Keywords: neural regeneration, neuroimaging, MRI, post-traumatic stress disorder, voxel-based morphometry, pre-frontal lobe, parietal lobe, occipital lobe, follow-ups, grants-supported paper, neuroregeneration
Abstract
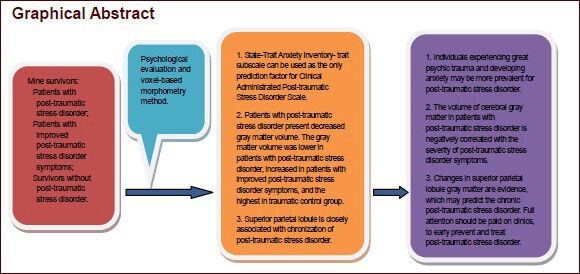
This study compared the difference in brain structure in 12 mine disaster survivors with chronic post-traumatic stress disorder, 7 cases of improved post-traumatic stress disorder symptoms, and 14 controls who experienced the same mine disaster but did not suffer post-traumatic stress disorder, using the voxel-based morphometry method. The correlation between differences in brain structure and post-traumatic stress disorder symptoms was also investigated. Results showed that the gray matter volume was the highest in the trauma control group, followed by the symptoms-improved group, and the lowest in the chronic post-traumatic stress disorder group. Compared with the symptoms-improved group, the gray matter volume in the lingual gyrus of the right occipital lobe was reduced in the chronic post-traumatic stress disorder group. Compared with the trauma control group, the gray matter volume in the right middle occipital gyrus and left middle frontal gyrus was reduced in the symptoms-improved group. Compared with the trauma control group, the gray matter volume in the left superior parietal lobule and right superior frontal gyrus was reduced in the chronic post-traumatic stress disorder group. The gray matter volume in the left superior parietal lobule was significantly positively correlated with the State-Trait Anxiety Inventory subscale score in the symptoms-improved group and chronic post-traumatic stress disorder group (r = 0.477, P = 0.039). Our findings indicate that (1) chronic post-traumatic stress disorder patients have gray matter structural damage in the prefrontal lobe, occipital lobe, and parietal lobe, (2) after post-traumatic stress, the disorder symptoms are improved and gray matter structural damage is reduced, but cannot recover to the trauma-control level, and (3) the superior parietal lobule is possibly associated with chronic post-traumatic stress disorder. Post-traumatic stress disorder patients exhibit gray matter abnormalities.
INTRODUCTION
What kinds of psychological trauma occur when humans experience huge disasters? Is the psychological trauma associated with changes in brain structure? Can brain structural damage alter as symptoms improve? Which neuroimaging technology can assist the diagnosis?
Wars, earthquakes, major traffic accidents, and terrorist attacks may bring about profound spiritual pains, and even cause extreme fear and helplessness for people that have experienced or witnessed these unusual threats or disasters. This persistent and constant mental disorder caused by psychological trauma is termed post-traumatic stress disorder (PTSD). PTSD is characterized by re-experiencing, avoidance, and high vigilance symptoms[1,2]. There is increasing interest in PTSD due to its high incidence and serious consequences. In the United States, the PTSD lifetime prevalence rate is 7.8%, with females showing higher prevalence (10.4%) than males (5%)[3]. PTSD may lead to severe social dysfunction, and at least 1/3 of affected patients lose their working and living ability[4]. PTSD patients are at high risk for suicide[5], which is six times higher than the normal population[6]. Therefore, the mechanisms underlying PTSD are a hot topic in psychiatry research.
