Abstract
Hypoxia-inducible factor 1 (HIF-1) attenuates amyloid-beta protein neurotoxicity and decreases apoptosis induced by oxidative stress or hypoxia in cortical neurons. In this study, we constructed a recombinant adeno-associated virus (rAAV) vector expressing the human HIF-1α gene (rAAV-HIF-1α), and tested the assumption that rAAV-HIF-1α represses hippocampal neuronal apoptosis induced by amyloid-beta protein. Our results confirmed that rAAV-HIF-1α significantly reduces apoptosis induced by amyloid-beta protein in primary cultured hippocampal neurons. Direct intracerebral rAAV-HIF-1α administration also induced robust and prolonged HIF-1α production in rat hippocampus. Single rAAV-HIF-1α administration resulted in decreased apoptosis of hippocampal neurons in an Alzheimer's disease rat model established by intracerebroventricular injection of aggregated amyloid-beta protein (25–35). Our in vitro and in vivo findings demonstrate that HIF-1 has potential for attenuating hippocampal neuronal apoptosis induced by amyloid-beta protein, and provides experimental support for treatment of neurodegenerative diseases using gene therapy.
Keywords: nerve regeneration, Alzheimer's disease, adeno-associated virus, hypoxia-inducible factor 1α, apoptosis, gene therapy, calcium concentration, transduction, intracerebroventricular injection, NSFC grant, neural regeneration
Introduction
Alzheimer's disease is characterized by progressive loss of cognitive function, resulting in disturbances of language and akinesia. The characteristic neuropathology of Alzheimer's disease includes senile plaques composed of aggregated amyloid-beta peptide, neurofibrillary tangles, severe brain atrophy, neuronal loss, inflammation, and neurotoxic responses (Palop and Mucke, 2009; Marchesi, 2011). With the global increase in human life expectancy, Alzheimer's disease will be an ever-increasing burden to society. Despite an intensive search for therapeutic intervention, no drug has yet proven effective in combating this devastating neurodegenerative disease. Recently, much attention has been focused on gene therapies for Alzheimer's disease treatment, and several such approaches have been examined (Braddock, 2005; Tuszynski, 2007).
In terms of a gene therapy target for Alzheimer's disease, the transcriptional regulator, hypoxia-inducible factor 1 (HIF-1), has potential neuroprotective effects and is a rational choice, as brain HIF-1 levels are reduced in Alzheimer's disease patients compared with age-matched controls (Liu et al., 2008). HIF-1 prevents neuronal apoptosis induced by hypoxia (Soucek et al., 2003; Greijer and Van der Wall, 2004; Maxwell, 2005; Piret et al., 2005). Furthermore, neuroprotective activity of HIF-1 against amyloid-beta peptide toxicity has been observed (Avramovich-Tirosh et al., 2010). A critical step in successful gene therapy for Alzheimer's disease is selection of an appropriate viral vector. Recombinant adeno-associated virus (rAAV), reconstructed from a non-pathogenic wild-type adeno-associated virus (AAV), is presently one of the most widely applicable carrier systems with the desired features of a good safety profile, wide host range, and weak immunogenicity (Zhang et al., 2011). Thus, in this study we constructed a rAAV vector containing the human HIF-1α gene (rAAV-HIF-1α), and determined its effect on apoptosis in amyloid-beta-induced hippocampal neurons in vitro. We also investigated the effect of rAAV-HIF-1α on hippocampal neuronal apoptosis in vivo, using a rat model of Alzheimer's disease, obtained by intracerebroventricular injection of amyloid-beta protein (25–35).
Materials and Methods
Hippocampal neuronal culture
One-day-old Sprague-Dawley rats were purchased from Hebei Medical University, China. The Animal Care and Use Committee of the Hebei Science and Technical Bureau in the People's Republic of China approved the experimental protocol. Methods for culturing hippocampal neurons have been described previously (Banker and Cowan, 1977; Dotti et al., 1988). Briefly, brains of 1-day-old rats were dissected, the meninges removed and hippocampus isolated (Wray et al., 1991). Hippocampi were transferred to tissue culture tubes, with a volume of 1–2 mL of Ca-Mg-free Hanks’ balanced salt solution in each tube. Hippocampi were mechanically dissociated and treated with 0.125% trypsin for 20 minutes at 37°C. Subsequently, hippocampal cells were centrifuged at 1,000 × g for 5 minutes, resuspended in Dulbecco's modified Eagle's medium (DMEM) (Invitrogen, Carlsbad, CA, USA) containing 10% fetal bovine serum and 5% horse serum, and counted using a hemocytometer (EMD Millipore Corporation, Billerica, MA, USA). Approximately 5 × 105 hippocampal cells per well were seeded into 6-well plates coated with poly-L-lysine (0.05 mg/mL), and cultured in DMEM enriched with B-27 (Invitrogen). Hippocampal cells were maintained at 37°C in a 5% CO2/95% O2 air atmosphere. The medium was replaced after 24 hours, when the cells had attached to the substrate, and then changed every 3–4 days thereafter. Two days after plating, cultures were exposed to 10 μmol/L cytosine arabinoside (Sigma-Aldrich, St. Louis, MO, USA) to inhibit non-neuronal cell growth. Experimental studies were performed on hippocampal cells maintained in vitro for 7 to 8 days, to ensure adequate neuronal development.
Intracerebroventricular injection of amyloid-beta peptide (25–35)
Male, specific pathogen-free, Sprague-Dawley rats weighing 250 g and aged 4 months were purchased from Hebei Medical University, China (Animals license No. 1105007), and housed in stainless steel rust-free cages at 22–24°C with 45–55% relative humidity. All animals were allowed free access to food and distilled water. The experimental protocol was approved by the Animal Care and Use Committee of Hebei Science and Technical Bureau.
The procedure for amyloid-beta peptide infusion has been described previously (Yamaguchi et al., 2006; Stepanov et al., 2007; Zhang et al., 2013). Briefly, amyloid-beta peptide (25–35) (Sigma-Aldrich) was dissolved in sterile saline at a concentration of 10 mg/mL and stored at –20°C. To obtain the neurotoxic form of amyloid-beta peptide (25–35), the peptide solution was incubated at 37°C for 4 days. Rats were anesthetized with an intraperitoneal injection of pentobarbital (50 mg/kg), and 2 μL aggregated amyloid-beta peptide (25–35) (10 mg/mL) gradually delivered into the right lateral ventricle (1.5 mm lateral to the midline, 0.8 mm posterior to bregma) over a 5 minute period, using a 10 μL microsyringe (Hamilton, Reno, NV, USA) inserted to a depth of 4.0 mm.
