Abstract
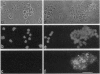
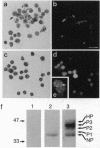
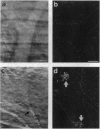
Levels and subcellular distribution of connexin 43 (Cx43), a gap junction protein, were studied in hamster leukocytes before and after activation with endotoxin (lipopolysaccharide, LPS) both in vitro and in vivo. Untreated leukocytes did not express Cx43. However, Cx43 was clearly detectable by indirect immunofluorescence in cells treated in vitro with LPS (1 micrograms/ml, 3 hr). Cx43 was also detected in leukocytes obtained from the peritoneal cavity 5-7 days after LPS-induced inflammation. In some leukocytes that formed clusters Cx43 immunoreactivity was present at appositional membranes, suggesting formation of homotypic gap junctions. In cell homogenates of activated peritoneal macrophages, Cx43, detected by Western blot analysis, was mostly unphosphorylated. A second in vivo inflammatory condition studied was that induced by ischemia-reperfusion of the hamster cheek pouch. In this system, leukocytes that adhered to venular endothelial cells after 1 hr of ischemia, followed by 1 hr of reperfusion, expressed Cx43. Electron microscope observations revealed small close appositions, putative gap junctions, at leukocyte-endothelial cell and leukocyte-leukocyte contacts. These results indicate that the expression of Cx43 can be induced in leukocytes during an inflammatory response which might allow for heterotypic or homotypic intercellular gap junctional communication. Gap junctions may play a role in leukocyte extravasation.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albelda S. M., Buck C. A. Integrins and other cell adhesion molecules. FASEB J. 1990 Aug;4(11):2868–2880. [PubMed] [Google Scholar]
- Aronson F. R., Libby P., Brandon E. P., Janicka M. W., Mier J. W. IL-2 rapidly induces natural killer cell adhesion to human endothelial cells. A potential mechanism for endothelial injury. J Immunol. 1988 Jul 1;141(1):158–163. [PubMed] [Google Scholar]
- Bennett M. V., Barrio L. C., Bargiello T. A., Spray D. C., Hertzberg E., Sáez J. C. Gap junctions: new tools, new answers, new questions. Neuron. 1991 Mar;6(3):305–320. doi: 10.1016/0896-6273(91)90241-q. [DOI] [PubMed] [Google Scholar]
- Bennett M. V., Verselis V. K. Biophysics of gap junctions. Semin Cell Biol. 1992 Feb;3(1):29–47. doi: 10.1016/s1043-4682(10)80006-6. [DOI] [PubMed] [Google Scholar]
- Berthoud V. M., Rook M. B., Traub O., Hertzberg E. L., Sáez J. C. On the mechanisms of cell uncoupling induced by a tumor promoter phorbol ester in clone 9 cells, a rat liver epithelial cell line. Eur J Cell Biol. 1993 Dec;62(2):384–396. [PubMed] [Google Scholar]
- Bevilacqua M. P., Pober J. S., Mendrick D. L., Cotran R. S., Gimbrone M. A., Jr Identification of an inducible endothelial-leukocyte adhesion molecule. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9238–9242. doi: 10.1073/pnas.84.24.9238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyer E. C., Paul D. L., Goodenough D. A. Connexin family of gap junction proteins. J Membr Biol. 1990 Jul;116(3):187–194. doi: 10.1007/BF01868459. [DOI] [PubMed] [Google Scholar]
- Beyer E. C., Steinberg T. H. Evidence that the gap junction protein connexin-43 is the ATP-induced pore of mouse macrophages. J Biol Chem. 1991 May 5;266(13):7971–7974. [PubMed] [Google Scholar]
- Borić M. P., Donoso V., Fournier A., St Pierre S., Huidobro-Toro J. P. Endothelin reduces microvascular blood flow by acting on arterioles and venules of the hamster cheek pouch. Eur J Pharmacol. 1990 Nov 6;190(1-2):123–133. doi: 10.1016/0014-2999(90)94119-i. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bruzzone R., Haefliger J. A., Gimlich R. L., Paul D. L. Connexin40, a component of gap junctions in vascular endothelium, is restricted in its ability to interact with other connexins. Mol Biol Cell. 1993 Jan;4(1):7–20. doi: 10.1091/mbc.4.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butcher E. C. Leukocyte-endothelial cell recognition: three (or more) steps to specificity and diversity. Cell. 1991 Dec 20;67(6):1033–1036. doi: 10.1016/0092-8674(91)90279-8. [DOI] [PubMed] [Google Scholar]