The advent of brain imaging technology raises the possibility of studying the brain mechanisms of PTSD in vivo. Currently, brain imaging studies can be roughly divided into functional imaging and structural imaging[7]. Existing studies examining PTSD brain structures have focused on the hippocampus, prefrontal cortex, amygdala, temporal lobe, anterior cingulate cortex, and other brain areas[8]. Using magnetic resonance technology, Bremner et al[9] found that the right hippocampus volume decreased by 8% in Vietnam veterans with PTSD, which was correlated with deficits in verbal declarative memory. Patients with PTSD after a motorcycle accident were also reported to have decreased thickness of the triangular area in the left medial frontal lobe and left inferior frontal gyrus, and reduced thickness in the right superior temporal gyrus cortex[10]. Furthermore, compared with normal miners, PTSD patients who experienced a mine disaster presented cortical thinning in the right inferior temporal gyrus, and the cortical thickness was negatively correlated with Clinical Administrated PTSD Scale scores[11]. The gray matter volume in the bilateral superior and interior frontal lobe, as well as the right anterior cingulate, in PTSD patients had a negative correlation with Clinical Administrate PTSD Scale scores[12]. Lyoo et al[13] also found that PTSD rehabilitee patients exhibited increased dorsolateral prefrontal cortical thickness at 1.42 years after a subway accident in South Korea, and cortical thickness was highly correlated with the decrease of PTSD symptoms. Diffusion tensor imaging, another method for assessing brain structure, also revealed that the fractional anisotropy value of the left superior frontal gyrus was higher in PTSD patients than healthy controls[14]. Furthermore, in PTSD patients who had experienced the Vietnam and Persian Gulf wars, the size and thickness of the parahippocampal gyrus, superior temporal cortex, lateral orbitofrontal cortex, and inferior frontal orbital cortex were reduced[15]. Additionally, the bilateral superior, bilateral middle and left inferior frontal gyrus, and left superior temporal gyrus were thinner[16]. Rauch et al[17] further demonstrated that PTSD female patients had a significantly lower anterior cingulate cortex volume than female controls who experienced trauma but did not suffer from PTSD. Similar findings have been reported in other studies[18,19]. Overall, these data indicate that PTSD brain structure results are not consistent, although a recent meta-analysis of PTSD whole-brain structure supports that the PTSD is highly correlated with the reduction of gray matter volume in the anterior cingulate, prefrontal cortex, left temporal pole/middle temporal gyrus, and left hippocampus[20]. Therefore, brain structural damage is definitely detectable in PTSD patients.
The imaging performance of PTSD patients has been frequently reported during follow-ups[21,22,23]. Hakamata et al[22] performed a longitudinal follow-up study on changes in brain structure of cancer-related PTSD patients, and reported that the right orbitofrontal cortex gray matter volume in patients was significantly lower than the trauma control and healthy control groups, while the PTSD diagnosis had no interaction with the determination time of right orbitofrontal cortex gray matter volume. As cancer-related PTSD progresses, it is hypothesized that orbitofrontal cortex gray matter volume remains unchanged. De Bellis et al[21] found no changes in temporal lobe, amygdala, or hippocampus volume between adolescent children with abuse-caused PTSD and healthy controls during a 2-year follow-up; these negative results may be explained by the small sample size. The hippocampal volume of a PTSD candidate was also reported to remain unchanged at six months after trauma[23]. As PTSD progresses, brain structure inevitably changes, however, no PTSD symptoms have been clearly described in previous PTSD imaging studies.
Understanding the brain areas highly involved in the improvement of PTSD symptoms will assist the judgment of PTSD patient prognosis. Growing evidence shows that PTSD clinical symptoms were improved after treatment[24,25]. For example, after 6–9 months of psychotherapy, the right subgenual anterior cingulate cortex thickness can predict the degree of PTSD symptom improvement[24]. When memory tasks are performed, the activity of the hippocampus and subgenual anterior cingulate cortex reflects the improvement of PTSD symptoms, suggesting that the right subgenual anterior cingulate cortex is a neuroimaging indicator for PTSD symptom improvement[25]. Although the aforementioned studies have attempted to explore the potential neuroimaging mechanisms of PTSD rehabilitation, they cannot eliminate the impacts of treatment (medication and/or psychotherapy) on brain structure/function. Only one study[26] has examined the brain structure changes of PTSD patients during its natural course (without any treatment), with a 2-year follow-up on 25 soldiers with PTSD and 22 controls. In that study, the degree of brain atrophy in PTSD patients was similar to controls. Furthermore, PTSD patients were divided into subgroups of improved symptoms and decreased symptoms, with only a small portion of the left parietal lobe in the symptoms-decreased group exhibiting loss of more nerve fibers than the control group, while whole brain atrophy in the symptoms-improved group was significantly more prevalent compared with the control group, especially in the brain stem and frontal and temporal lobe[26].