HIF-1α expression vector construction
The method used has been described previously (Wu et al., 2002; Zhang et al., 2003). In brief, the HIF-1α gene, obtained from pBSKhHIF1αT7 (a generous gift from Max Gassmann from University of Zurich, Zurich, Switzerland), was digested using the restriction endonuclease enzymes, KpnI and BamHI, and inserted into KpnI and BglII-digested pSNAV2.0 (Vector Gene Technology Company, Beijing, China) to obtain an AAV vector shuttle plasmid containing the human HIF-1α gene (pSNAV-HIF-1α) (Figure 1A). Next, pSNAV-HIF-1α was transfected into BHK-21 cells (Vector Gene Technology Company, Beijing, China) using Lipofectamine 2000 (Invitrogen), and G418-selected to obtain a BHK/pSNAV-HIF-1α cell line. BHK/pSNAV-HIF-1α cells were then infected with HSV1-rc/ΔUL2 (Vector Gene Technology Company, Beijing, China), which expresses the rep and cap genes of wild-type AAV. After chloroform treatment, PEG/NaCl precipitation, chloroform extraction, and bag filter concentration, confirmation of the human HIF-1α gene in rAAV-HIF-1α was determined by PCR using appropriate forward (5′-TAA GAA ACC TAT GAC CT-3′) and reverse (5′-TGA GTT TCA ACC CAG ACA TA-3′) primers. Amplification was performed with an initial denaturation at 94°C for 5 minutes, followed by 30 cycles of 94°C for 45 seconds, 55°C for 45 seconds, and 72°C for 45 seconds, with a single final extension at 72°C for 10 minutes. PCR products were analyzed on 1% agarose gels using Gel-Pro Analyzer® Analysis software (Media Cybernetics Inc., Bethesda, MD, USA). The expected size of the HIF-1α PCR product was 324 bp. The rAAV-HIF-1α titer was obtained by dot-blot using a digoxin labeled cytomegalovirus probe (Musiani et al., 1991).
Figure 1.
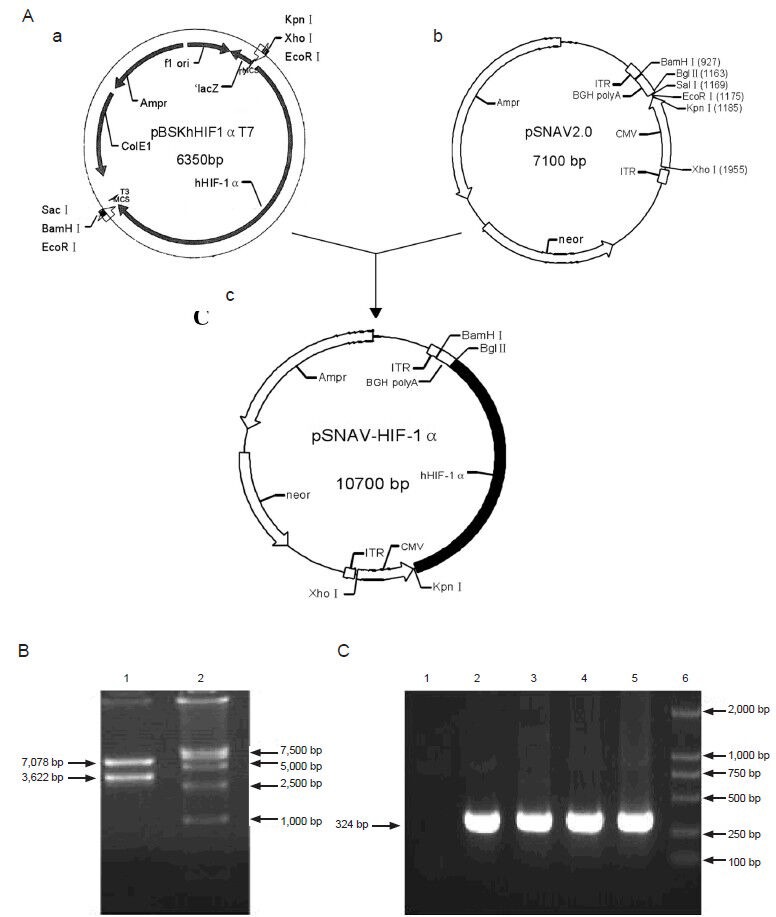
Construction and confirmation of rAAV-HIF-1α.
(A) Schematic outline of pSNAV-HIF-1α construction. (a) pBSKhHIF1αT7; (b) pSNAV2.0; and (c) pSNAV-HIF-1α. (B) Identification of pSNAV-HIF-1α by double restriction enzyme digestion and electrophoretic analysis. 1: pSNAV-HIF-1α digested with BglII/KpnI; 2: DNA marker. (C) PCR identification of rAAV-HIF-1α. 1: Negative control; 2: pBSKhHIF1αT7; 3: HIF-1 α cDNA; 4: pSNAV-HIF-1α; 5: rAAV-HIF-1α; 6: DNA marker. ITR: Inverted terminal repeat of adeno-associated virus; CMV: enhancer/promoter of cytomegalovirus immediate early genes; hHIF-1α: human hypoxia inducible factor-1α gene; BGH poly A: bovine growth hormone polyadenylation signals; Ampr: ampicillin resistant gene; neor: neomycin resistant gene; HIF-1: hypoxia-inducible factor 1; rAAV: recombinant adeno-associated virus.
HIF-1α protein expression detected by western blot assay in rAAV-HIF-1α transduced primary cultured hippocampal neurons
To detect HIF-1α protein expression in rAAV-HIF-1α transduced primary cultured hippocampal neurons, three groups were established: normal group, untreated primary hippocampal neurons; vector group, rAAV2/lacZ (Vector Gene Technology Company, Beijing, China) transduced primary hippocampal neurons (1 × 105 vector genomes (v.g.)/cell); and rAAV-HIF-1α group, rAAV-HIF-1α transduced primary hippocampal neurons (1 × 105 v.g./cell). To detect HIF-1α expression by western blot assay, total protein was extracted from hippocampal neurons at 3 days post-transfection.
