons, predominantly located in the lateral hypothalamic area (feeding center) and the lateral arcuate nucleus, modulate feeding behavior, and are closely correlated with obesity, diabetes, and other metabolic disorders. Glucose-inhibited neurons express neuropeptide Y, insulin, leptin, and other receptors[6], and peripheral leptin and glucose signals converge in the feeding center[7]. In addition, leptin and insulin control feeding regulation via the activity of glucose-inhibited neurons. Therefore, glucose-inhibited neurons may be important targets of leptin, in which the inhibitory effect on the feeding center is regulated by activity of glucose-inhibited neurons[4].
The majority of obese patients present adipose tissue functional disorders[8] and abnormal secretion of adipocyte factor. Electroacupuncture is regarded as an effective and safe means for treatment of simple obesity[9,10]. For example, electroacupuncture was reported to reduce food intake of the diet-induced obese rats[11]. However, the inhibitory mechanism of electroacupuncture on feeding remains unclear. In this study, we examined adipocyte factors, feeding-related factors, and the activity of hypothalamic glucose-inhibited neurons to determine the influence of electroacupuncture at different acupoints on the adipose tissue-hypothalamic feeding pathways in obese rats, and to explore the weight reduction mechanism of electroacupuncture at lower limbs and abdominal cavity.
RESULTS
Quantitative analysis of experimental animals
Sprague-Dawley rats at 21 days of age were randomly divided into a normal diet group (n = 10) and a high fat diet group (n = 90). In the high fat diet group, 30 obesity rats were successfully established (33% success rate), which were then divided randomly into the model group (no electroacupuncture intervention), Zusanli/Inner Court group [electroacupuncture at Zusanli (ST36)/Inner Court (ST44) acupoints at the left side], and the Tianshu/Tianshu group [electroacupuncture at bilateral Tianshu (ST25) acupoints] (n = 10 per group).
Electroacupuncture effectively reduced the body weight of obese rats
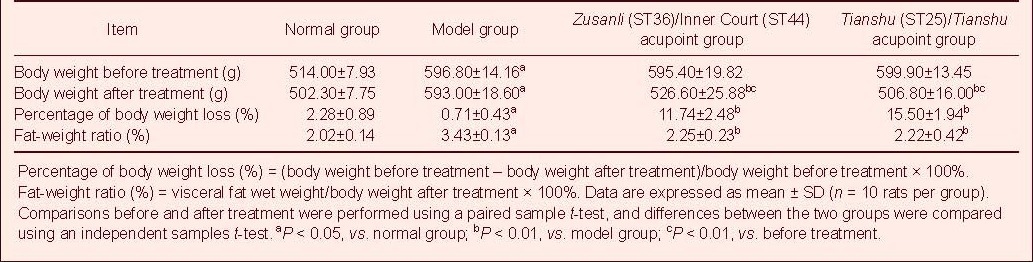
The body weight of experimental rats was increased after high fat diet (P < 0.05). Electroacupuncture at Zusanli/Inner Court or Tianshu/Tianshu acupoints significantly reduced the body weight of obese rats (P < 0.01) close to normal. There was no significant difference in body weight between the Zusanli/Inner Court group and the Tianshu/Tianshu group (P > 0.05, Table 1).
Table 1.
Effect of electroacupuncture at various acupoints on body weight and fat-weight ratio of rats
Effect of electroacupuncture on adipocyte factors of obese rats
After rats were fed with high fat diet, the serum leptin, resistin, and tumor necrosis factor-α levels were increased, while adiponectin levels were decreased (P < 0.05). Electroacupuncture at Zusanli/Inner Court or Tianshu/Tianshu acupoints decreased the serum leptin, resistin, and tumor necrosis factor-α levels, but increased adiponectin levels, in the obese rats (P < 0.01). Acupuncture at Zusanli/Inner Court acupoints showed a stronger effect than that at bilateral Tianshu acupoints (P < 0.05; Figure 1).
Figure 1.
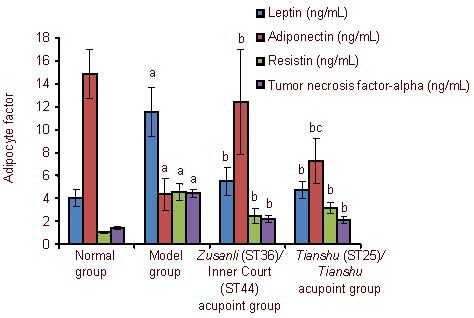
Effect of electroacupuncture on adipocyte factors of obese rats.