Voxel-based morphometry is a potential method to investigate the brain structure factors leading to chronic PTSD. With the development of MRI technology and computer image processing, the use of voxel in MRI analysis of brain structure was first proposed in 1995[27,28], which can detect differences in brain gray matter and white matter on MRI scanning. In 2000, Ashburner and Friston[29] formally introduced the concept of voxel-based morphometry, a quantitative method to compare the volume of brain areas through calculating gray matter, white matter, and cerebrospinal fluid, thus accurately displaying morphological changes of brain tissue.
Thus, in the present study, we proposed the hypothesis that brain structural damage in the symptoms-improved group lies between the PTSD patient group and the trauma control group. To verify this hypothesis, we investigated the brain structure of mine disaster survivors in the Hunan Province of China with PTSD symptoms-improved or persistent within the recent two years, and mine disaster survivors without PTSD, using the voxel-based morphometry method.
RESULTS
Quantitative analysis of participants
Eighteen patients with chronic PTSD were included in this study. Three cases were excluded on survey according to the exclusion criteria and seven cases were transferred to symptoms-improved group. Thus, five PTSD patients were included. Additionally one chronic PTSD patient was excluded due to imaging data mirror caused by head movement. Finally, 12 cases in the patient group and 7 cases in the symptoms-improved group were included in our analyses. Fourteen miners who also experienced the same trauma, but did not suffer from PTSD, were included in the trauma control group.
Demographic and psychological assessment
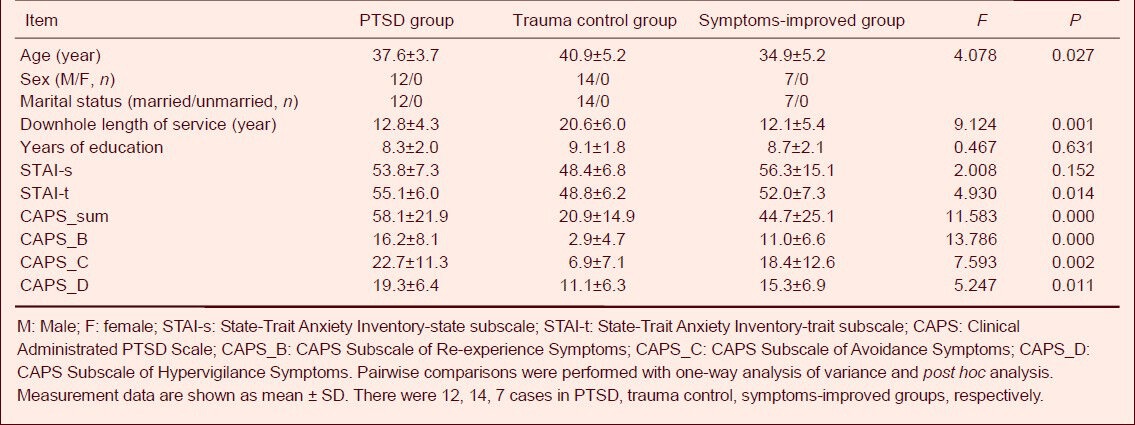
The baseline data and psychological assessment results of all subjects in three groups are shown in Table 1.
Table 1.
General conditions and psychological assessment results of the post-traumatic stress disorder (PTSD) group, trauma control group, and symptoms-improved group.
