Abstract
Basement membrane degradation and blood-brain barrier damage appear after cerebral infarction, severely impacting neuronal and brain functioning; however, the underlying pathogenetic mechanisms remain poorly understood. In this study, we induced cerebral infarction in stroke-prone spontaneously hypertensive rats by intragastric administration of high-sodium water (1.3% NaCl) for 7 consecutive weeks. Immunohistochemical and immunofluorescence assays demonstrated that, compared with the non-infarcted contralateral hemisphere, stroke-prone spontaneously hypertensive rats on normal sodium intake and Wistar-Kyoto rats, matrix metalloproteinase-9 expression, the number of blood vessels with discontinuous collagen IV expression and microvessel density were significantly higher, and the number of continuous collagen IV-positive blood vessels was lower in the infarct border zones of stroke-prone spontaneously hypertensive rats given high-sodium water. Linear correlation analysis showed matrix metalloproteinase-9 expression was positively correlated with the number of discontinuously collagen IV-labeled blood vessels and microvessel density in cerebral infarcts of stroke-prone spontaneously hypertensive rats. These results suggest that matrix metalloproteinase-9 upregulation is associated with increased regional angiogenesis and degradation of collagen IV, the major component of the basal lamina, in stroke-prone spontaneously hypertensive rats with high-sodium water-induced focal cerebral infarction.
Keywords: nerve regeneration, cerebral infarction, matrix metalloproteinase-9, collagen IV, microvessel density, angiogenesis, basement membrane degradation, high sodium, stroke-prone spontaneously hypertensive, China Medical Board Project, neural regeneration
Introduction
Cerebral infarction is a common complication of hypertension (Qureshi, 2008; Sun et al., 2011). The major underlying initiating cause of infarction is small vessel structural changes (i.e., vessel wall thickening, disruption and eventual breakdown), leading to damage to the blood-brain barrier, consequently resulting in stroke (Fukuda et al., 2004; Liu et al., 2006; Bailey et al., 2009; Bernas et al., 2010; Goldstein et al., 2011; Iadecola and Anrather, 2011; Liu et al., 2011; Dirnagl, 2012; Hossmann, 2012; Yenari and Han, 2012). Angiogenesis is a major factor associated with improved neurologic recovery after stroke (Chopp et al., 2007; Zhao et al., 2007; Bosomtwi et al., 2008; Teng et al., 2008; Li et al., 2010; Potente et al., 2011; Reitmeir et al., 2012; Espinera et al., 2013; Xiong et al., 2013; Zechariah et al., 2013; Zhang et al., 2013; Kono et al., 2014; Omote et al., 2014).
Studies have demonstrated that matrix metalloproteinase-9, a member of the matrix metalloproteinase family of zinc- and calcium-dependent enzymes, is thought to play key roles in the pathogenesis of blood-brain barrier breakdown following stroke (Asahi et al., 2001; Aoki et al., 2002; Maier et al., 2004; Gidday et al., 2005; Kelly et al., 2006; Rosell et al., 2006; Kamada et al., 2007; Rosell et al., 2008; Feiler et al., 2011; Kumari et al., 2011; Shichi et al., 2011; Wang et al., 2011; Graham et al., 2012; Lee et al., 2012; Liu et al., 2012; Ralay Ranaivo et al., 2012; Suofu et al., 2012; Wu et al., 2012; Xiang et al., 2012; Alam and Shuaib, 2013; Morancho et al., 2013; Russell et al., 2014; Wu et al., 2014), and it appears to be involved in ischemic stroke. Matrix metalloproteinase-9 is able to degrade the major components of the basement membrane around cerebral blood vessels, such as collagen IV (Rosell et al., 2008; Luo et al., 2011), which increases the risk of cerebral hemorrhage (Alam et al., 2011). Recent studies (Lee et al., 2004; Mira et al., 2004; Yang et al., 2013; Yousuf et al., 2014) indicate that matrix metalloproteinase-9, in particular, plays a central role during angiogenesis. Mira et al. (2004) confirmed a relationship between matrix metalloproteinase-9 and angiogenesis in the invasion and metastasis of malignant tumors. Others have shown that matrix metalloproteinase-9 expression is upregulated by growth factors, including vascular endothelial growth factor, and that the enzyme plays an important role in vasculogenesis and vascular remodeling in response to injury (Lee et al., 2004), such as stroke (Yang et al., 2013). Matrix metalloproteinase-9 may also mediate neurovascular remodeling via lipoprotein receptor signaling in the peri-infarct cortical region, and could be a useful biomarker (Xiong et al., 2013). However, it remains poorly understood whether matrix metalloproteinase-9 is involved in basement membrane degradation following infarction and angiogenesis induced by hypertension in the stroke-prone spontaneously hypertensive rat model. In this study, we investigate the relationship between matrix metalloproteinase-9, collagen IV expression and microvessel density in stroke-prone spontaneously hypertensive rats.
Materials and Methods
Experimental animals and establishment of cerebral infarction model
Experiments were performed in 20 male stroke-prone spontaneously hypertensive rats, weighing 230–280 g, and 20 male non-hypertensive Wistar-Kyoto rats weighing 300–350 g. All rats, aged 9 weeks, were obtained from the Shanghai SLAC Laboratory Animal Co., Ltd. (Shanghai, China). The rats were maintained at the Laboratory Animal Center, Medical School of Xi’an Jiaotong University, China (license No. SYXK (Shaan) 2007-003). The rats were housed in a room at 22 ± 1°C with a 12-hour light/dark cycle. All protocols were performed in accordance with the European Communities Council Directive of 24 November, 1986 (86/609/EEC), or with the Guidelines laid down by the NIH in the US regarding the care and use of animals for experimental procedures.
Stroke-prone spontaneously hypertensive rats and Wis-tar-Kyoto rats received either high sodium intake (10 stroke-prone spontaneously hypertensive rats, 10 Wistar-Kyoto rats) or normal sodium intake (10 stroke-prone spontaneously hypertensive rats, 10 Wistar-Kyoto rats) starting at 9 weeks of age to accelerate stroke onset. Rats receiving normal sodium intake were given 0.9% NaCl, and rats receiving high sodium intake were given 1.3% NaCl to drink, with daily weigh-ins. All rats were fed with standard rat chow. Systolic blood pressure of conscious rats was measured over 5-second intervals every 10 minutes by tail-cuff plethysmography (Kvetnaflsky et al., 1977). We calculated the mean weekly systolic blood pressure values for each animal. We began the experiments at 3 weeks after the rats showed major stroke-associated signs, such as hyperirritability, paroxysm, palsy or hemiplegia.