Data are expressed as mean ± SD (n = 10 rats per group). Differences between the two groups were compared using independent samples t-test. aP < 0.05, vs. normal group; bP < 0.01, vs. model group; cP < 0.01, vs. Zusanli/Inner Court acupoint group.
Effect of electroacupuncture on serum feeding-related peptides in obesity rats
After rats were fed with a high fat diet, the serum neuropeptide Y level increased and cholecystokinin-8 level decreased (P < 0.01). Electroacupuncture treatment decreased the neuropeptide Y level and increased cholecystokinin-8 level in obese rats (P < 0.05; Figure 2).
Figure 2.
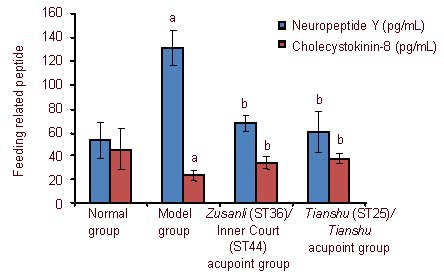
Effect of electroacupuncture at various acupoints on the feeding related peptide in obese rats.
Data are expressed as mean ± SD (n =10 rats per group). Comparison before and after treatment was performed using a paired sample t-test, and differences between the two groups were compared using an independent samples t-test. aP < 0.05, vs. normal group; bP < 0.01, vs. model group.
Effect of electroacupuncture on the discharge of glucose-inhibited neurons in lateral hypothalamus area of food deprived rats
There were 13 glucose-inhibited neurons recorded in the normal group, with a discharge frequency of 4.01 ± 1.62 Hz, and 15 glucose-inhibited neurons recorded in the food deprivation group, with a discharge frequency of 7.36 ± 2.02 Hz. The discharge frequency of recorded neurons was higher in the food deprivation group than in the normal group (P < 0.05), suggesting that the activity of glucose-inhibited neurons was altered by appetite hyperthyroidism pathology.
After electroacupuncture at the Zusanli/Inner Court acupoints, 31 glucose-inhibited neurons were completely recorded, 27 of which had a significantly altered discharge activity (27/31, 87%). Of these, electroacupuncture inhibited discharge activity in 22 neurons (22/27, 82%, P < 0.01) and completely inhibited activity in six neurons. After electroacupuncture at Tianshu/Tianshu acupoints, 22 glucose-inhibited neurons were completely recorded, 16 of which had a significantly altered discharge activity (16/22, 73%). Of these, 11 neurons were excited by the electroacupuncture (11/16, 69%; Table 2, Figure 3). These data suggest that electroacupuncture can alter the electrical activity of glucose-inhibited neurons. Furthermore, electroacupuncture at Zusanli/Inner Court acupoints exerts predominantly inhibitory effects, while electroacupuncture at Tianshu/Tianshu exerts predominantly excitatory effects role.
Table 2.
Effect of electroacupuncture at various acupoints on activity of glucose-inhibited neurons in the lateral hypothalamic area of food deprived rats [number of neurons (%)]
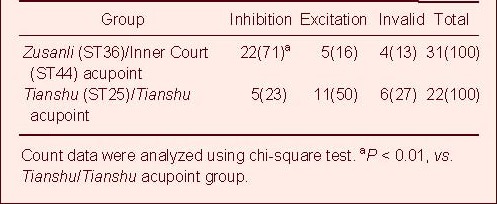
Figure 3.
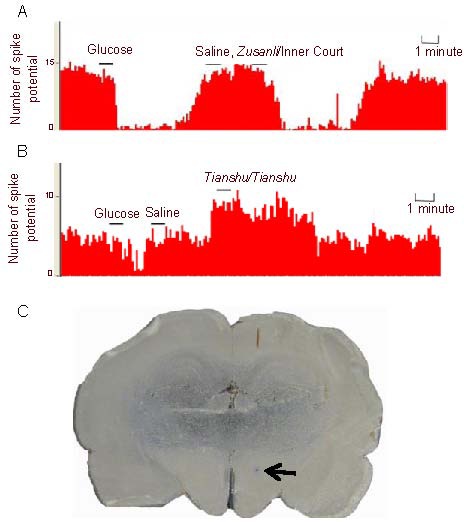
Effect of electroacupuncture at Zusanli (ST36)/Inner Court (ST44) and Tianshu (ST25)/Tianshu acupoints on activity of glucose-inhibited neurons.
(A) In the electroacupuncture at Zusanli/Inner Court point group, the discharge frequency of glucose-inhibited neurons decreased significantly after intravenous injection of glucose, and was not changed after saline injection, while glucose-inhibited neurons were inhibited by electroacupuncture at Zusanli/Inner Court points.