The western blot procedure has been described previously (Kong et al., 2008). Hippocampal neurons were harvested and homogenized by supersonication in 1 mL Tris-buffered saline (TBS) (pH 8.0) buffer. Total cell extract proteins (30 μg) were separated by 10% sodium dodecyl sulphate-polyacrylamide gel electrophoresis under reducing conditions and transferred by electroblotting onto nitrocellulose membranes (Trans-Blot® Transfer Medium Pure Nitrocellulose Membrane, pore size 0.45 μm; Bio-Rad, Hercules, CA, USA) for 45 minutes at 4°C. After incubation in 5% blocking reagent (Amersham Biosciences, Piscataway, NJ, USA) in a TBS solution with Tween-20 (TBS-T) for 2 hours at room temperature, blots were separately incubated with mouse anti-rat or human HIF-1α antiserum (1:5,000; Sigma-Aldrich) overnight at 4°C. After washing with TBS-T, blots were incubated in horseradish peroxide-conjugated rabbit anti-mouse secondary antibody (1:5,000; Amersham Biosciences) for 1.5 hours at room temperature. Immunoreactive proteins were detected by enhanced chemiluminescence (Amersham Biosciences) using the Chemiluminescence Imaging System (Omega 12iC; UltraLum, Inc., Claremont, CA, USA). To determine HIF-1α protein enrichment in hippocampal neurons, immunoreactive proteins were quantified by absorbance using volume integration with the Gel-Pro Analyzer® Analysis software (Media Cybernetics). To ensure equal sample loading, membranes were also probed with rabbit anti-human β-actin polyclonal antibody (1:5,000; Sigma-Aldrich), and HIF-1α protein concentration normalized to β-actin expression.
HIF-1α protein expression detected by western blot assay in rAAV-HIF-1α transduced rat hippocampal tissue
Male rats were divided randomly into three groups: normal group, untreated healthy animals; vector group, rats received right intracerebroventricular injections of rAAV2/lacZ (1 × 1012 v.g./mL); and rAAV-HIF-1α group, rats received right intracerebroventricular injections of 10 μL rAAV-HIF-1α (1 × 1012 v.g./mL). To detect HIF-1α expression in hippocampal tissues by western blot assay, rats were sacrificed 4 weeks after intracerebroventricular injections.
Effect of rAAV-HIF-1α and amyloid-beta peptide (25–35) treatment on hippocampal neurons in vivo and in vitro
To determine the effect of rAAV-HIF-1α transduction on apoptosis in primary cultured hippocampal neurons exposed to amyloid-beta peptide (25–35), three groups were established (n = 6 per group): normal group, untreated primary hippocampal neurons; amyloid-beta peptide group, primary hippocampal neurons treated with 10 μmol/L amyloid-beta peptide (25–35) for 24 hours; rAAV-HIF-1α group, primary hippocampal neurons transduced with rAAV-HIF-1α for 3 days and treated with 10 μmol/L amyloid-beta peptide (25–35) for 24 hours.
To determine the effect of intracerebroventricular rAAV-HIF-1α injection on hippocampal neuronal apoptosis in an Alzheimer's disease animal model, male Sprague-Dawley rats were divided randomly into three groups (n = 8 per group): normal group, untreated healthy male animals; Alzheimer's disease group, rats received right intracerebroventricular injections of 2 μL amyloid-beta peptide (25–35) (10 mg/mL); Alzheimer's disease + rAAV-HIF-1α group, rats received right intracerebroventricular injections of 2 μL amyloid-beta peptide (25–35) (10 mg/mL), and then 1 week later, right intracerebroventricular injections of 10 μL rAAV-HIF-1α (1 × 1012 v.g./mL). To detect apoptosis in hippocampal neurons, rats were sacrificed 5 weeks after intracerebroventricular amyloid-beta peptide (25–35) injections.
Analysis of apoptosis in hippocampal neurons by flow cytometry
Flow cytometry analysis of apoptotic cells has been described previously (Zhou and Zhu, 2000). In brief, hippocampal neurons (1 × 106 cells) were collected by centrifugation, washed with PBS, and fixed in 70% ethanol. Fixed cells were centrifuged at 200 × g for 10 minutes, resuspended in PBS containing 50 mg/mL RNaseA, followed by incubation at 37°C for 1 hour. Next, cells were stained with 100 mg/mL propidium iodide (Sigma) at 4°C for 30 minutes before analysis on a FACScan instrument (Becton Dickinson FACStar Plus flow cytometer, San Jose, CA, USA).
Analysis of apoptosis in hippocampal neurons by transmission electron microscopy
In accordance with previously described methods (Beneduci et al., 2005), hippocampal neuronal suspensions were centrifuged and pellets fixed in 3% glutaraldehyde solution (pH 7.3) for approximately 2 hours at 4°C. Cell suspensions were rinsed twice with PBS, post-fixed in 1% osmium tetraoxide solution (pH 7.3), and incubated for 2 hours at 4°C. Cells were dehydrated in a graded alcohol series for 5 minutes each incubation. Dehydrated pellets were embedded three times with propylene oxide for 1 hour each time, infiltrated with a resin/propylene oxide mixture (1:1 ratio) for 2 hours, and then resin only for 12 hours at ambient temperature. The inclusion was made with Epon 812 and Araldite (Electron Microscopy Sciences, Hatfield, PA, USA), and polymerization performed at 60°C for 48 hours. Sections were cut parallel to the culture substrate at a thickness of 70 nm, and collected on 300-mesh copper grids. Ultrathin sections were stained with uranyl acetate and lead citrate counterstained, before examination with an EM900 Zeiss transmission electron microscope (Zeiss, Oberkochen, Germany).
Determination of intracellular calcium concentration by confocal laser scanning microscopy
Hippocampal neuronal intracellular calcium concentration ([Ca2+]i) was determined by confocal laser scanning microscopy, as described previously (Zhao et al., 2013). [Ca2+]i was measured using the intracellular calcium indicator, Fluo-3/AM (Invitrogen). Primary cultured hippocampal neurons were loaded in the dark with 5 μmol/L Fluo-3/AM for 30 minutes at 37°C. Cells were then washed with physiological 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid buffer, and viewed using a confocal microscope (400 ×; Olympus, Tokyo, Japan). Fluo-3/AM fluorescence was excited at 488 nm and emitted at 530 nm. [Ca2+]i was represented by fluorescence intensity.