A logistic regression analysis was conducted taking Clinical Administrated PTSD Scale (CAPS) total score as the independent variable, and the age, downhole length of service, years of education, STAI-s, and STAI-t as the dependent variables. STAI-t was introduced into the regression equation (R = 0.45, R2 = 0.216, adjusted R2 = 0.191) and regression model analysis of variance (F = 8.562, P = 0.006). The STAI-t is regarded as the only predictive factor for CAPS total scores.
Radiological changes of PTSD brain structures
The gray matter volume in the right occipital lingual gyrus (Brodmann 18 area) of PTSD patients was decreased compared with the symptoms-improved group, and no brain areas showed a larger gray matter volume than the symptoms-improved group. The gray matter volume in the left superior parietal lobule (Brodmann 7 area) and the right superior frontal gyrus (Brodmann 8 area) of PTSD patients was decreased compared with the trauma control group, while no brain areas showed larger gray matter volumes than the trauma control group. The gray matter volume in the right middle occipital gyrus (Brodmann 19 area) and left middle frontal gyrus (Brodmann 11 area) of the symptoms-improved group was also reduced compared with trauma control group, and no brain areas showed larger gray matter volumes than the trauma control group (Table 2, Figure 1). The gray matter volume was the smallest in PTSD patients, followed by the symptoms-improved group, but was largest in the trauma control group.
Table 2.
Comparison of gray matter volume change in brain areas
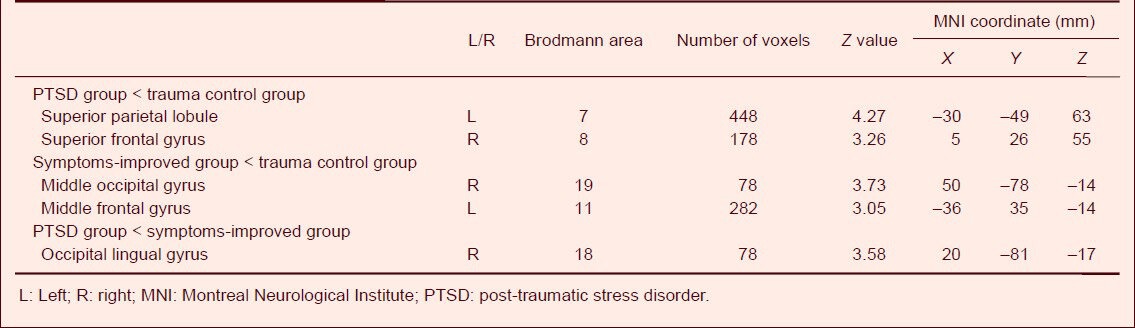
Figure 1.
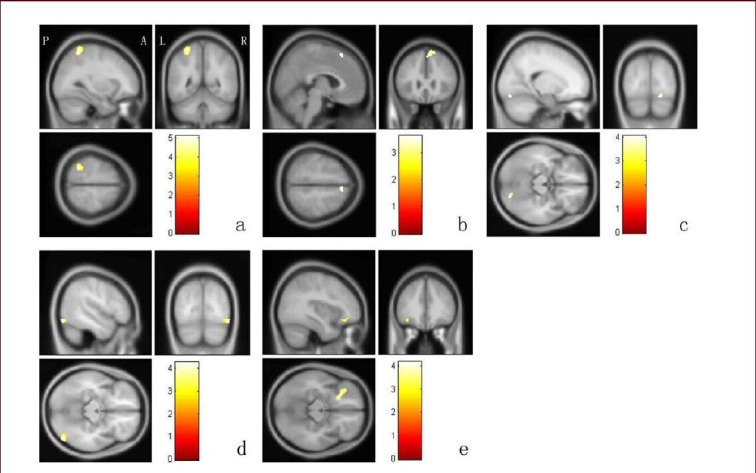
Comparison of gray matter volume in brain areas using voxel-based morphometry analysis.