(B) In the electroacupuncture at Tianshu/Tianshu acupoint group, glucose-inhibited neurons were excited by electroacupuncture at Tianshu.
(C) After the experiment was complete, pontamine sky blue was applied to label the microelectrode recording sites. Arrow indicates pontamine sky blue exudation point (lateral hypothalamic area). The intervals are denoted by “-”.
DISCUSSION
Electroacupuncture stimulation can effectively reduce the body weight and body mass index of obese patients[12,13,14,15]. In addition, electroacupuncture can reduce body weight and food intake in obese rats, suggesting that central nuclei such as the lateral hypothalamic area, hypothalamic ventromedial nucleus, and arcuate nucleus, are likely to be involved in these regulatory mechanisms[16,17]. However, the specific neuronal types involved in these pathways remain unclear.
The hypothalamus plays a key role in controlling food intake, maintaining energy balance, and regulating neurohumor. Furthermore, the hypothalamus may integrate peripheral body fat signals such as leptin and insulin, as well as metabolic signals such as glucose and lipid[18], to modulate feeding behavior. The lateral hypothalamic area is considered an important feeding center[19], and more than 33% of neurons within this area are glucose-sensitive neurons[20]. Thus, glucose-inhibited neurons may regulate feeding behavior caused by reduced circulating glucose levels. These actions of glucose-inhibited neurons are likely mediated by leptin[18], which can inhibit feeding through inhibition of glucose-inhibited neurons. Signaling via adenosine monophosphate-activated protein kinase may play a key role in this pathway, as leptin inhibits hypothalamic adenosine monophosphate-activated protein kinase activity, while knockout of adenosine monophosphate-activated protein kinase in the hypothalamus reduces the effect of leptin on lowering food intake. There is also growing evidence to suggest that the excitability effect of hypoglycemia on glucose-inhibited neurons is similar to that of the adenosine monophosphate-activated protein kinase agonist 5-amino-imidazole-4-carboxamide riboside, which can be blocked by the adenosine monophosphate-activated protein kinase antagonist Compound C[21]. Similarly, the inhibitory effects of leptin on glucose-inhibited neurons can be reversed by 5-amino-imidazole-4-carboxamide riboside.
Neuropeptide Y is the strongest pro-appetite peptide, and can be suppressed by leptin binding with the leptin receptor, thus inhibiting food intake and regulating energy metabolism. Hypothalamic neuropeptide Y neurons respond to extracellular glucose levels, suggesting that neuropeptide Y plays a crucial role in glucose sensing. The increase of extracellular glucose concentration may lead to the reduction in activation of agouti-related peptide/neuropeptide Y neurons[22] and the release of neuropeptide Y. In vivo and in vitro experiments suggest that insulin-induced hypoglycemia can elevate the release of neuropeptide Y in the paraventricular nucleus of the hypothalamus in normal rats[23]. Furthermore, 40% of neuropeptide Y neurons were reported to be glucose-inhibited neurons, and in the non-neuropeptide Y neurons, their inhibition in response to glucose was less than 5%[22]. When sufficient energy is supplied, leptin reduces the activity of neuropeptide Y/glucose-inhibited neurons and food intake. In the fasting state, both leptin and glucose activate adenosine monophosphate-activated protein kinase in neuropeptide Y/glucose-inhibited neurons, leading to neuronal depolarization and increased neuropeptide Y release, increased food intake, reduced energy consumption, and maintenance of energy balance[24]. Therefore, these data suggest that when starvation leads to reduced blood glucose levels, the excitability of glucose-inhibited neurons increases, triggering the release of neuropeptide Y and other pro-appetite substances, thus resulting in feeding behavior[25].
Cholecystokinin-8 is an important satiety signal[26], and functions to transfer satiety signals to the nucleus of solitary tract and the lateral hypothalamic area, as well as regulate feeding termination through activation of the gastric vagal nerve. There are two pathways involved in cholecystokinin-8 regulation of feeding: (1) gastric vagal nerve signals can directly transfer gastrointestinal hormones including cholecystokinin-8 to the glucose sensitive neurons in lateral hypothalamic area[20,27], and (2) leptin exerts intervention effects on cholecystokinin-8[28], whereby leptin can improve the central satiety response to cholecystokinin, thus inhibiting feeding[29,30].