Statistical analysis
Data are expressed as mean ± SD. Statistical analysis was performed using the SPSS statistics package (version 15.0, SPSS, Chicago, IL, USA). Differences between mean values of two groups were assessed using independent samples t-tests. One-way analysis of variance was used where appropriate. Probability values of P < 0.05 were taken to be statistically significant.
Results
Identification of recombinant pSNAV-HIF-1α plasmid
Recombinant pSNAV-HIF-1α plasmid was double digested with BglII and KpnI, and digestion confirmed by 0.5% agarose gel electrophoresis. Fully digested pSNAV-HIF-1α plasmid produces two bands of 7,078 bp and 3,622 bp (Figure 1B). Electrophoretic analysis confirmed correct insertion of the HIF-1α gene into the pSNAV plasmid.
Confirmation of rAAV-HIF-1α
PCR analysis identified a distinct band of 324 bp in pBSKhHIF1αT7, HIF-1 α cDNA, pSNAV-HIF-1α, and rAAV-HIF-1α, confirming that rAAV-HIF-1α contained the human HIF-1α gene (Figure 1C).
HIF-1α expression in rAAV-HIF-1α-transduced primary cultured hippocampal neurons
Western blot assays showed HIF-1α expression in rAAV-HIF-1α-transduced primary cultured hippocampal neurons was significantly higher than in normal and vector groups (P < 0.05). There was no significant difference between normal and vector groups (P > 0.05; Figure 2A).
Figure 2.
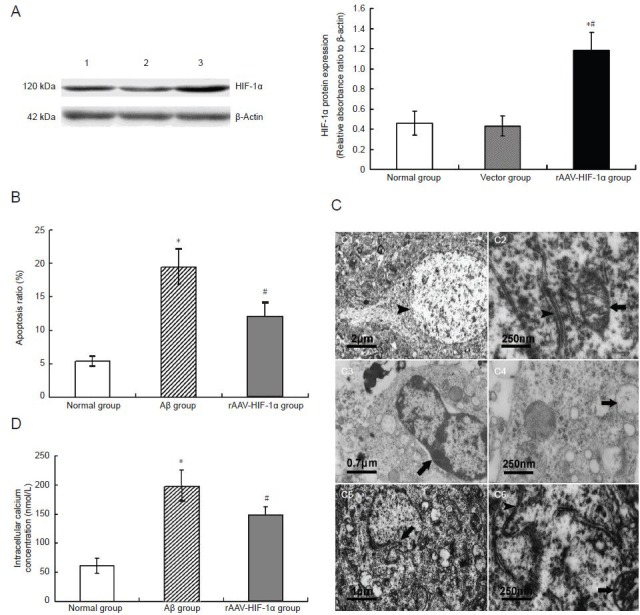
rAAV-HIF-1α expresses HIF-1α protein efficiently in primary cultured hippocampal neurons and inhibits Aβ25-35-induced apoptosis of hippocampal neurons.
(A) HIF-1α expression in rAAV-HIF-1α-transduced primary hippocampal neurons. HIF-1α expression was detected by western blot assay. 1: Normal group; 2: vector group; 3: rAAV-HIF-1α group. *P < 0.05, vs. normal group. #P < 0.05, vs. vector group.
(B) Effect of rAAV-HIF-1α transduction on apoptosis induced by Aβ25-35 in primary hippocampal neurons. Proportions of apoptotic hippocampal neurons were detected by flow cytometry. *P < 0.05, vs. normal group; #P < 0.05, vs. Aβ group.
(C) Effect of rAAV-HIF-1α transduction on ultrastructural changes determined by transmission electron microscopy in primary hippocampal neurons induced by Aβ25-35. (C1, 2) Normal group, hippocampal neuronal chromatin (arrow) is well-distributed (C1), endoplasmic reticulum smooth and continuous (arrowhead), and mitochondrial (MT) cristae (arrow) clearly visible (C2). (C3, 4) Aβ group, hippocampal neurons show striking apoptotic changes involving chromatin condensation (arrow) into compact patches with peripheral migration (C3), disintegration of the contents of cytoplasmic organelles (including endoplasmic reticulum and mitochondria) into non-visible membranous debris, non-descript particulate matter, or vacuoles (arrow) (C4). (C5, 6) rAAV-HIF-1α group, hippocampal neurons show only slight morphological changes associated with apoptosis e.g. nuclear envelope blebbing (arrow) (C5), while the cytoplasmic organelles, e.g. endoplasmic reticulum (arrowhead) and mitochondria (arrow), are relatively normal (C6). Bars: (C1) 2 μm; (C2, 4, 6) 250 nm; (C3) 0.7 μm; and (C5) 1 μm.
(D) Effect of rAAV-HIF-1α transduction on [Ca2+]i in primary hippocampal neurons induced by Aβ25-35. [Ca2+]i in hippocampal neurons was determined by laser scanning confocal microscopy using Fluo-3/AM as the fluorescent dye. *P < 0.05, vs. normal group; #P < 0.05, vs. Aβ group.
(A, B, D) Results are expressed as mean ± SD (n= 6). Differences between mean values of two groups were assessed by one-way analysis of variance and independent samples t-tests.
rAAV: Recombinant adeno-associated virus; HIF-1α: hypoxia-inducible factor 1α; Aβ: amyloid-beta protein.
Effect of rAAV-HIF-1α transduction on amyloid-beta peptide (25–35)-induced apoptosis of primary cultured hippocampal neurons
Flow cytometry analysis showed that the rate of apoptosis in hippocampal neurons in the amyloid-beta peptide group was significantly higher than in the normal group (P < 0.05), and the proportion of apoptotic hippocampal neurons in the rAAV-HIF-1α group was significantly lower than in the amyloid-beta peptide group (P < 0.05; Figure 2B).
Transmission electron photomicrographs of the normal group showed well-distributed hippocampal neuronal chromatin (Figure 2C1), with smooth, continuous endoplasmic reticulum and clearly visible mitochondrial cristae (Figure 2C2). In the amyloid-beta peptide group, hippocampal neurons showed striking apoptotic changes, including chromatin condensation into compact patches and peripheral migration (Figure 2C3). Cytoplasmic organelles, such as endoplasmic reticulum and mitochondria, were undergoing transformation of their contents, with disintegrating membranous debris and non-descript particulate matter or vacuoles observed (Figure 2C4). However, in the rAAV-HIF-1α group, hippocampal neurons showed only slight morphological changes associated with apoptosis, for example, nuclear envelope blebbing (Figure 2C5), while cytoplasmic organelles appeared relatively normal (Figure 2C6).