Expression of collagen IV, matrix metalloproteinase-9 and factor VIII in rat brain as detected by immunohistochemical staining
Five stroke-prone spontaneously hypertensive rats with brain infarction, five stroke-prone spontaneously hypertensive rats without brain infarction, five Wistar-Kyoto rats given high sodium intake and five Wistar-Kyoto rats given normal sodium intake were given an intraperitoneal injection of 10% chloral hydrate (400 mg/kg) and then intracardially perfused with 100 mL of PBS, followed by 60 mL of fixative (4% paraformaldehyde, 2% sucrose in PBS; pH 7.5). Dissected brains were stored in the same fixative at 4°C overnight, followed by 10% sucrose for 12 hours, 20% sucrose for 12 hours, and 30% sucrose for 12 hours. Fixed brains were sectioned coronally 3 mm anterior and 3 mm posterior to the mid-coronal plane. Serial transverse sections of frozen brain (4-μm-thick) were made using a cryostat and treated with 3-aminopropyl-triethoxysilane. One section from each experimental animal was stained with hematoxylin and eosin, and the other sections were stored at −80°C for further use.
Immunohistochemical staining using the streptavidin-peroxidase method was performed after sectioning. Sections (4-μm-thick) containing the frontoparietal cortex from infarcted stroke-prone spontaneously hypertensive rats and Wistar-Kyoto rats were incubated with rabbit anti-rat collagen IV monoclonal antibody (1:50; Dako, Carpinteria, CA, USA), rabbit anti-rat matrix metalloproteinase-9 monoclonal antibody (1:200; Dako) or rabbit anti-rat factor VIII monoclonal antibody (1:300; Dako) at 4°C overnight, followed by goat anti-rabbit IgG (1:100; Dako) at 37°C for 30 minutes. After three additional washes, sections were visualized using 3,3′-diaminobenzidine solution. PBS was used instead of 3,3′-diaminobenzidine for negative controls. Larynx squamous cell carcinoma was used as a positive control. We evaluated matrix metalloproteinase-9 expression by counting the number of matrix metalloproteinase-9-positive cells and matrix metalloproteinase-9-positive blood vessels in different areas of rat brain from 10 high-power fields (× 400), and the values were averaged for each parameter. All collagen IV-positive staining was found in the basal lamina of cerebral blood vessels. Two patterns of labeling were found: continuous or discontinuous around microvessels. The evaluation of collagen IV immunostaining was similar to that for matrix metalloproteinase-9. Microvessel density was measured by counting the number of single or clustered factor VIII-positive endothelial cells in different areas of the brain. The mean microvessel density was obtained from five high-power fields (× 400) by light microscopy (Leica, Solms, Germany) (Ritz et al., 2009).
Expression of collagen IV, matrix metalloproteinase-9 and factor VIII in rat brain as determined by immunofluorescence labeling
All rats were intraperitoneally injected with 10% chloral hydrate (400 mg/kg). FITC-dextran (2 × 106 molecular weight; Sigma, St. Louis, MO, USA; 50 mg/mL, 1 mL) was injected into rats (five stroke-prone spontaneously hypertensive rats with brain infarction, five stroke-prone spontaneously hypertensive rats without brain infarction, five high sodium intake Wistar-Kyoto rats and five normal sodium intake Wistar-Kyoto rats) via the left femoral vein. One minute later, the rats were sacrificed by decapitation. The brains were rapidly removed and fixed in 4% paraformaldehyde at 4°C for 24 hours. Serial transverse sections of frozen brain (30-μm-thick) were cut with a cryostat (JungCM 1900, Leica Instruments, Nussloch, Germany). Sections were treated with 3-aminopropyl-triethoxysilane and stored at −80°C for further use.
Sections were dried for an hour at room temperature and immersed in 3% hydrogen peroxide for 10 minutes to suppress endogenous peroxidase activity. After three 5-minute rinses in PBS, sections were incubated at 4°C overnight with rabbit anti-rat collagen IV monoclonal antibody (1:50) or rabbit anti-rat matrix metalloproteinase-9 monoclonal antibody (1:100). After three 5-minute washes in PBS, sections were incubated with streptavidin-fluorescein (or Texas red)-labeled goat anti-rabbit IgG (1:800; Dako) in the dark at room temperature for 30 minutes. PBS was substituted for the negative control. Larynx squamous cell carcinoma was used as a positive control. Matrix metalloproteinase-9-positive blood vessels and collagen IV-positive blood vessels (red fluorescence) were visualized by confocal laser microscopy (Leica) (excitation, 488 nm). Imaging was performed under the same conditions, and laser excitation lasted for an hour.
Statistical analysis
All data were presented as mean ± SD. Statistical analysis was performed with the Social Sciences Statistical package 13.0 (SPSS, Chicago, IL, USA). Data with two-group variables were analyzed by independent t-tests. Differences among multiple groups were assessed using univariate analysis of variance. The relationships between markers were evaluated with linear correlation analysis (Pearson correlation). Differences between means were considered statistically significant at P < 0.05.
Results
Morphological changes in brain tissues of stroke-prone spontaneously hypertensive rats with cerebral infarction
Stroke-prone spontaneously hypertensive rats maintained on high-sodium water (1.3% NaCl) had a mean arterial blood pressure of 201 ± 8 mmHg. Decreased weight with a depressed state was apparent at 7 weeks. In comparison, stroke-prone spontaneously hypertensive rats given normal water had a mean arterial systolic blood pressure of 151 ± 4 mmHg, without a reduction in weight or depressive signs. Wistar-Kyoto rats on high-sodium water or normal water did not show abnormal symptoms and had a mean arterial systolic blood pressure of 115 ± 7 mmHg and 110 ± 5 mmHg, respectively.
There were no hemorrhages or infarcts in the brains of any control Wistar-Kyoto or stroke-prone spontaneously hypertensive rats with normal sodium intake (Figure 1A). Gross and histopathologic examination of the brain showed that they were normal. However, in all stroke-prone spontaneously hypertensive rats that ingested high-sodium water, gross pathologic examination showed softening in the frontoparietal or temporal cortex and surface bleeding, and the incidence rate of brain infarction was 100%. Hematoxylin-eosin-stained sections showed that the center of the softened area contained a cavity, and the boundary zone contained a small, softened area speckled with hemorrhages in stroke-prone spontaneously hypertensive rats with cerebral infarction. In the boundary zone, we observed proliferative changes in inflammatory cells, and degenerating astrocytes, neurons, gitter cells and capillary vessels (Figure 1E). No hemorrhages or infarction were observed in the non-infarcted hemisphere or in any control Wistar-Kyoto or stroke-prone spontaneously hypertensive rats with normal sodium intake.