In the present study we found that electroacupuncture produced marked regulatory effects on body weight, fat-weight ratio, body fat signal leptin, and the feeding-related factors neuropeptide Y and cholecystokinin-8. Obese rats exhibited an abnormal increase of leptin suggestive of leptin resistance, while the increased levels of neuropeptide Y and decreased cholecystokinin-8 level indicate hyperthyroidism appetite and reduced perception to satiety signals in obese rats. Gong et al[31] confirmed that electroacupuncture could improve leptin resistance in obese rats, indicating that acupuncture can regulate the levels of leptin, neuropeptide Y, and cholecystokinin-8. Acupuncture can also suppress hyperthyroidism appetite and simultaneously reduce perception to satiety signals, thus enhancing the satiety feeling. However, the central mechanism remains unclear. Thus, the aim of our study was to examine glucose-inhibited neurons in the lateral hypothalamic area to further explore the difference of electroacupuncture at various acupoints and its possible central mechanism.
In the fasting state, the activity of glucose-inhibited neurons is similar to that of the obesity rat model[24]. Thus, we adopted experimental rats that had been fasted for 24 hours to simulate pathological models of hyperthyroidism appetite. We found that the discharge frequency of glucose-inhibited neurons increased in the food deprivation group, suggesting that the excitation of glucose-inhibited neurons may reflect hyperthyroidism appetite after food deprivation. The role of glucose-inhibited neurons in feeding has been previously demonstrated. However, there are limited data on the actions of electroacupuncture on glucose-inhibited neurons. Our experimental findings showed that electroacupuncture at Zusanli/Inner Court and Tianshu/Tianshu markedly altered the electrical activity of glucose-inhibited neurons in the lateral hypothalamic area. These data suggest that electroacupuncture at various acupoints can affect the activity of glucose-inhibited neurons and modulate feeding behavior. Thus, glucose-inhibited neurons may be a key target of electroacupuncture-mediated modulation of feeding behavior. In addition, the glucose-inhibited neurons were inhibited by electroacupuncture at the abdominal cavity and excited by electroacupuncture at the lower cavity. In summary, we suggest that the lower extremity acupoints may inhibit feeding behavior through leptin/glucose-inhibited neurons/neuropeptide Y neuron pathway, and can terminate feeding behavior by increasing cholecystokinin-8 levels and enhancing the satiety feeling. However, the mechanism associated with abdominal acupuncture requires further investigation. In addition, electroacupuncture plays a crucial role in modulating adipocyte factors. Endogenous tumor necrosis factor-α is the main source of adipose tissues[32], and is closely related to lipid metabolism and glucose metabolism, and functions to promote lipolysis, increase energy expenditure, and reduce insulin sensitivity. Thus, tumor necrosis factor-α is considered one of the main factors leading to fat tissue insulin resistance[33]. Adiponectin possesses anti-inflammatory and anti-insulin resistance effects[34]. The levels of adiponectin are decreased in obese and type 2 diabetes patients, and the plasma concentration may be positively correlated with body mass index and fasting plasma glucose and triglyceride in the normal population. The biological role of resistin remains unclear, although animal trials demonstrate that resistin levels are elevated in diet-induced obese mice, and are positively related to insulin, leading to impaired glucose tolerance. Thus, it has been suggested to be involved in diet-induced obesity and insulin resistance-associated obesity[35,36]. A previous study confirmed that electroacupuncture can change abnormally secreted products in adipose tissue of obese rats[37], while Sun et al[38] reported that electroacupuncture improved lipid metabolism in experimental obese rats, with sex differences. In the present study, we also found significant differences in the regulation of adiponectin levels between electroacupuncture at Zusanli/Inner Court and Tianshu/Tianshu acupoints.
Overall, our data suggest that electroacupuncture at various acupoints may reduce body fat and act on the abnormal pathological link in fat-hypothalamic pathways by improving the abnormal secretion of adipose tissue, regulating body fat signals and feeding factors such as neuropeptide Y and cholecystokinin-8, and thereby adjusting abnormal feeding behaviors including hyperthyroidism appetite and reduced perception to satiety. In addition, the hypothalamic glucose-inhibited neurons can modulate energy balance.
MATERIALS AND METHODS
Design
A randomized, controlled, animal experiment.
Time and setting
Experiments were performed from March 2010 to November 2011 in the Experimental Animal Center of Nanjing University of Chinese Medicine, China.
Materials
Sprague-Dawley male rats (specific pathogen free level) at neonatal 21 days (weaned), and 170 healthy male adult Wistar rats (specific pathogen free level) at 8–12 weeks old (250–350 g) were provided by Shanghai SLAC Experiment Animal Co., Ltd., China [license No. SCXK (Hu) 2010-0010]. All experimental animals were raised in the Experimental Animal Center of Nanjing University of Chinese Medicine, China. All experimental procedures were in accordance with the Regulations for the Administration of Affairs Concerning Experimental Animals in Jiangsu Province, China[39].