In recent years, evidence has accumulated indicating that enhanced neuronal Ca2+ is a key stage in activating the intrinsic apoptopic mitochondrial pathway, leading to neuronal death (Fernaández-Morales et al., 2012). Laser scanning confocal microscopy showed that in the amyloid-beta peptide group, hippocampal neuronal [Ca2+]i was significantly higher than in the normal group (P < 0.05). Moreover, [Ca2+]i in the rAAV-HIF-1α group was significantly lower than in the amyloid-beta peptide group (P < 0.05; Figure 2D).
rAAV-mediated HIF-1α expression in rat hippocampus
Western blot assays showed HIF-1α expression in rat hippocampus of the rAAV-HIF-1α group was significantly higher than in normal and vector groups (P < 0.05). No significant difference was detected between the normal and vector groups (P > 0.05; Figure 3A). These observations demonstrate that rAAV-HIF-1α can induce efficient HIF-1α protein expression in rat hippocampus.
Figure 3.
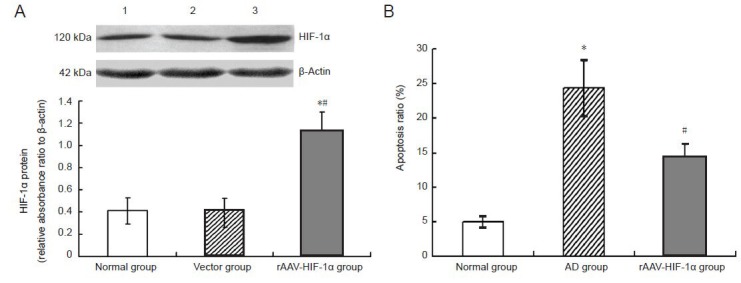
rAAV-HIF-1α expresses HIF-1α protein efficiently in rat hippocampus and inhibits Aβ25–35 induced apoptosis of hippocampal neurons in an Alzheimer's disease model.
(A) HIF-1α expression in rat hippocampus (western blot assay), 4 weeks after intracerebroventricular rAAV-HIF-1α injection. 1: Normal group; 2: vector group; 3: rAAV-HIF-1α group. HIF-1α protein concentration in each group was normalized to β-actin expression. *P < 0.05, vs. normal group. #P < 0.05, vs. vector group.
(B) Effect of intracerebroventricular rAAV-HIF-1α injection on hippocampal neuronal apoptosis in rats, 5 weeks after intracerebroventricular Aβ25–35 injection. The ratio of hippocampal neuronal apoptosis was determined by flow cytometry. *P < 0.05, vs. normal group. #P < 0.05, vs. AD group. Results are expressed as mean ± SD (n = 8 rats in each group). Differences between mean values of two groups were assessed by one-way analysis of variance and independent samples t-tests.
rAAV: recombinant adeno-associated virus; HIF-1α: hypoxia-inducible factor 1α. Aβ: amyloid-beta protein; AD: Alzheimer's disease.
Effect of intracerebroventricular rAAV-HIF-1α injection on amyloid-beta peptide (25–35)-induced apoptosis in rat hippocampal neurons in vivo
Apoptosis of hippocampal neurons was determined by flow cytometry. The ratio of apoptotic hippocampal neurons in the Alzheimer's disease group was significantly higher than in the normal group (P < 0.05). The proportion of apoptotic hippocampal neurons in the rAAV-HIF-1α group was significantly lower than in the Alzheimer's disease group (P < 0.05; Figure 3B).
Discussion
Gene therapy, developed since the 1980s, is a method to introduce human normal or therapeutic genes into human target cells to remedy defective genes or enhance a specific function. There have been many encouraging results in Alzheimer's disease gene therapy (Hong et al., 2006; Carty et al., 2008). For example, gene delivery of the neprilysin gene has contributed to decreased amyloid-beta peptide aggregation in an Alzheimer's disease transgenic mouse model (Hong et al., 2006). Similarly, AAV vector mediated gene delivery of endothelin-converting enzyme reduces amyloid-beta peptide deposits in transgenic mice with Alzheimer's disease (Carty et al., 2008). These gene therapy studies mainly focused on reduction of amyloid-beta peptide deposition, because abnormal processing and extracellular deposition of amyloid-beta peptide are believed to be central to Alzheimer's disease pathogenesis (Pike et al., 1992).
Although use of amyloid-beta peptide degrading enzyme genes for Alzheimer's therapy has proved effective, neuronal injury induced by amyloid-beta peptide has already formed in these mouse models. Many studies have shown that peptides containing the highly hydrophobic 25–35 amino acid region form stable aggregates and mediate neuronal death by necrosis or apoptosis (Pike et al., 1995). Animals studies also found that 1 week after amyloid-beta peptide (25–35) injection in rats, a reduction in neuronal number and cognitive dysfunction were observed (Stepanichev et al., 2003; Stepanichev et al., 2004; Stepanichev et al., 2006; Zussy et al., 2011). However, treatment strategies for neuronal protection and repair are still poor. HIF-1 is a novel neuroprotective factor best known for its ability to suppress neuronal cell death caused by hypoxia or oxidative stress, and to protect against amyloid-beta peptide toxicity, factors that support the potential of HIF-1 for Alzheimer's disease therapy. In this study, we determined the effect of HIF-1α gene delivery using rAAV on rat hippocampal neuronal apoptosis induced by amyloid-beta peptide (25–35), both in vitro and in vivo. Our results show that morphological changes associated with amyloid-beta peptide (25–35)-induced apoptosis in hippocampal neurons are diminished by rAAV-HIF-1α transduction. Flow cytometry showed a significantly lower ratio of apoptotic primary cultured hippocampal neurons in the rAAV-HIF-1α + amyloid-beta peptide group than in the normal + amyloid-beta peptide group. The effect of intracerebroventricular rAAV-HIF-1α injection on hippocampal neuronal apoptosis in a rat model of Alzheimer's disease was also determined. Similar to the changes observed in vitro, hippocampal neuronal apoptosis in the Alzheimer's disease + rAAV-HIF-1α group was significantly lower than in the Alzheimer's disease group. Altogether, our in vitro and in vivo results suggest that HIF-1α gene delivery using rAAV has potential for attenuating amyloid-beta peptide-induced cognitive deficits by reducing hippocampal neuronal apoptosis.