- Carlos T. M., Harlan J. M. Membrane proteins involved in phagocyte adherence to endothelium. Immunol Rev. 1990 Apr;114:5–28. doi: 10.1111/j.1600-065x.1990.tb00559.x. [DOI] [PubMed] [Google Scholar]
- De Bruyn P. P., Cho Y., Michelson S. Endothelial attachment and plasmalemmal apposition in the transcellular movement of intravascular leukemic cells entering the myeloid parenchyma. Am J Anat. 1989 Oct;186(2):115–126. doi: 10.1002/aja.1001860202. [DOI] [PubMed] [Google Scholar]
- Dermietzel R., Spray D. C. Gap junctions in the brain: where, what type, how many and why? Trends Neurosci. 1993 May;16(5):186–192. doi: 10.1016/0166-2236(93)90151-b. [DOI] [PubMed] [Google Scholar]
- Geng J. G., Bevilacqua M. P., Moore K. L., McIntyre T. M., Prescott S. M., Kim J. M., Bliss G. A., Zimmerman G. A., McEver R. P. Rapid neutrophil adhesion to activated endothelium mediated by GMP-140. Nature. 1990 Feb 22;343(6260):757–760. doi: 10.1038/343757a0. [DOI] [PubMed] [Google Scholar]
- Grabstein K. H., Urdal D. L., Tushinski R. J., Mochizuki D. Y., Price V. L., Cantrell M. A., Gillis S., Conlon P. J. Induction of macrophage tumoricidal activity by granulocyte-macrophage colony-stimulating factor. Science. 1986 Apr 25;232(4749):506–508. doi: 10.1126/science.3083507. [DOI] [PubMed] [Google Scholar]
- Granger D. N., Benoit J. N., Suzuki M., Grisham M. B. Leukocyte adherence to venular endothelium during ischemia-reperfusion. Am J Physiol. 1989 Nov;257(5 Pt 1):G683–G688. doi: 10.1152/ajpgi.1989.257.5.G683. [DOI] [PubMed] [Google Scholar]
- Guinan E. C., Smith B. R., Davies P. F., Pober J. S. Cytoplasmic transfer between endothelium and lymphocytes: quantitation by flow cytometry. Am J Pathol. 1988 Sep;132(3):406–409. [PMC free article] [PubMed] [Google Scholar]
- Hülser D. F., Peters J. H. Contact cooperation in stimulated lymphocytes. II. Electrophysiological investigations on intercellular communication. Exp Cell Res. 1972 Oct;74(2):319–326. doi: 10.1016/0014-4827(72)90383-7. [DOI] [PubMed] [Google Scholar]
- Hülser D. F., Peters J. H. Intercellular communication in phytohemagglutinin-induced lymphocyte agglutinates. Eur J Immunol. 1971 Dec;1(6):494–495. doi: 10.1002/eji.1830010618. [DOI] [PubMed] [Google Scholar]
- Issekutz T. B., Issekutz A. C., Movat H. Z. The in vivo quantitation and kinetics of monocyte migration into acute inflammatory tissue. Am J Pathol. 1981 Apr;103(1):47–55. [PMC free article] [PubMed] [Google Scholar]
- Jongen W. M., Fitzgerald D. J., Asamoto M., Piccoli C., Slaga T. J., Gros D., Takeichi M., Yamasaki H. Regulation of connexin 43-mediated gap junctional intercellular communication by Ca2+ in mouse epidermal cells is controlled by E-cadherin. J Cell Biol. 1991 Aug;114(3):545–555. doi: 10.1083/jcb.114.3.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keane R. W., Mehta P. P., Rose B., Honig L. S., Loewenstein W. R., Rutishauser U. Neural differentiation, NCAM-mediated adhesion, and gap junctional communication in neuroectoderm. A study in vitro. J Cell Biol. 1988 Apr;106(4):1307–1319. doi: 10.1083/jcb.106.4.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Larson D. M., Haudenschild C. C., Beyer E. C. Gap junction messenger RNA expression by vascular wall cells. Circ Res. 1990 Apr;66(4):1074–1080. doi: 10.1161/01.res.66.4.1074. [DOI] [PubMed] [Google Scholar]
- Lasky L. A. A 'roll' in acute inflammation. Curr Biol. 1993 Oct 1;3(10):680–682. doi: 10.1016/0960-9822(93)90067-x. [DOI] [PubMed] [Google Scholar]