Methods
Establishment of obese models
Sprague-Dawley rats were adapted to normal feed for 1 week, and then given a high fat diet containing 60% complete formula feed, 12% pork fat, 5% sucrose, 5% milk, 5% peanut, 10% egg, 1% sesame oil, and 2% salt (Suzhou Shuangshi Laboratory Animal Feed Science Co., Ltd., Suzhou, Jiangsu Province, China) for 3 months[40]. Obesity was evaluated by an increase of 15% body weight higher than that of normal diet group (given complete formula feed; Suzhou Shuangshi Laboratory Animal Feed Science Co., Ltd.).
Establishment of hyperthyroidism appetite models by food deprivation
Wistar rats were fasted for 24 hours prior to the experiment, with only access to sugar and water[24].
Electroacupuncture intervention at various acupoints
Acupuncture intervention at the Zusanli acupoint (5 mm below the capitulum fibulae, at lateral stifle joint) and the Inner Court acupoint (between the second and third metatarsus, posterior to the edge of toe web) was administered at a depth 0.5 mm on the left side. Bilateral Tianshu acupoints (5 mm lateral to the intersection between the upper 2/3 and the lower 1/3 in the line between xiphoid process and pubic symphysis upper border) was administered at a depth 0.5 mm. The acupuncture interventions were applied using a Han electroacupuncture therapeutic apparatus (LH402A; Beijing Huawei Industrial Development Corporation, Beijing, China) at 2 Hz and 15 Hz alternating frequencies (intensity of 2 mA) for 15 minutes, once per day. The model group rats were only fixed, without acupuncture interventions. All experimental rats were weighed before each intervention, and the intervention was given for 6 successive days and allowed to rest for one day. During the 38-day observations, rats were treated or fixed for a total of 33 times, and allowing free access to normal diet.
Calculation of fat-weight ratio
All experimental rats were fasted for 12 hours after the interventions were given, and their body weight was recorded (g). Under 20% urethane (0.8 g/kg) anesthesia, rats were euthanized and blood samples (3 mL) were collected by the retrobulbar capillary method. The abdominal cavity was then opened, and perirenal and epididymal fat (representative of visceral fat content) were harvested and weighed. The fat-weight ratio was calculated as: fat-weight ratio = visceral fat wet weight / weight after treatment × 100%.
Enzyme linked immunosorbent assay for the measurement of serum leptin, neuropeptide Y, cholecystokinin-8, adiponectin, resistin, and tumor necrosis factor alpha levels
Serum leptin, neuropeptide Y, cholecystokinin-8, adiponectin, resistin, and tumor necrosis factor alpha levels were determined by enzyme linked immunosorbent assay[37], according to the instructions of assay kit (Shanghai Touching Technology Co., Ltd., Shanghai, China).
Discharge of glucose-inhibited neurons in lateral hypothalamus area
Wistar rats were anesthetized with an intraperitoneal injection of 20% urethane and 1.625% α-chloralose (65 mg/kg; Sigma, Milwaukee, WI, USA), and then received external jugular vein intubation and tracheal intubation. Animals were fixed onto a brain stereotaxic instrument (David Kopf Instruments, Tujunga, CA, USA), and according to the Rat Brain in Stereotaxic Coordinates[41], brain tissue above the lateral hypothalamic area was exposed by craniotomy, and neuronal discharge was recorded using glass microelectrode (Nanjing Spring Teaching Experimental Instrument Factory, Nanjing, Jiangsu Province, China; containing 2% pontamine sky blue, Sigma, and 0.5 M sodium acetate; DC impedance 5–10 MΩ). External jugular vein injection of glucose (0.4 M) and saline was applied to identify glucose-inhibited neurons[42,43]; nerves exhibiting a response to glucose and no response to saline were considered to be the target nerves. After the discharge frequency was stable for 2 minutes, the baseline recordings were collected. The normal group and food deprivation group were recorded without interventions, while the Zusanli/Inner Court and Tianshu/Tianshu acupoint groups were given electroacupuncture during neuronal discharge recording. During the experiment, animal temperature was maintained at 37 ± 0.5°C using an electric heating board. After the end of the experiment, pontamine sky blue was dialyzed to the recording site at the electrode tip using the cathode current (10 μA, 10 minutes). Brain tissues were then fixed with 10% formalin solution, and sectioned using a freezing microtome. The recording sites beyond the lateral hypothalamic area were not included in the statistics. The neuronal discharge signals were amplified (A-M systems, Carlsborg, WA, USA) and monitored using an oscilloscope (DF4351A; Ningbo Zhongce Electronics, Ningbo, China), and then recorded with a physiological signal acquisition system (RM6240; Chengdu Instrument Factory, Chengdu, Sichuan Province, China). A time histogram before and after stimulation was plotted, and a change of more than 20% of baseline data in the discharge frequency before and after stimulation was used to indicate a neuronal response to the stimulus.