Figure 1.
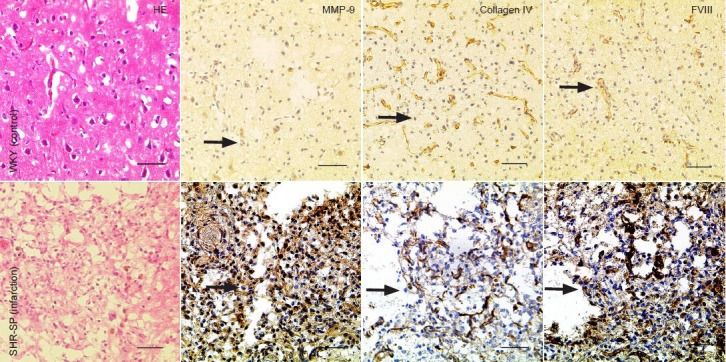
Histological changes in brain tissues of stroke-prone spontaneously hypertensive rats with cerebral infarction.
Proliferative changes were visible in tissue. Cytoplasmic staining for matrix metalloproteinase-9 (MMP-9) is visible in vascular endothelial cells, astro-cytes, neurons, gitter cells and inflammatory cells. Moderate disruption of the microvessel basal lamina was revealed by staining for collagen IV. There was strong cytoplasmic staining for factor VIII (FVIII) in vascular endothelial cells in stroke-prone spontaneously hypertensive rats. Arrows show pos-itive expression. Scale bars: 50 μm. HE: Hematoxylin and eosin staining; WKY: Wistar-Kyoto; SHR-SP: stroke-prone spontaneously hypertensive.
Expression of matrix metalloproteinase-9 and collagen IV in the brain of stroke-prone spontaneously hypertensive rats with cerebral infarction
Immunohistochemical staining showed that matrix metalloproteinase-9 was mainly expressed in the cell membrane and cytoplasm of vascular endothelial cells, astrocytes, neurons, gitter cells and inflammatory cells (Figure 1B and F). The numbers of matrix metalloproteinase-9-positive cells and blood vessels were significantly higher in infarct border zones compared to the non-infarcted contralateral hemisphere, stroke-prone spontaneously hypertensive rats in normal water or Wistar-Kyoto rats (P < 0.01; Table 1). Immunofluorescence staining for matrix metalloproteinase-9 was especially robust and easily discerned in microvessels in experimental rats after injecting FITC (Figure 2A).
Table 1.
Expression of MMP-9 (n/400 × field of view), collagen IV (n/400 × field of view) and microvesel density (n/400 × field of view) in stroke-prone spontaneously hypertensive rats with or without cerebral infarction and in WKY rats
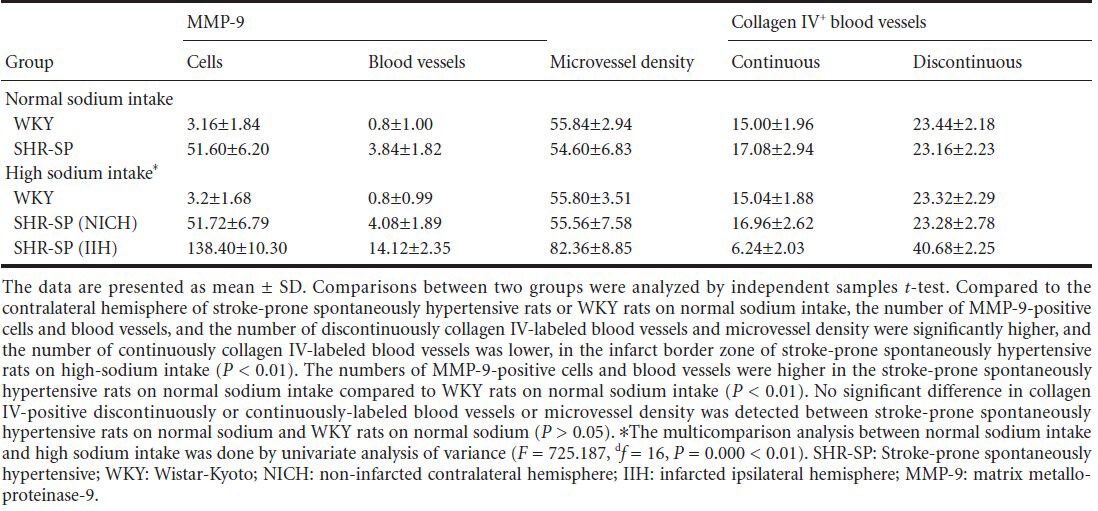
Figure 2.
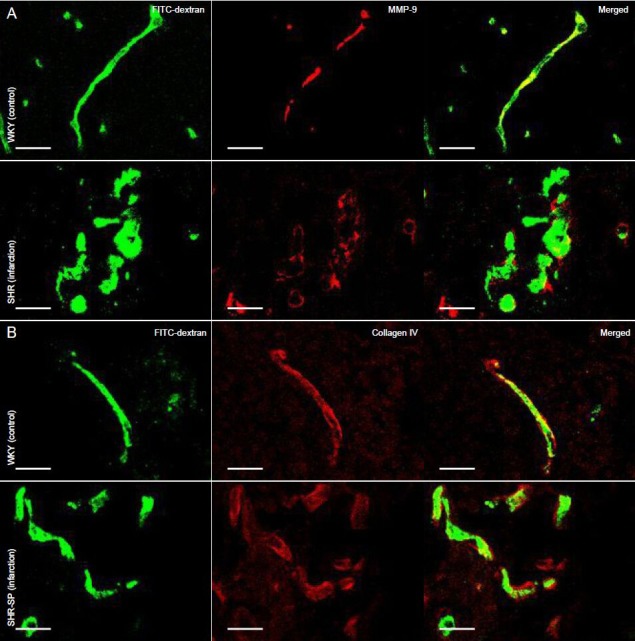
Expression of MMP-9 (A) and collagen IV (B) in the brain of stroke-prone spontaneously hypertensive rats with cerebral infarction (immunofluorescence staining).