Successful Alzheimer's disease gene therapy undoubtedly requires use of an appropriate viral vector to effectively deliver therapeutic genes into neurons at sufficient expression levels. AAV, a dependovirus of the Parvoviridae family, is a non-pathogenic defective single stranded DNA virus. AAV persistently expresses exogenous gene(s) by integration into host cell chromosomes and/or as stable extrachromosomal episomes (Carty et al., 2008). We demonstrate that the rAAV-HIF-1α vector constructed in this study, efficiently expresses HIF-1α protein in primary cultured hippocampal neurons and rat hippocampal tissue. The exact mechanism by which HIF-1 improves amyloid-beta peptide-induced apoptosis of hippocampal neurons and cognitive impairment is not well understood, and several hypotheses have been proposed. First, amyloid-beta peptide interaction with the plasma membrane results in elevated cytoplasmic Ca2+ concentrations and increases neuronal vulnerability to excitotoxicity (Mattson et al., 1992). Therefore, rAAV-HIF-1α transduction may inhibit amyloid-beta peptide (25–35)-induced apoptosis of primary cultured hippocampal neurons by suppressing up-regulation of intracellular Ca2+ concentrations. Second, amyloid-beta peptide-dependent oxidative damage induces mitochondrial dysfunction, a central feature of neuronal apoptosis, and HIF-1α stabilization prevents neuronal death by reducing the mitochondrial membrane potential and cytosolic accumulation of cytochrome C, causing caspase-9 and caspase-3 inactivation. In addition, HIF-1α overexpression increases antiapoptotic Bcl-2 and Bcl-X(L) levels and decreases pro-apoptotic Bax and Bak levels (Sasabe et al., 2005). Third, amyloid-beta peptide-dependent astrocyte activation and reduced glucose metabolism in selective brain areas are additional pathological features of Alzheimer's disease. Maintenance of HIF-1α levels reverses amyloid-beta peptide-induced glial activation and glycolytic changes (Soucek et al., 2003; Schubert et al., 2009), suggesting HIF-1 may mediate a neuroprotective response to amyloid-beta peptide by decreasing inflammation and maintaining metabolic integrity. Moreover, the mammalian brain is extremely sensitive to hypoxia. Hypoxic challenges cause severe and detrimental alterations in brain function, and can trigger neuronal cell death within minutes, and potentially exacerbate Alzheimer's disease progression (Manukhina et al., 2010; Murray et al., 2011). HIF-1 is a major transcription factor and has been shown to increase capillary network density and improve blood circulation in living tissue by regulating expression of proteins such as erythropoietin and vascular endothelial growth factor (Nordal et al., 2004). Thus, HIF-1 participates widely in hypoxia-induced adaptive reactions to restore cellular homeostasis. Gene delivery of human HIF-1α may have potential utility in decreasing hypoxia-induced neuronal injury.
In summary, HIF-1α overexpression by rAAV-HIF-1α transduction dramatically reduces hippocampal neuronal apoptosis induced by amyloid-beta peptide (25–35) in vitro. Moreover, direct intracerebral administration of rAAV-HIF-1α induced prolonged, robust HIF-1α production in rat hippocampus, resulting in decreased neuronal apoptosis. These findings suggest that rAAV-HIF-1α has therapeutic potential as a protective agent against amyloid-beta peptide-induced cognitive dysfunction. However, further experiments are required before clinical application of HIF-1α gene therapy can be implemented. First, our findings should be confirmed in other animal models of Alzheimer's disease e.g. amyloid precursor protein transgenic mice. Second, extensive apoptotic cells in the cortex were also observed in amyloid-beta peptide (25–35)-injected animals. It has been demonstrated that the brain permeable iron chelator (M30) protects cultured cortical neurons against amyloid-beta peptide (25–35) toxicity by promoting HIF-1α expression levels and increasing transcription of HIF-1α-dependent genes (Avramovich-Tirosh et al., 2010). Further experiments are needed to investigate the protective effect of rAAV-HIF-1α transduction against amyloid-beta peptide (25–35)-induced neurotoxicity in cortical neurons and potential mechanisms. Finally, the safety profile of the rAAV-HIF-1α vector and a safe strategy for its delivery to target cells must be determined. This could be achieved using a bispecific F(ab’)2 antibody that mediates a novel interaction between the AAV vector and specific cell surface receptors expressed on human hippocampal neurons (Bartlett et al., 1999). Furthermore, a highly efficient regulatory virus vector system, such as an AAV vector with a tetracycline-responsive promoter, with expression of foreign genes modulated in response to tetracycline (Pluta et al., 2005), should also be established for reversible control of HIF-1 gene expression in neurons. With these further investigations, gene therapy using rAAV to deliver HIF-1 may ultimately provide a new option for clinical treatment of Alzheimer's disease.
Acknowledgments:
We thank Max Gassmann (University of Zurich, Zurich, Switzerland) for providing the pBSKhHIF1αT7 plasmid, and the Vector Gene Technology Company Ltd. (Beijing, China) for the Construction of pSNAV-HIF-1α plasmid.
Footnotes
Conflicts of interest: None declared.
Funding: This study was supported by the National Natural Science Foundation of China, No. 81273983; the Natural Science Foundation of Hebei Province of China, No. C2010001471; and the Scientific Research Fund of Hebei Provincial Education Department in China, No. Q2012036.