Statistical analysis
Data are expressed as mean ± SD, and were analyzed using SPSS 17.0 software (SPSS, Chicago, IL, USA). Differences before and after treatment were compared using a paired sample t-test, the two groups were compared using an independent samples t-test, and comparison among groups was performed using analysis of variance. Neuronal discharge was analyzed by independent sample t-test, and count data were compared using the chi-square test. A P value less than 0.05 value was considered statistically significant.
Acknowledgments
We thank Xiliu Zhang and Lijun Jia from Nanjing University of Chinese Medicine for help with photographs, Jianjun Wang, Hongzhao Li, Bin Li, and Jingning Zhu from Nanjing University for experimental and technical support.
Footnotes
Funding: This study was supported by the National Basic Research Program of China (973 Program), No. 2011CB505206, the National Natural Science Foundation of China, No. 30873307 and No. 81202744; the Natural Science Research Project of Higher Education of Jiangsu Province, No. 11KJB360008; the Distinctive Discipline of Jiangsu Province; and the Scientific and Technological Innovation Group of “Qinglan Project” of Jiangsu Province.
Conflicts of interest: None declared.
Ethical approval: Experimental procedures were approved by the Institutional Animal Care and Use Committee, Nanjing University of Traditional Chinese Medicine, China.
(Edited by Ma J, Liang FX/Yang Y/Wang L)
REFERENCES
- [1].Wozniak SE, Gee LL, Wachtel MS, et al. Adipose tissue: the new endocrine organ? A review article. Dig Dis Sci. 2009;54(9):1847–1856. doi: 10.1007/s10620-008-0585-3. [DOI] [PubMed] [Google Scholar]
- [2].Galic S, Oakhill JS, Steinberg GR. Adipose tissue as an endocrine organ. Mol Cell Endocrinol. 2010;316(2):129–139. doi: 10.1016/j.mce.2009.08.018. [DOI] [PubMed] [Google Scholar]
- [3].Cammisotto PG, Levy E, Bukowiecki LJ, et al. Cross-talk between adipose and gastric leptins for the control of food intake and energy metabolism. Prog Histochem Cytochem. 2010;45(3):143–200. doi: 10.1016/j.proghi.2010.06.001. [DOI] [PubMed] [Google Scholar]
- [4].Valassi E, Scacchi M, Cavagnini F. Neuroendocrine control of food intake. Nutr Metab Cardiovasc Dis. 2008;18(2):158–168. doi: 10.1016/j.numecd.2007.06.004. [DOI] [PubMed] [Google Scholar]
- [5].Burdakov D, González JA. Physiological functions of glucose-inhibited neurones. Acta Physiol (Oxf) 2009;195(1):71–78. doi: 10.1111/j.1748-1716.2008.01922.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Shiraishi T, Oomura Y, Sasaki K, et al. Effects of leptin and orexin-A on food intake and feeding related hypothalamic neurons. Physiol Behav. 2000;71(3-4):251–261. doi: 10.1016/s0031-9384(00)00341-3. [DOI] [PubMed] [Google Scholar]
- [7].Li B, Guo CL, Tang J, et al. Cerebellar fastigial nuclear inputs and peripheral feeding signals converge on neurons in the dorsomedial hypothalamic nucleus. Neurosignals. 2009;17(2):132–143. doi: 10.1159/000197913. [DOI] [PubMed] [Google Scholar]
- [8].Blüher M. Adipose tissue dysfunction in obesity. Exp Clin Endocrinol Diabetes. 2009;117(6):241–250. doi: 10.1055/s-0029-1192044. [DOI] [PubMed] [Google Scholar]
- [9].Sui Y, Zhao HL, Wong VC, et al. A systematic review on use of Chinese medicine and acupuncture for treatment of obesity. Obes Rev. 2012;13(5):409–430. doi: 10.1111/j.1467-789X.2011.00979.x. [DOI] [PubMed] [Google Scholar]
- [10].Cho SH, Lee JS, Thabane L, et al. Acupuncture for obesity: a systematic review and meta-analysis. Int J Obes (Lond) 2009;33(2):183–196. doi: 10.1038/ijo.2008.269. [DOI] [PubMed] [Google Scholar]