(a) The left superior parietal lobule (post-traumatic stress disorder patients group < trauma control group); (b) the right superior frontal gyrus (post-traumatic stress disorder patients group < trauma control group); (c) the right lingual gyrus (post-traumatic stress disorder patients group < symptoms-improved group); (d) the right middle occipital gyrus (symptoms-improved group < trauma control group); (e) the left middle frontal gyrus (symptoms-improved group < trauma control group). A: Anterior; P: posterior; L: left; R: right. Data were analyzed using one-way analysis of covariance and adjusted with Alphasim. The color bar in each image represents the T value, and the higher T values indicate greater differences.
Correlation between brain structural damage and psychological assessment indicators
There was no correlation in the left superior parietal lobule, the right superior frontal gyrus, and the right lingual gyrus with the scale scores (CAPS total scores and STAI-s, STAI-t subscale scores) in the PTSD patients (Table 3). In the trauma control group, the gray matter volume in the right middle occipital gyrus was negatively correlated with STAI-t scores (r = –0.669, P = 0.009; Table 3, Figure 2); the left superior parietal lobule, the right superior frontal gyrus, and the left middle frontal gyrus had no correlation with the scale scores (CAPS total score and STAI-s, STAI-t subscale scores) (Table 3).
Table 3.
Correlation between gray matter volume changes and psychological assessment in post-traumatic stress disorder (PTSD) patients
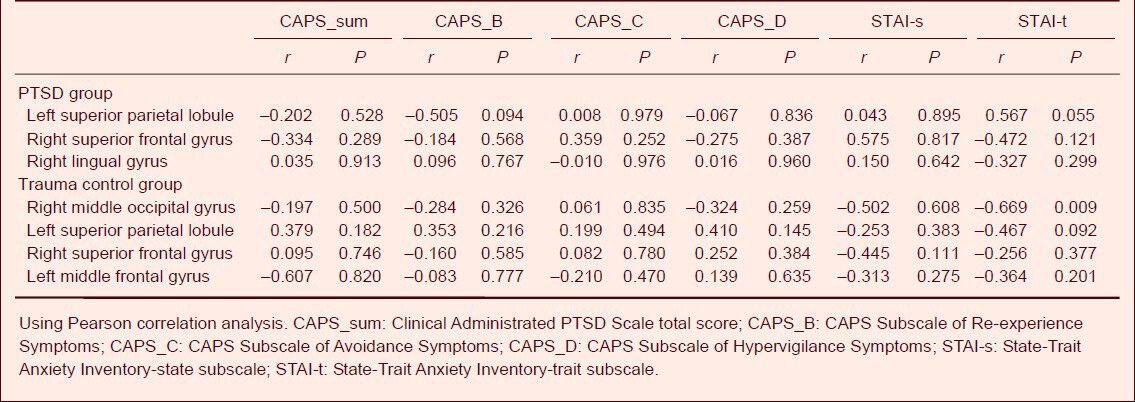
Figure 2.
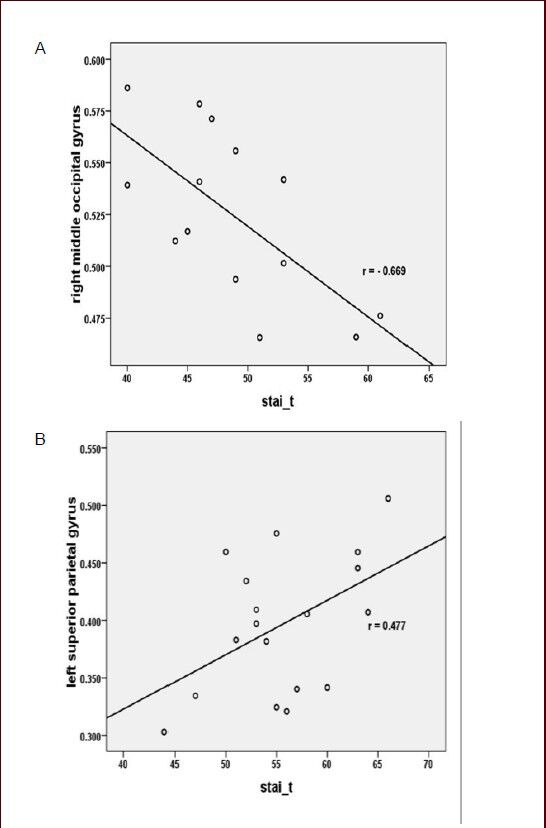
Scatter plot for correlation analysis between gray matter volume changes and psychological assessment in post-traumatic stress disorder (PTSD) patients.