FITC-dextran was injected into rats via the left femoral vein before being sacrificed. The architecture of microvessels is displayed with FITC dextran (green), and with MMP-9 and collagen IV immunofluorescence labeling (red). MMP-9 expression was increased in microvessels and endothelial cells in the infarct border zone in stroke-prone spontaneously hypertensive rats, and collagen IV was discontinuous in basal lamina of microvessels, compared to the corresponding areas of WKY rats. Scale bars: 20 µm. MMP-9: Matrix metalloproteinase-9; SHR-SP: stroke-prone spontaneously hypertensive; WKY: Wistar-Kyoto.
To assess basal lamina integrity in blood vessels, we stained brain sections for collagen IV. Two distinct patterns of collagen IV immunofluorescence were visible around microvessels-continuous and discontinuous (Figure 2B). Numerous blood vessels with continuous collagen IV labeling were present in the non-infarcted contralateral hemisphere and in stroke-prone spontaneously hypertensive rats on normal sodium intake and in Wistar-Kyoto rats (Figure 1C). A lower number was observed in infarct border zones (Figure 1G). This difference was statistically significant (P < 0.01). The profile of discontinuous collagen IV labeling in blood vessels was essentially opposite to that of continuous collagen IV labeling (P < 0.01; Table 1).
Microvessel density in the brain of stroke-prone spontaneously hypertensive rats with cerebral infarction
Factor VIII expression was observed in the cytoplasm of cerebral vascular endothelial cells in all groups (Figure 1D and H). The number of factor VIII-positive microvessels and microvessel density were significantly higher in infarct border zones compared with the contralateral hemisphere, stroke-prone spontaneously hypertensive rats on normal sodium or Wistar-Kyoto rats (P < 0.01; Table 1).
Correlation of matrix metalloproteinase-9 with collagen IV and microvessel density
Linear correlation analysis showed that the number of matrix metalloproteinase-9-positive cells and blood vessels positively correlated with microvessel density and the number of blood vessels with discontinuous collagen IV labeling (matrix metalloproteinase-9-positive cells versus microvessel density: r = 0.754, P < 0.01, Figure 3A; matrix metalloproteinase-9-positive cells versus blood vessels discontinuously labeled for collagen IV: r = 0.845, P < 0.01, Figure 3B; matrix metalloproteinase-9-positive microvessels versus microvessel density: r = 0.767, P < 0.01, Figure 3C; matrix metalloproteinase-9-positive microvessels versus blood vessels discontinuously labeled for collagen IV: r = 0.871, P < 0.01, Figure 3D). In addition, the number of blood vessels with continuous collagen IV labeling was negatively correlated with the number of matrix metalloproteinase-9-positive cells (r = –0.672, P < 0.01; Figure 3E) and with the number of matrix metalloproteinase-9-positive blood vessels (r = –0.719, P < 0.01; Figure 3F). These results demonstrate that matrix metalloproteinase-9 expression is positively correlated with collagen IV degradation and angiogenesis in the cerebral infarct area of stroke-prone spontaneously hypertensive rats administered high-sodium drinking water.
Figure 3.
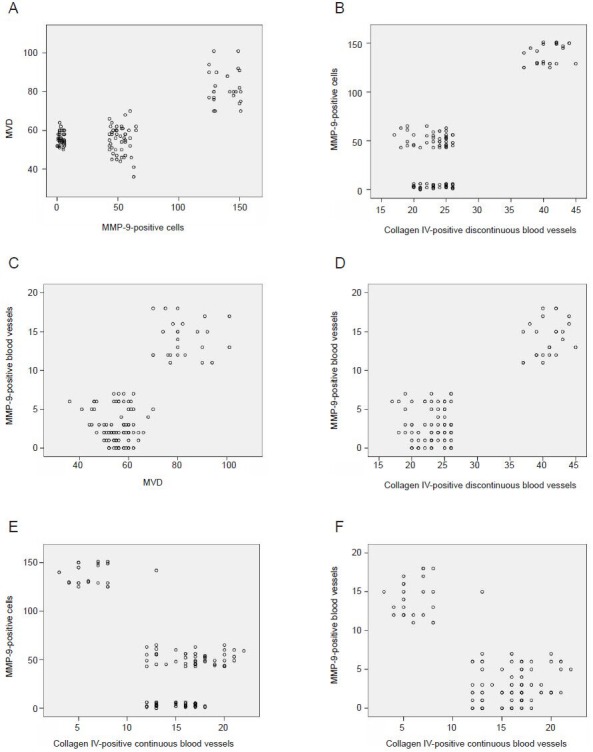
Correlation of MMP-9 with collagen IV and microvessel density in the brain of stroke-prone spontaneously hypertensive rats with cerebral infarction.
Linear correlation analysis showed that the number of MMP-9-positive cells and blood vessels positively correlated with microvessel density and the number of collagen IV-positive discontinuously-labeled blood vessels. The correlations were positive for MMP-9-positive cells versus microves-sel density (r = 0.754; A) and versus collagen IV-positive discontinuously-labeled blood vessels (r = 0.845; B); and for MMP-9-positive microvessels versus microvessel density (r = 0.767; C) and versus collagen IV-positive discontinuously-labeled blood vessels (r = 0.871; D). The correlations were negative for the number of collagen IV-positive continuously-labeled blood vessels versus MMP-9-positive cells (r = –0.672; E) and versus MMP-9-positive blood vessels (r = –0.719; F). MVD: Microvessel density; MMP-9: matrix metalloproteinase-9.
Discussion
Stroke-prone spontaneously hypertensive rats are used as a stroke model because they exhibit cerebral hemorrhage and infarction similar to hypertensive humans (Bailey et al., 2009). However, they have a relatively low incidence rate of brain infarction, which is a concern in a model system (Yenari and Han, 2012). Studies reported a high proportion of strokes in stroke-prone spontaneously hypertensive rats on a high-salt diet, and they have severe hypertension and rapid stroke onset (Cho et al., 2007; Thoene-Reineke et al., 2011). Our observations of neurological changes in stroke-prone spontaneously hypertensive rats given high sodium (1.3%) drinking water showed mainly brain infarctions with macroscopic and microscopic pathology, and the incidence rate of brain infarction was 100%. Thus, we suggest that stroke-prone spontaneously hypertensive rats on a high-salt regimen typically have hypertension and neocortical strokes, and may be a satisfactory experimental model of stroke.