Copyedited by James R, Yajima W, Yu J, Qiu Y, Li CH, Song LP, Zhao M
References
- 1.Avramovich-Tirosh Y, Bar-Am O, Amit T, Youdim M, Weinreb O. Up-regulation of hypoxia-inducible factor (HIF)-1 and HIF-target genes in cortical neurons by the novel multifunctional iron chelator anti-Alzheimer drug, M30. Curr Alzheimer Res. 2010;7:300–306. doi: 10.2174/156720510791162403. [DOI] [PubMed] [Google Scholar]
- 2.Banker GA, Cowan WM. Rat hippocampal neurons in dispersed cell culture. Brain Res. 1977;126:397–425. doi: 10.1016/0006-8993(77)90594-7. [DOI] [PubMed] [Google Scholar]
- 3.Bartlett JS, Kleinschmidt J, Boucher RC, Samulski RJ. Targeted adeno-associated virus vector transduction of nonpermissive cells mediated by a bispecific F(ab’gamma)2 antibody. Nat Biotechnol. 1999;17:181–186. doi: 10.1038/6185. [DOI] [PubMed] [Google Scholar]
- 4.Beneduci A, Chidichimo G, Tripepi S, Perrotta E. Transmission electron microscopy study of the effects produced by wide-band low-power millimeter waves on MCF-7 human breast cancer cells in culture. Anticancer Res. 2005;25:1009–1013. [PubMed] [Google Scholar]
- 5.Braddock M. Safely slowing down the decline in Alzheimer's disease: gene therapy shows potential. Expert Opin Investig Drugs. 2005;14:913–915. doi: 10.1517/13543784.14.7.913. [DOI] [PubMed] [Google Scholar]
- 6.Carty NC, Nash K, Lee D, Mercer M, Gottschall PE, Meyers C, Muzyczka N, Gordon MN, Morgan D. Adeno-associated viral (AAV) serotype 5 vector mediated gene delivery of endothelin-converting enzyme reduces Abeta deposits in APP + PS1 transgenic mice. Mol Ther. 2008;16:1580–1586. doi: 10.1038/mt.2008.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dotti CG, Sullivan CA, Banker GA. The establishment of polarity by hippocampal neurons in culture. J Neurosci. 1988;8:1454–1468. doi: 10.1523/JNEUROSCI.08-04-01454.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fernaández-Morales JC, Arranz-Tagarro JA, Calvo-Gallardo E, Maroto M, Padín JF, García AG. Stabilizers of neuronal and mitochondrial calcium cycling as a strategy for developing a medicine for Alzheimer's disease. ACS Chem Neurosci. 2012;3:873–883. doi: 10.1021/cn3001069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Greijer A, Van der Wall E. The role of hypoxia inducible factor 1 (HIF-1) in hypoxia induced apoptosis. J Clin Pathol. 2004;57:1009–1014. doi: 10.1136/jcp.2003.015032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hong CS, Goins WF, Goss JR, Burton EA, Glorioso JC. Herpes simplex virus RNAi and neprilysin gene transfer vectors reduce accumulation of Alzheimer's disease-related amyloid-β peptide in vivo. Gene Ther. 2006;13:1068–1079. doi: 10.1038/sj.gt.3302719. [DOI] [PubMed] [Google Scholar]
- 11.Kong WN, Zhao SE, Duan XL, Yang Z, Qian ZM, Chang YZ. Decreased DMT1 and increased ferroportin 1 expression is the mechanisms of reduced iron retention in macrophages by erythropoietin in rats. J Cell Biochem. 2008;104:629–641. doi: 10.1002/jcb.21654. [DOI] [PubMed] [Google Scholar]
- 12.Liu Y, Liu F, Iqbal K, Grundke-Iqbal I, Gong CX. Decreased glucose transporters correlate to abnormal hyperphosphorylation of tau in Alzheimer disease. FEBS Lett. 2008;582:359–364. doi: 10.1016/j.febslet.2007.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Manukhina E, Goryacheva A, Barskov I, Viktorov I, Guseva A, Pshennikova M, Khomenko I, Mashina SY, Pokidyshev D, Malyshev IY. Prevention of neurodegenerative damage to the brain in rats in experimental Alzheimer's disease by adaptation to hypoxia. Neurosci Behav Physiol. 2010;40:737–743. doi: 10.1007/s11055-010-9320-6. [DOI] [PubMed] [Google Scholar]
- 14.Marchesi VT. Alzheimer's dementia begins as a disease of small blood vessels, damaged by oxidative-induced inflammation and dysregulated amyloid metabolism: implications for early detection and therapy. FASEB J. 2011;25:5–13. doi: 10.1096/fj.11-0102ufm. [DOI] [PubMed] [Google Scholar]
- 15.Mattson MP, Cheng B, Davis D, Bryant K, Lieberburg I, Rydel RE. beta-Amyloid peptides destabilize calcium homeostasis and render human cortical neurons vulnerable to excitotoxicity. J Neurosci. 1992;12:376–389. doi: 10.1523/JNEUROSCI.12-02-00376.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maxwell PH. Hypoxia-inducible factor as a physiological regulator. Exp Physiol. 2005;90:791–797. doi: 10.1113/expphysiol.2005.030924. [DOI] [PubMed] [Google Scholar]
- 17.Murray IV, Proza JF, Sohrabji F, Lawler JM. Vascular and metabolic dysfunction in Alzheimer's disease: a review. Exp Biol Med (Maywood) 2011;236:772–782. doi: 10.1258/ebm.2011.010355. [DOI] [PubMed] [Google Scholar]
- 18.Musiani M, Zerbini M, Gibellini D, Gentilomi G, La Placa M, Ferri E, Girotti S. Chemiluminescent assay for the detection of viral and plasmid DNA using digoxigenin-labeled probes. Anal Biochem. 1991;194:394–398. doi: 10.1016/0003-2697(91)90247-q. [DOI] [PubMed] [Google Scholar]
- 19.Nordal RA, Nagy A, Pintilie M, Wong CS. Hypoxia and hypoxia-inducible factor-1 target genes in central nervous system radiation injury a role for vascular endothelial growth factor. Clin Cancer Res. 2004;10:3342–3353. doi: 10.1158/1078-0432.CCR-03-0426. [DOI] [PubMed] [Google Scholar]
- 20.Palop JJ, Mucke L. Epilepsy and cognitive impairments in Alzheimer disease. Arch Neurol. 2009;66:435–440. doi: 10.1001/archneurol.2009.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pike CJ, Cummings BJ, Cotman CW. [beta]-Amyloid induces neuritic dystrophy in vitro: similarities with Alzheimer pathology. Neuroreport. 1992;3:769–772. doi: 10.1097/00001756-199209000-00012. [DOI] [PubMed] [Google Scholar]