- Lefer A. M., Lefer D. J. Pharmacology of the endothelium in ischemia-reperfusion and circulatory shock. Annu Rev Pharmacol Toxicol. 1993;33:71–90. doi: 10.1146/annurev.pa.33.040193.000443. [DOI] [PubMed] [Google Scholar]
- Levy J. A., Weiss R. M., Dirksen E. R., Rosen M. R. Possible communication between murine macrophages oriented in linear chains in tissue culture. Exp Cell Res. 1976 Dec;103(2):375–385. doi: 10.1016/0014-4827(76)90273-1. [DOI] [PubMed] [Google Scholar]
- Mayadas T. N., Johnson R. C., Rayburn H., Hynes R. O., Wagner D. D. Leukocyte rolling and extravasation are severely compromised in P selectin-deficient mice. Cell. 1993 Aug 13;74(3):541–554. doi: 10.1016/0092-8674(93)80055-j. [DOI] [PubMed] [Google Scholar]
- Mege R. M., Matsuzaki F., Gallin W. J., Goldberg J. I., Cunningham B. A., Edelman G. M. Construction of epithelioid sheets by transfection of mouse sarcoma cells with cDNAs for chicken cell adhesion molecules. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7274–7278. doi: 10.1073/pnas.85.19.7274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musil L. S., Cunningham B. A., Edelman G. M., Goodenough D. A. Differential phosphorylation of the gap junction protein connexin43 in junctional communication-competent and -deficient cell lines. J Cell Biol. 1990 Nov;111(5 Pt 1):2077–2088. doi: 10.1083/jcb.111.5.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navab M., Liao F., Hough G. P., Ross L. A., Van Lenten B. J., Rajavashisth T. B., Lusis A. J., Laks H., Drinkwater D. C., Fogelman A. M. Interaction of monocytes with cocultures of human aortic wall cells involves interleukins 1 and 6 with marked increases in connexin43 message. J Clin Invest. 1991 May;87(5):1763–1772. doi: 10.1172/JCI115195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polacek D., Lal R., Volin M. V., Davies P. F. Gap junctional communication between vascular cells. Induction of connexin43 messenger RNA in macrophage foam cells of atherosclerotic lesions. Am J Pathol. 1993 Feb;142(2):593–606. [PMC free article] [PubMed] [Google Scholar]
- Reed K. E., Westphale E. M., Larson D. M., Wang H. Z., Veenstra R. D., Beyer E. C. Molecular cloning and functional expression of human connexin37, an endothelial cell gap junction protein. J Clin Invest. 1993 Mar;91(3):997–1004. doi: 10.1172/JCI116321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risley M. S., Tan I. P., Roy C., Sáez J. C. Cell-, age- and stage-dependent distribution of connexin43 gap junctions in testes. J Cell Sci. 1992 Sep;103(Pt 1):81–96. doi: 10.1242/jcs.103.1.81. [DOI] [PubMed] [Google Scholar]
- Willecke K., Hennemann H., Dahl E., Jungbluth S., Heynkes R. The diversity of connexin genes encoding gap junctional proteins. Eur J Cell Biol. 1991 Oct;56(1):1–7. [PubMed] [Google Scholar]
- Yamamoto T., Ochalski A., Hertzberg E. L., Nagy J. I. LM and EM immunolocalization of the gap junctional protein connexin 43 in rat brain. Brain Res. 1990 Feb 5;508(2):313–319. doi: 10.1016/0006-8993(90)90415-8. [DOI] [PubMed] [Google Scholar]
- Yasuhara H., Hobson R. W., 2nd, Dillon P. K., Durán W. N. A new model for studying ischemia-reperfusion injury in hamster cheek pouch. Am J Physiol. 1991 Nov;261(5 Pt 2):H1626–H1629. doi: 10.1152/ajpheart.1991.261.5.H1626. [DOI] [PubMed] [Google Scholar]
- Zimmerman G. A., Hill H. R. Inflammatory mediators stimulate granulocyte adherence to cultured human endothelial cells. Thromb Res. 1984 Jul 15;35(2):203–217. doi: 10.1016/0049-3848(84)90215-9. [DOI] [PubMed] [Google Scholar]
- el-Sabban M. E., Pauli B. U. Cytoplasmic dye transfer between metastatic tumor cells and vascular endothelium. J Cell Biol. 1991 Dec;115(5):1375–1382. doi: 10.1083/jcb.115.5.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]