(A) In the trauma control group, the right middle occipital gyrus gray matter volume was negatively correlated with State-Trait Anxiety Inventory-trait subscale (STAI-t) scores (r = –0.669, P = 0.009). (B) While the PTSD patients group and the symptoms-improved group were joined into the PTSD symptoms group, the left superior parietal lobule gray matter volume was positively correlated with STAI-t (r = 0.477, P = 0.039).
In the symptoms-improved group, the right lingual gyrus, the right middle occipital gyrus, and the left middle frontal gyrus were not correlated with the scale scores (CAPS total score and STAI-s, STAI-t subscale scores; Table 3). While the patients group and the symptoms-improved group were joined into the PTSD symptoms group for correlation analysis between brain areas and psychological assessment, and the left superior parietal lobule gray matter volume was positively correlated with STAI-t (r = 0.477, P = 0.039; Figure 2, Table 3).
DISCUSSION
PTSD is accompanied by advanced neurological impairment and abnormalities in cerebral blood flow and metabolism[7]. PTSD patients present brain gray matter and white matter abnormalities, which are the basis for functional abnormalities. Using the voxel-based morphometry method, MRI examination found that the gray matter volume in the superior parietal lobule, frontal lobe, and occipital lobe of PTSD patients was reduced, suggesting that gray matter abnormalities are present in PTSD patients.
Previous structural imaging studies found that the volumes of the bilateral orbitofrontal cortex, left rostral middle frontal lobe[30], and the prefrontal cortex, hippocampus, and lingual gyrus volume were decreased[31] in adult PTSD patients compared with a trauma control group. Chronic PTSD patients present thinner prefrontal cortex[32] and a decline in the prefrontal lobe volume, including the orbitofrontal cortex, compared with normal people[33]. The whole brain volume and the left ventral prefrontal volume were also markedly decreased in PTSD symptoms group compared with a normal control group[34]. Veterans with PTSD show a reduced volume in the left occipital lobe gray matter compared with normal veterans, and the volume was negatively correlated with the severity of PTSD symptoms[35]. As for untreated PTSD patients who were all drug-naive, had experienced traumas during adulthood, and had a single diagnosis of PTSD without any other current or lifetime diagnosis, whole brain gray matter volume was reduced, especially in the frontal and occipital lobe, compared with normal controls[36]. A reduced volume was also observed in the occipital and frontal lobe of female PTSD patients who suffered from partner violence[37]. Overall, these studies support our experimental findings, although we did not detect changes in hippocampal volume.
The majority of PTSD symptoms may be improved over time, although a small portion of PTSD patients exhibit chronic progression and the symptoms cannot be alleviated[38]. The pathogenesis of chronic PTSD can be explained by a strong response to traumatic events such that these patients cannot recover[39]. Therefore, targeting the rehabilitation of PTSD disease and exploring the neuropathological mechanisms of PTSD rehabilitation are of great clinical significance, which is why we included the PTSD symptoms group.