Hitherto, the exact molecular mechanism responsible for clearing damaged tissue following stroke remained unknown. Studies demonstrated that matrix metalloproteinase-9 is upregulated early in injured tissue, suggesting a detrimental role, and that it is involved in brain damage in animal models of cerebral ischemia (Feiler et al., 2011; Kumari et al., 2011; Wang et al., 2011; Lee et al., 2012; Wu et al., 2012). We performed immunohistochemical staining for matrix metalloproteinase-9 expression in stroke-prone spontaneously hypertensive rats given high-sodium drinking water, and found that matrix metalloproteinase-9 expression was increased in the cell membranes and cytoplasm of all positive cell types, including astrocytes, neurons, gitter cells, vascular endothelial cells and inflammatory cells in different regions of interest. We also found that the number of matrix metalloproteinase-9-positive cells and matrix metalloproteinase-9-positive blood vessels was high in infarct border zones. These findings are similar to those of a previous investigation by Shichi et al. (2011), which showed that protein levels of matrix metalloproteinase-9 were significantly increased 1 and 3 days after middle cerebral artery occlusion in a mouse model of cerebral infarction. Their results also demonstrated that matrix metalloproteinase-9 plays a detrimental role in brain damage in animal models of stroke.
Interestingly, matrix metalloproteinase-9 is associated with collagen IV degradation in cerebral stroke. The loss of basal lamina integrity has been postulated to be the primary cause of stroke, because matrix metalloproteinase-9 can degrade the main components of basal lamina, such as laminin, fibronectin and collagen IV (Rosell et al., 2008). Hence, matrix metalloproteinase-9 upregulation leads to a breakdown of the blood-brain barrier, and this ultimately results in brain hemorrhage and infarction. Studies have implicated matrix metalloproteinase-9 in cerebral stroke events and basal lamina destruction in rat models (Alam et al., 2011). Fukuda et al. (2004) showed that matrix metalloproteinase-9 significantly reduces levels of microvessel-associated collagen IV, laminin and heparan sulfate proteoglycans, and is acutely responsible for vascular matrix degradation in infarcted cerebral tissues after middle cerebral artery occlusion in adolescent male baboons. We found that the number of discontinuously collagen IV-labeled blood vessels mirrored the pattern of matrix metalloproteinase-9 expression. However, the number of continuously collagen IV-labeled blood vessels was low in infarct border zones. Linear correlation analysis showed that the number of matrix metalloproteinase-9-positive cells and matrix metalloproteinase-9-positive blood vessels were positively correlated with discontinuously collagen IV-labeled blood vessels, and it was negatively correlated with the number of continuously collagen IV-labeled blood vessels. Therefore, our findings confirm the association between matrix metalloproteinase-9 overexpression and collagen IV degradation in infarct areas in stroke-prone spontaneously hypertensive rats, suggesting that matrix metalloproteinase-9 may be a key contributor to collagen IV degradation in brain after stroke.
Although many studies have reported matrix metalloproteinase-9 expression in vascular endothelial cells (Asahi et al., 2001; Aoki et al., 2002; Lee et al., 2004; Maier et al., 2004; Yang et al., 2013), the association between matrix metalloproteinase-9 expression and angiogenesis in infarcted brain tissue is still unclear. Zhao et al. (2007) showed that, in the peri-infarct area of the cortex, matrix metalloproteinases might mediate neurovascular remodeling via lipoprotein receptor signaling, and suggested its use as a biomarker. Therefore, to gain more insight into the mechanisms of the microvascular proliferative effects of matrix metalloproteinase-9 in stroke-prone spontaneously hypertensive brain infarcts (Kvetnaflsky et al., 1977; Bosomtwi et al., 2008), we measured markers such as microvessel density. Our results show that microvessel density mirrored the pattern of matrix metalloproteinase-9-positive blood vessels; i.e., microvessel density was significantly higher in infarct border zones compared with the non-infarcted contralateral hemisphere and corresponding areas of stroke-prone spontaneously hypertensive rats on normal sodium or Wistar-Kyoto rats. Furthermore, microvessel density was positively correlated with the number of matrix metalloproteinase-9-positive cells and matrix metalloproteinase-9-positive blood vessels using linear correlation analysis, which suggests that matrix metalloproteinase-9 upregulation is associated with regional angiogenesis in the stroke-prone spontaneously hypertensive rat model of brain infarction. This is supported by the results of Li et al. (2010), which showed that vessel density and matrix metalloproteinase-9 protein levels were augmented in the Goto-Kakizaki rat model of type 2 diabetes, and further demonstrated that matrix metalloproteinase-9 augments angiogenesis at the capillary level in the stroke-prone spontaneously hypertensive rat model of brain infarction.
Taken together, our findings demonstrate a relationship between matrix metalloproteinase-9 upregulation, a reduction in continuous collagen IV labeling of microvessels and increased microvessel density in cerebral infarcts in stroke-prone spontaneously hypertensive rats given high-sodium (1.3%) drinking water. Furthermore, this study suggests that high-sodium water induces focal brain infarction in stroke-prone spontaneously hypertensive rats. Our findings suggest that matrix metalloproteinase-9 may play a key role in accelerating regional angiogenesis and degrading collagen IV.
Footnotes
Conflicts of interest: None declared.
Funding: This work was supported by the China Medical Board Project, No. 82-143.