- [11].Wang F, Tian DR, Han JS. Electroacupuncture in the treatment of obesity. Neurochem Res. 2008;33(10):2023–2027. doi: 10.1007/s11064-008-9822-6. [DOI] [PubMed] [Google Scholar]
- [12].Tong J, Chen JX, Zhang ZQ, et al. Clinical observation on simple obesity treated by acupuncture. Zhongguo Zhen Jiu. 2011;31(8):697–701. [PubMed] [Google Scholar]
- [13].Abdi H, Zhao B, Darbandi M, et al. The effects of body acupuncture on obesity: anthropometric parameters, lipid profile, and inflammatory and immunologic markers. ScientificWorldJournal 2012. 2012 doi: 10.1100/2012/603539. 603539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Yang JJ, Xing HJ, Wang SJ, et al. Effects of acupuncture combined with dietary adjustments and aerobic exercise on body weight, body mass index and serum leptin level in simple obesity patients. Zhen Ci Yan Jiu. 2010;35(6):453–457. [PubMed] [Google Scholar]
- [15].Kim SK, Bae H, Lee G, et al. The endogenous CCK mediation of electroacupuncture stimulation-induced satiety in rats. Peptides. 2008;29(4):564–570. doi: 10.1016/j.peptides.2008.01.002. [DOI] [PubMed] [Google Scholar]
- [16].Zhao M, Yuan JH, Li J, et al. Effect of acupuncture on feeding center of hypothalamus in experimental fat rats. Zhongguo Zhen Jiu. 2001;21(5):305–306. [Google Scholar]
- [17].Liu ZC, Sun FM. The experimental study on acupuncture treatment of simple obesity in rats. Zhen Ci Yan Jiu. 1998;23(1):69–74. [Google Scholar]
- [18].Jordan SD, Könner AC, Brüning JC. Sensing the fuels: glucose and lipid signaling in the CNS controlling energy homeostasis. Cell Mol Life Sci. 2010;67(19):3255–3273. doi: 10.1007/s00018-010-0414-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Corbett SW, Keesey RE. Energy balance of rats with lateral hypothalamic lesions. Am J Physiol. 1982;242(4):E273–279. doi: 10.1152/ajpendo.1982.242.4.E273. [DOI] [PubMed] [Google Scholar]
- [20].Zhang YP, Ma C, Wen YQ, et al. Convergence of gastric vagal and cerebellar fastigial nuclear inputs on glycemiasensitive neurons of lateral hypothalamic area in the rat. Neurosci Res. 2003;45(1):9–16. doi: 10.1016/s0168-0102(02)00192-x. [DOI] [PubMed] [Google Scholar]
- [21].Mountjoy PD, Bailey SJ, Rutter GA. Inhibition by glucose or leptin of hypothalamic neurons expressing neuropeptide Y requires changes in AMP-activated protein kinase activity. Diabetologia. 2007;50(1):168–177. doi: 10.1007/s00125-006-0473-3. [DOI] [PubMed] [Google Scholar]
- [22].Fioramonti X, Contié S, Song Z, et al. Characterization of glucosensing neuron subpopulations in the arcuate nucleus: integration in neuropeptide Y and pro-opio melanocortin networks? Diabetes. 2007;56(5):1219–1227. doi: 10.2337/db06-0567. [DOI] [PubMed] [Google Scholar]
- [23].Gozali M, Pavia JM, Morris MJ. Involvement of neuropeptide Y in glucose sensing in the dorsal hypothalamus of streptozotocin diabetic rats - in vitro and in vivo studies of transmitter release. Diabetologia. 2002;45(9):1332–1339. doi: 10.1007/s00125-002-0890-x. [DOI] [PubMed] [Google Scholar]
- [24].Murphy BA, Fioramonti X, Jochnowitz N, et al. Fasting enhances the response of arcuate neuropeptide Y-glucose-inhibited neurons to decreased extracellular glucose. Am J Physiol Cell Physiol. 2009;296(4):C746–756. doi: 10.1152/ajpcell.00641.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Levin BE, Routh VH, Kang L, et al. Neuronal glucosensing: what do we know after 50 years? Diabetes. 2004;53(10):2521–2528. doi: 10.2337/diabetes.53.10.2521. [DOI] [PubMed] [Google Scholar]