- 22.Pike CJ, Walencewicz-Wasserman AJ, Kosmoski J, Cribbs DH, Glabe CG, Cotman CW. Structure-activity analyses of beta-amyloid peptides: contributions of the beta 25-35 region to aggregation and neurotoxicity. J Neurochem. 1995;64:253–265. doi: 10.1046/j.1471-4159.1995.64010253.x. [DOI] [PubMed] [Google Scholar]
- 23.Piret JP, Minet E, Cosse JP, Ninane N, Debacq C, Raes M, Michiels C. Hypoxia-inducible factor-1-dependent overexpression of myeloid cell factor-1 protects hypoxic cells against tert-butyl hydroperoxide-induced apoptosis. J Biol Chem. 2005;280:9336–9344. doi: 10.1074/jbc.M411858200. [DOI] [PubMed] [Google Scholar]
- 24.Pluta K, Luce MJ, Bao L, Agha-Mohammadi S, Reiser J. Tight control of transgene expression by lentivirus vectors containing second-generation tetracycline-responsive promoters. J Gene Med. 2005;7:803–817. doi: 10.1002/jgm.712. [DOI] [PubMed] [Google Scholar]
- 25.Sasabe E, Tatemoto Y, Li D, Yamamoto T, Osaki T. Mechanism of HIF-1alpha-dependent suppression of hypoxia-induced apoptosis in squamous cell carcinoma cells. Cancer Sci. 2005;96:394–402. doi: 10.1111/j.1349-7006.2005.00065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schubert D, Soucek T, Blouw B. The induction of HIF-1 reduces astrocyte activation by amyloid beta peptide. Eur J Neurosci. 2009;29:1323–1334. doi: 10.1111/j.1460-9568.2009.06712.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Soucek T, Cumming R, Dargusch R, Maher P, Schubert D. The regulation of glucose metabolism by HIF-1 mediates a neuroprotective response to amyloid beta peptide. Neuron. 2003;39:43–56. doi: 10.1016/s0896-6273(03)00367-2. [DOI] [PubMed] [Google Scholar]
- 28.Stepanichev MY, Zdobnova IM, Yakovlev AA, Onufriev MV, Lazareva NA, Zarubenko II, Gulyaeva NV. Effects of tumor necrosis factor-alpha central administration on hippocampal damage in rat induced by amyloid beta-peptide (25–35) J Neurosci Res. 2003;71:110–120. doi: 10.1002/jnr.10469. [DOI] [PubMed] [Google Scholar]
- 29.Stepanichev MY, Zdobnova IM, Zarubenko II, Moiseeva YV, Lazareva NA, Onufriev MV, Gulyaeva NV. Amyloid-beta (25-35)-induced memory impairments correlate with cell loss in rat hippocampus. Physiol Behav. 2004;80:647–655. doi: 10.1016/j.physbeh.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 30.Stepanichev MY, Zdobnova IM, Zarubenko II, Lazareva NA, Gulyaeva NV. Studies of the effects of central administration of β-amyloid peptide (25–35):pathomorphological changes in the hippocampus and impairment of spatial memory. Neurosci Behav Physiol. 2006;36:101–106. doi: 10.1007/s11055-005-0167-1. [DOI] [PubMed] [Google Scholar]
- 31.Stepanov II, Kuznetsova NN, Klement’ev BI, Sapronov NS. Effects of intracerebroventricular administration of beta-amyloid on the dynamics of learning in purebred and mongrel rats. Neurosci Behav Physiol. 2007;37:583–590. doi: 10.1007/s11055-007-0056-x. [DOI] [PubMed] [Google Scholar]
- 32.Tuszynski MH. Nerve growth factor gene therapy in Alzheimer disease. Alzheimer Dis Assoc Disord. 2007;21:179–189. doi: 10.1097/WAD.0b013e318068d6d2. [DOI] [PubMed] [Google Scholar]
- 33.Wray S, Baisden RH, Woodruff ML. Neurochemical anatomy of fetal hippocampus transplanted into large lesion cavities made in the adult rat brain. Exp Neurol. 1991;111:36–48. doi: 10.1016/0014-4886(91)90048-h. [DOI] [PubMed] [Google Scholar]
- 34.Wu Z, Wu X, Cao H, Dong X, Wang H, Hou Y. A novel and highly efficient production system for recombinant adeno-associated virus vector. Sci China C Life Sci. 2002;45:96–104. doi: 10.1360/02yc9011. [DOI] [PubMed] [Google Scholar]
- 35.Yamaguchi Y, Miyashita H, Tsunekawa H, Mouri A, Kim HC, Saito K, Matsuno T, Kawashima S, Nabeshima T. Effects of a Novel Cognitive Enhancer, Spiro [imidazo-[1, 2-a] pyridine-3, 2-indan]-2 (3H)-one (ZSET1446), on Learning Impairments Induced by Amyloid-β1–40 in the Rat. J Pharmacol Exp Ther. 2006;317:1079–1087. doi: 10.1124/jpet.105.098640. [DOI] [PubMed] [Google Scholar]
- 36.Zhang J, Wu X, Qin C, Qi J, Ma S, Zhang H, Kong Q, Chen D, Ba D, He W. A novel recombinant adeno-associated virus vaccine reduces behavioral impairment and β-amyloid plaques in a mouse model of Alzheimer's disease. Neurobiol Dis. 2003;14:365–379. doi: 10.1016/j.nbd.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 37.Zhang J, Yu Z, Yu Z, Yang Z, Zhao H, Liu L, Zhao J. rAAV-mediated delivery of brain-derived neurotrophic factor promotes neurite outgrowth and protects neurodegeneration in focal ischemic model. Int J Clin Exp Pathol. 2011;4:496. [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang J, Zhen YF, Song LG, Kong WN, Shao TM, Li X, Chai XQ. Salidroside attenuates beta amyloid-induced cognitive deficits via modulating oxidative stress and inflammatory mediators in rat hippocampus. Behav Brain Res. 2013;244:70–81. doi: 10.1016/j.bbr.2013.01.037. [DOI] [PubMed] [Google Scholar]
- 39.Zhao L, Wu J, Zhang X, Kuang H, Guo Y, Ma L. The effect of Shenmai injection on the proliferation of Rat airway smooth muscle cells in asthma and underlying mechanism. BMC Complement Altern Med. 2013;13:221. doi: 10.1186/1472-6882-13-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhou LJ, Zhu XZ. Reactive oxygen species-induced apoptosis in PC12 cells and protective effect of bilobalide. J Pharmacol Exp Ther. 2000;293:982–988. [PubMed] [Google Scholar]
- 41.Zussy C, Brureau A, Delair B, Marchal S, Keller E, Ixart G, Naert G, Meunier J, Chevallier N, Maurice T, Givalois L. Time-course and regional analyses of the physiopathological changes induced after cerebral injection of an amyloid β fragment in rats. Am J Pathol. 2011;179:315–334. doi: 10.1016/j.ajpath.2011.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]