Copyedited by Patel B, Yu J, Qiu Y, Li CH, Song LP, Zhao M
References
- 1.Alam M, Shuaib A. Complexity in differentiating the expression of truncated or matured forms of MMP-2 and MMP-9 through zymography in rat brain tissues after acute ischaemic stroke. J Neurosci Methods. 2013;216:22–27. doi: 10.1016/j.jneumeth.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 2.Alam M, Mohammad A, Rahman S, Todd K, Shuaib A. Hyperthermia up-regulates matrix metalloproteinases and accelerates basement membrane degradation in experimental stroke. Neurosci Lett. 2011;495:135–139. doi: 10.1016/j.neulet.2011.03.056. [DOI] [PubMed] [Google Scholar]
- 3.Aoki T, Sumii T, Mori T, Wang X, Lo EH. Blood-brain barrier disruption and matrix metalloproteinase-9 expression during reperfusion injury mechanical versus embolic focal ischemia in spontaneously hypertensive rats. Stroke. 2002;33:2711–2717. doi: 10.1161/01.str.0000033932.34467.97. [DOI] [PubMed] [Google Scholar]
- 4.Asahi M, Wang X, Mori T, Sumii T, Jung JC, Moskowitz MA, Fini ME, Lo EH. Effects of matrix metalloproteinase-9 gene knock-out on the proteolysis of blood-brain barrier and white matter components after cerebral ischemia. J Neurosci. 2001;21:7724–7732. doi: 10.1523/JNEUROSCI.21-19-07724.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bailey EL, McCulloch J, Sudlow C, Wardlaw JM. Potential animal models of lacunar stroke a systematic review. Stroke. 2009;40:e451–458. doi: 10.1161/STROKEAHA.108.528430. [DOI] [PubMed] [Google Scholar]
- 6.Bernas MJ, Cardoso FL, Daley SK, Weinand ME, Campos AR, Ferreira AJG, Hoying JB, Witte MH, Brites D, Persidsky Y, Ramirez SH, Brito MA. Establishment of primary cultures of human brain microvascular endothelial cells to provide an in vitro cellular model of the blood-brain barrier. Nat Protoc. 2010;5:1265–1272. doi: 10.1038/nprot.2010.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bosomtwi A, Jiang Q, Ding GL, Zhang L, Zhang ZG, Lu M, Ewing JR, Chopp M. Quantitative evaluation of microvascular density after stroke in rats using MRI. J Cereb Blood Flow Metab. 2008;28:1978–1987. doi: 10.1038/jcbfm.2008.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cho TM, Peng N, Clark JT, Novak L, Roysommuti S, Prasain J, Wyss JM. Genistein attenuates the hypertensive effects of dietary NaCl in hypertensive male rats. Endocrinology. 2007;148:5396–5402. doi: 10.1210/en.2007-0245. [DOI] [PubMed] [Google Scholar]
- 9.Chopp M, Zhang ZG, Jiang Q. Neurogenesis, angiogenesis, and MRI indices of functional recovery from stroke. Stroke. 2007;38:827–831. doi: 10.1161/01.STR.0000250235.80253.e9. [DOI] [PubMed] [Google Scholar]
- 10.Dirnagl U. Pathobiology of injury after stroke: the neurovascular unit and beyond. Ann N Y Acad Sci. 2012;1268:21–25. doi: 10.1111/j.1749-6632.2012.06691.x. [DOI] [PubMed] [Google Scholar]
- 11.Espinera AR, Ogle ME, Gu X, Wei L. Citalopram enhances neurovascular regeneration and sensorimotor functional recovery after ischemic stroke in mice. Neuroscience. 2013;247:1–11. doi: 10.1016/j.neuroscience.2013.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feiler S, Plesnila N, Thal SC, Zausinger S, Schöller K. Contribution of matrix metalloproteinase-9 to cerebral edema and functional outcome following experimental subarachnoid hemorrhage. Cerebrovasc Dis. 2011;32:289–295. doi: 10.1159/000328248. [DOI] [PubMed] [Google Scholar]
- 13.Fukuda S, Fini CA, Mabuchi T, Koziol JA, Eggleston LL, del Zoppo GJ. Focal cerebral ischemia induces active proteases that degrade microvascular matrix. Stroke. 2004;35:998–1004. doi: 10.1161/01.STR.0000119383.76447.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gidday JM, Gasche YG, Copin JC, Shah AR, Perez RS, Shapiro SD, Chan PH, Park TS. Leukocyte-derived matrix metalloproteinase-9 mediates blood-brain barrier breakdown and is proinflammatory after transient focal cerebral ischemia. Am J Physiol Heart Circ Physiol. 2005;289:H558–568. doi: 10.1152/ajpheart.01275.2004. [DOI] [PubMed] [Google Scholar]
- 15.Goldstein LB, Bushnell CD, Adams RJ, Appel LJ, Braun LT, Chaturvedi S, Creager MA, Culebras A, Eckel RH, Hart RG, Hinchey JA, Howard VJ, Jauch EC, Levine SR, Meschia JF, Moore WS, Nixon JV, Pearson TA. Guidelines for the Primary Prevention of Stroke A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2011;42:517–584. doi: 10.1161/STR.0b013e3181fcb238. [DOI] [PubMed] [Google Scholar]
- 16.Graham CA, Chan RW, Chan D, Chan CP, Wong LK, Rainer TH. Matrix metalloproteinase 9 mRNA: an early prognostic marker for patients with acute stroke. Clin Biochem. 2012;45:352–355. doi: 10.1016/j.clinbiochem.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 17.Hossmann KA. The two pathophysiologies of focal brain ischemia: implications for translational stroke research. J Cereb Blood Flow Metab. 2012;32:1310–1316. doi: 10.1038/jcbfm.2011.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iadecola C, Anrather J. Stroke research at a crossroad: asking the brain for directions. Nat Neurosci. 2011;14:1363–1368. doi: 10.1038/nn.2953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kamada H, Yu F, Nito C, Chan PH. Influence of hyperglycemia on oxidative stress and matrix metalloproteinase-9 activation after focal cerebral ischemia/reperfusion in rats relation to blood-brain barrier dysfunction. Stroke. 2007;38:1044–1049. doi: 10.1161/01.STR.0000258041.75739.cb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kelly MA, Shuaib A, Todd KG. Matrix metalloproteinase activation and blood-brain barrier breakdown following thrombolysis. Exp Neurol. 2006;200:38–49. doi: 10.1016/j.expneurol.2006.01.032. [DOI] [PubMed] [Google Scholar]