- [26].Morton GJ, Cummings DE, Baskin DG, et al. Central nervous system control of food intake and body weight. Nature. 2006;443(7109):289–295. doi: 10.1038/nature05026. [DOI] [PubMed] [Google Scholar]
- [27].Dockray GJ. Cholecystokinin and gut-brain signalling. Regul Pept. 2009;155(1-3):6–10. doi: 10.1016/j.regpep.2009.03.015. [DOI] [PubMed] [Google Scholar]
- [28].Emond M, Schwartz GJ, Ladenheim EE, et al. Central leptin modulates behavioral and neural responsivity to CCK. Am J Physiol. 1999;276(5 Pt 2):R1545–1549. doi: 10.1152/ajpregu.1999.276.5.R1545. [DOI] [PubMed] [Google Scholar]
- [29].Elmquist JK, Bjørbaek C, Ahima RS, et al. Distributions of leptin receptor mRNA isoforms in the rat brain. J Comp Neurol. 1998;395(4):535–547. [PubMed] [Google Scholar]
- [30].Grill HJ, Schwartz MW, Kaplan JM, et al. Evidence that the caudal brainstem is a target for the inhibitory effect of leptin on food intake. Endocrinology. 2002;143(1):239–246. doi: 10.1210/endo.143.1.8589. [DOI] [PubMed] [Google Scholar]
- [31].Gong M, Wang X, Mao Z, et al. Effect of electroacupuncture on leptin resistance in rats with diet-induced obesity. Am J Chin Med. 2012;40(3):511–520. doi: 10.1142/S0192415X12500395. [DOI] [PubMed] [Google Scholar]
- [32].Fonseca-Alaniz MH, Takada J, Alonso-Vale MI, et al. Adipose tissue as an endocrine organ: from theory to practice. J Pediatr (Rio J) 2007;83(5 Suppl):S192–203. doi: 10.2223/JPED.1709. [DOI] [PubMed] [Google Scholar]
- [33].Zeyda M, Stulnig TM. Obesity, inflammation, and insulin resistance--a mini-review. Gerontology. 2009;55(4):379–386. doi: 10.1159/000212758. [DOI] [PubMed] [Google Scholar]
- [34].Maury E, Brichard SM. Adipokine dysregulation, adipose tissue inflammation and metabolic syndrome. Mol Cell Endocrinol. 2010;314(1):1–16. doi: 10.1016/j.mce.2009.07.031. [DOI] [PubMed] [Google Scholar]
- [35].Antuna-Puente B, Feve B, Fellahi S, et al. Adipokines: the missing link between insulin resistance and obesity. Diabetes Metab. 2008;34(1):2–11. doi: 10.1016/j.diabet.2007.09.004. [DOI] [PubMed] [Google Scholar]
- [36].Barnes KM, Miner JL. Role of resistin in insulin sensitivity in rodents and humans. Curr Protein Pept Sci. 2009;10(1):96–107. doi: 10.2174/138920309787315239. [DOI] [PubMed] [Google Scholar]
- [37].Fan YJ, Xu B, Xiang XR, et al. Effects of electroacupuncture versus sibutramine on adipocyte products in obesity rats. Zhongguo Zuzhi Gongcheng Yanjiu yu Linchuang Kangfu. 2008;12(11):2189–2192. [Google Scholar]
- [38].Sun DY, Sun LH, Liang YL, et al. Effect of electroacupuncture on lipid metabolism in male and female obesity rats. Zhen Ci Yan Jiu. 2012;37(3):206–210. [PubMed] [Google Scholar]
- [39].China: People's Government of Jiangsu Province. Regulations for the Administration of Affairs Concerning Experimental Animals in Jiangsu Province. 2008-09-02. [Google Scholar]
- [40].Sun Z, Zhang ZC, Liu ZC. Experimental study of diet-induced obesity animal model. Zhongyao Tongbao. 2002;18(4):466–467. [Google Scholar]
- [41].Paxinos G, Watson C. 5th ed. London: Academic Press; 2005. The Rat Brain in Stereotaxic Coordinates. [Google Scholar]
- [42].Min BI, Oomura Y, Katafuchi T. Responses of rat lateral hypothalamic neuronal activity to fastigial nucleus stimulation. J Neurophysiol. 1989;61(6):1178–1184. doi: 10.1152/jn.1989.61.6.1178. [DOI] [PubMed] [Google Scholar]
- [43].Pu YM, Wang JJ, Wang T, et al. Cerebellar interpositus nucleus modulates neuronal activity of lateral hypothalamic area. Neuroreport. 1995;6(7):985–988. doi: 10.1097/00001756-199505090-00009. [DOI] [PubMed] [Google Scholar]