- 21.Kono S, Deguchi K, Omote Y, Yunoki T, Yamashita T, Kurata T, Ikeda Y, Abe K. Reducing hemorrhagic complication by dabigatran via neurovascular protection after recanalization with tissue plasminogen activator in ischemic stroke of rat. J Neurosci Res. 2014;92:46–53. doi: 10.1002/jnr.23302. [DOI] [PubMed] [Google Scholar]
- 22.Kumari R, Willing LB, Patel SD, Baskerville KA, Simpson IA. Increased cerebral matrix metalloprotease-9 activity is associated with compromised recovery in the diabetic db/db mouse following a stroke. J Neurochem. 2011;119:1029–1040. doi: 10.1111/j.1471-4159.2011.07487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kvetnaflsky R, Sun CL, Torda T. Plasma epinephrine and norepinephrine levels in stressed rats-effect of adrenalectomy. Pharmacologist. 1977;19:241–247. [Google Scholar]
- 24.Lee CZ, Xu B, Hashimoto T, McCulloch CE, Yang G-Y, Young WL. Doxycycline suppresses cerebral matrix metalloproteinase-9 and angiogenesis induced by focal hyperstimulation of vascular endothelial growth factor in a mouse model. Stroke. 2004;35:1715–1719. doi: 10.1161/01.STR.0000129334.05181.b6. [DOI] [PubMed] [Google Scholar]
- 25.Lee KY, Bae ON, Serfozo K, Hejabian S, Moussa A, Reeves M, Rumbeiha W, Fitzgerald SD, Stein G, Baek SH, Goudreau J, Kassab M, Majid A. Asiatic acid attenuates infarct volume, mitochondrial dysfunction, and matrix metalloproteinase-9 induction after focal cerebral ischemia. Stroke. 2012;43:1632–1638. doi: 10.1161/STROKEAHA.111.639427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li W, Prakash R, Kelly-Cobbs AI, Ogbi S, Kozak A, El-Remessy AB, Schreihofer DA, Fagan SC, Ergul A. Adaptive cerebral neovascularization in a model of type 2 diabetes relevance to focal cerebral ischemia. Diabetes. 2010;59:228–235. doi: 10.2337/db09-0902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu K, Li Z, Wu T, Ding S. Role of rho kinase in microvascular damage following cerebral ischemia reperfusion in rats. Int J Mol Sci. 2011;12:1222–1231. doi: 10.3390/ijms12021222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu XR, Luo M, Yan F, Zhang CC, Li SJ, Zhao HP, Ji XM, Luo YM. Ischemic postconditioning diminishes matrix metalloproteinase 9 expression and attenuates loss of the extracellular matrix proteins in rats following middle cerebral artery occlusion and reperfusion. CNS Neurosci Ther. 2012;18:855–863. doi: 10.1111/j.1755-5949.2012.00366.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu XS, Zhang ZG, Zhang L, Morris DC, Kapke A, Lu M, Chopp M. Atorvastatin downregulates tissue plasminogen activator-aggravated genes mediating coagulation and vascular permeability in single cerebral endothelial cells captured by laser microdissection. J Cereb Blood Flow Metab. 2006;26:787–796. doi: 10.1038/sj.jcbfm.9600227. [DOI] [PubMed] [Google Scholar]
- 30.Luo J, Tang T, Wu J, Zhou HJ, Wu J. Effect of method of supplementing Qi and activating blood circulation on the expression of type IV collagen in brains of intracerebral hemorrhage rats. Zhongguo Laonian Xue Zazhi. 2011;31:2882–2884. [Google Scholar]
- 31.Maier CM, Hsieh L, Yu F, Bracci P, Chan PH. Matrix metalloproteinase-9 and myeloperoxidase expression: quantitative analysis by antigen immunohistochemistry in a model of transient focal cerebral ischemia. Stroke. 2004;35:1169–1174. doi: 10.1161/01.STR.0000125861.55804.f2. [DOI] [PubMed] [Google Scholar]
- 32.Mira E, Lacalle RA, Buesa JM, de Buitrago GG, Jiménez-Baranda S, Gómez-Moutón C, Martínez-A C, Mañes S. Secreted MMP9 promotes angiogenesis more efficiently than constitutive active MMP9 bound to the tumor cell surface. J Cell Sci. 2004;117:1847–1857. doi: 10.1242/jcs.01035. [DOI] [PubMed] [Google Scholar]
- 33.Morancho A, Hernández-Guillamon M, Boada C, Barceló V, Giralt D, Ortega L, Montaner J, Rosell A. Cerebral ischaemia and matrix metalloproteinase-9 modulate the angiogenic function of early and late outgrowth endothelial progenitor cells. J Cell Mol Med. 2013;17:1543–1553. doi: 10.1111/jcmm.12116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Omote Y, Deguchi K, Kono S, Liu N, Liu W, Kurata T, Yamashita T, Ikeda Y, Abe K. Neurovascular protection of cilostazol in stroke-prone spontaneous hypertensive rats associated with angiogenesis and pericyte proliferation. J Neurosci Res. 2014;92:369–374. doi: 10.1002/jnr.23327. [DOI] [PubMed] [Google Scholar]
- 35.Potente M, Gerhardt H, Carmeliet P. Basic and therapeutic aspects of angiogenesis. Cell. 2011;146:873–887. doi: 10.1016/j.cell.2011.08.039. [DOI] [PubMed] [Google Scholar]
- 36.Qureshi AI. Acute hypertensive response in patients with stroke pathophysiology and management. Circulation. 2008;118:176–187. doi: 10.1161/CIRCULATIONAHA.107.723874. [DOI] [PubMed] [Google Scholar]
- 37.Ralay Ranaivo H, Hodge JN, Choi N, Wainwright MS. Albumin induces upregulation of matrix metalloproteinase-9 in astrocytes via MAPK and reactive oxygen species-dependent pathways. J Neuroinflammation. 2012;9:1–12. doi: 10.1186/1742-2094-9-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reitmeir R, Kilic E, Reinboth BS, Guo Z, ElAli A, Zechariah A, Kilic Ü, Hermann DM. Vascular endothelial growth factor induces contralesional corticobulbar plasticity and functional neurological recovery in the ischemic brain. Acta Neuropathol. 2012;123:273–284. doi: 10.1007/s00401-011-0914-z. [DOI] [PubMed] [Google Scholar]
- 39.Ritz MF, Fluri F, Engelter ST, Schaeren-Wiemers N, Lyrer PA. Cortical and putamen age-related changes in the microvessel density and astrocyte deficiency in spontaneously hypertensive and stroke-prone spontaneously hypertensive rats. Curr Neurovasc Res. 2009;6:279–287. doi: 10.2174/156720209789630311. [DOI] [PubMed] [Google Scholar]
- 40.Rosell A, Cuadrado E, Ortega-Aznar A, Hernández-Guillamon M, Lo EH, Montaner J. MMP-9-positive neutrophil infiltration is associated to blood-brain barrier breakdown and basal lamina type IV collagen degradation during hemorrhagic transformation after human ischemic stroke. Stroke. 2008;39:1121–1126. doi: 10.1161/STROKEAHA.107.500868. [DOI] [PubMed] [Google Scholar]
- 41.Rosell A, Ortega-Aznar A, Alvarez-Sabín J, Fernández-Cadenas I, Ribó M, Molina CA, Lo EH, Montaner J. Increased brain expression of matrix metalloproteinase-9 after ischemic and hemorrhagic human stroke. Stroke. 2006;37:1399–1406. doi: 10.1161/01.STR.0000223001.06264.af. [DOI] [PubMed] [Google Scholar]
- 42.Russell KL, Berman NE, Gregg PR, Levant B. Fish oil improves motor function, limits blood-rain barrier disruption, and reduces Mmp9 gene expression in a rat model of juvenile traumatic brain injury. Prostaglandins Leukot Essent Fatty Acids. 2014;90:5–11. doi: 10.1016/j.plefa.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shichi K, Fujita-Hamabe W, Harada S, Mizoguchi H, Yamada K, Nabeshima T, Tokuyama S. Involvement of matrix metalloproteinase-mediated proteolysis of neural cell adhesion molecule in the development of cerebral ischemic neuronal damage. J Pharmacol Exp Ther. 2011;338:701–710. doi: 10.1124/jpet.110.178079. [DOI] [PubMed] [Google Scholar]
- 44.Sun T, Liu R, Cao YX. Vasorelaxant and antihypertensive effects of formononetin through endothelium-dependent and -independent mechanisms. Acta Pharmacol Sin. 2011;32:1009–1018. doi: 10.1038/aps.2011.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Suofu Y, Clark JF, Broderick JP, Kurosawa Y, Wagner KR, Lu A. Matrix metalloproteinase-2 or -9 deletions protect against hemorrhagic transformation during early stage of cerebral ischemia and reperfusion. Neuroscience. 2012;212:180–189. doi: 10.1016/j.neuroscience.2012.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Teng H, Zhang ZG, Wang L, Zhang RL, Zhang L, Morris D, Gregg SR, Wu Z, Jiang A, Lu M, Zlokovic BV, Chopp M. Coupling of angiogenesis and neurogenesis in cultured endothelial cells and neural progenitor cells after stroke. J Cereb Blood Flow Metab. 2008;28:764–771. doi: 10.1038/sj.jcbfm.9600573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thoene-Reineke C, Rumschüssel K, Schmerbach K, Krikov M, Wengenmayer C, Godes M, Mueller S, Villringer A, Steckelings U, Namsolleck P, Unger T. Prevention and intervention studies with telmisartan, ramipril and their combination in different rat stroke models. PLoS One. 2011;6:e23646. doi: 10.1371/journal.pone.0023646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang Z, Leng Y, Tsai LK, Leeds P, Chuang DM. Valproic acid attenuates blood-brain barrier disruption in a rat model of transient focal cerebral ischemia: the roles of HDAC and MMP-9 inhibition. J Cereb Blood Flow Metab. 2011;31:52–57. doi: 10.1038/jcbfm.2010.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wu Y, Wang L, Dai C, Ma G, Zhang Y, Zhang X, Wu Z. Neuroprotection by platelet-activating factor acetylhydrolase in a mouse model of transient cerebral ischemia. Neurosci Lett. 2014;558:26–30. doi: 10.1016/j.neulet.2013.09.005. [DOI] [PubMed] [Google Scholar]
- 50.Wu Y, Wang YP, Guo P, Ye XH, Wang J, Yuan SY, Yao SL, Shang Y. A lipoxin A4 analog ameliorates blood-brain barrier dysfunction and reduces MMP-9 expression in a rat model of focal cerebral ischemia-reperfusion injury. J Mol Neurosci. 2012;46:483–491. doi: 10.1007/s12031-011-9620-5. [DOI] [PubMed] [Google Scholar]
- 51.Xiang J, Lan R, Tang YP, Chen YP, Cai DF. Apocynum venetum leaf extract attenuates disruption of the blood-brain barrier and upregulation of matrix metalloproteinase-9/-2 in a rat model of cerebral ischemia-reperfusion injury. Neurochem Res. 2012;37:1820–1828. doi: 10.1007/s11064-012-0796-z. [DOI] [PubMed] [Google Scholar]
- 52.Xiong Y, Hu Z, Han X, Jiang B, Zhang R, Zhang X, Lu Y, Geng C, Li W, He Y, Huo Y, Shibuya M, Luo J. Hypertensive stretch regulates endothelial exocytosis of Weibel-Palade bodies through VEGF receptor 2 signaling pathways. Cell Res. 2013;23:820–834. doi: 10.1038/cr.2013.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yang Y, Thompson JF, Taheri S, Salayandia VM, McAvoy TA, Hill JW, Yang Y, Estrada EY, Rosenberg GA. Early inhibition of MMP activity in ischemic rat brain promotes expression of tight junction proteins and angiogenesis during recovery. J Cereb Blood Flow Metab. 2013;33:1104–1114. doi: 10.1038/jcbfm.2013.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yenari MA, Han HS. Neuroprotective mechanisms of hypothermia in brain ischaemia. Nat Rev Neurosci. 2012;13:267–278. doi: 10.1038/nrn3174. [DOI] [PubMed] [Google Scholar]
- 55.Yousuf S, Sayeed I, Atif F, Tang H, Wang J, Stein DG. Delayed progesterone treatment reduces brain infarction and improves functional outcomes after ischemic stroke: a time-window study in middle-aged rats. J Cereb Blood Flow Metab. 2014;34:297–306. doi: 10.1038/jcbfm.2013.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zechariah A, ElAli A, Hagemann N, Jin F, Doeppner TR, Helfrich I, Mies G, Hermann DM. Hyperlipidemia attenuates vascular endothelial growth factor–induced angiogenesis, impairs cerebral blood flow, and disturbs stroke recovery via decreased pericyte coverage of brain endothelial cells. Arterioscler Thromb Vasc Biol. 2013;33:1561–1567. doi: 10.1161/ATVBAHA.112.300749. [DOI] [PubMed] [Google Scholar]
- 57.Zhang P, Yu H, Zhou N, Zhang J, Wu Y, Zhang Y, Bai Y, Jia J, Zhang Q, Tian S, Wu J, Hu Y. Early exercise improves cerebral blood flow through increased angiogenesis in experimental stroke rat model. J Neuroeng Rehabil. 2013;10:43. doi: 10.1186/1743-0003-10-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhao BQ, Tejima E, Lo EH. Neurovascular proteases in brain injury hemorrhage and remodeling after stroke. Stroke. 2007;38:748–752. doi: 10.1161/01.STR.0000253500.32979.d1. [DOI] [PubMed] [Google Scholar]