In the present study, gray matter volume in the trauma control group was higher than that in the symptoms-improved group and the PTSD patients group, while the symptoms-improved group was also higher than the PTSD patients group. As the PTSD symptoms improved, the partial occipital lobe (lingual gyrus) gray matter volume began to increase, while the gray matter volumes in the left frontal lobe and the right middle occipital gyrus were significantly decreased compared with the trauma control group. We speculate that PTSD patients still exhibited abnormal brain structures, despite an improvement in PTSD symptoms, although structural brain damage can be reduced, and was lower than normal levels. Changes in brain structure may be highly involved in the PTSD symptoms. For example, in 15 policemen who experienced trauma, the high re-experiencing scores were associated with smaller volumes in the amygdala, thalamus, hippocampus, and globus pallidus, while the high vigilance and avoidance symptoms were not associated with gray matter volume[40]. Furthermore, the higher scores of re-experience symptoms were attributed to reduced hippocampal volume[41]. In this study, no correlation between gray matter volume and PTSD symptoms was observed, possibly due to our relatively small sample size.
Although our experimental findings did not identify the brain area associated with key PTSD symptoms, we detected a trait anxiety-related brain area, the superior parietal lobule, while trait anxiety in this study was regarded as the only predictor of CAPS total scores. Therefore, the superior parietal lobule was associated with PTSD symptoms. A pairwise comparison among the PTSD patients group, the symptoms-improved group, and the trauma control group revealed that damage to the frontal structures was present in both the PTSD patients group and the symptoms-improved group, while changes in the superior parietal lobule volume was visible in only PTSD patients. Thus, the superior parietal lobule may be related to chronic PTSD.
Cardenas et al[26] found that the parietal lobe was involved in the improvement of PTSD symptoms, although the improvement was not evident. Trait anxiety may act as a personality trait to predict the severity of PTSD symptoms[42], which was consistent with our findings. Some authors have also reported that the superior parietal lobule is associated with the PTSD symptom severity. For example, the volume of the superior parietal lobule was negatively correlated with the flashback symptoms in PTSD[43]. Therefore, we speculate that abnormalities in the prefrontal lobe, occipital lobe, and superior parietal lobule structure may participate in the neural network mechanism for PTSD.
Some limitations of our study should be noted. (1) This study failed to capture brain structural data of subjects on the first survey due to technical difficulty. Thus, a longitudinal comparison of brain structures is missing. (2) The sample size for brain structure research is relatively small. (3) All mine survivors tested were males, so the results should be interpreted with caution.
In summary, structural damage occurs in the prefrontal lobe, occipital lobe, parietal lobule, and other brain areas in patients with PTSD, and the severity of damage may reduce as the PTSD symptoms improve. However, brain structural damage cannot be restored to normal levels in the trauma control group. Further studies with larger sample sizes or more follow-up data are needed to explore the changes in brain structure associated with improvement of PTSD symptoms, thus providing further evidence for the prevention and treatment of chronic PTSD.
SUBJECTS AND METHODS
Design
A case-control study.
Time and setting
Experiments were performed from June 2007 to January 2008 in the Second Xiangya Hospital, Central South University, China.
Subjects
Survivors from a terrible mine accident that occurred at Hunan Province of China in June 2005 were selected for epidemiological and radiological survey in August 2005 and April 2006, respectively[32]. This study reports the results of the third survey.
Inclusion criteria for PTSD patients
(1) PTSD patients met the criteria in the Diagnostic and Statistical Manual of Mental Disorders, Version IV, issued by American Psychiatric Association[33]; (2) aged 18–49 years; (3) males, right-handed; (4) all subjects volunteered to participate in this study, were informed of the experimental objects, methods, processes, possible discomfort and risks, and gave informed consent.
Exclusion criteria for PTSD patients
(1) Patients with or previous history of severe physical or nervous system disease; (2) patients with or previous history of drug abuse and dependence, mental retardation, schizophrenia, bipolar disorder, and other mental illness; (3) first-degree relatives have severe mental or nervous system disease; (4) patients with metal implants.
Inclusion criteria for control group
(1) Cases did not meet the criteria in the Diagnostic and Statistical Manual of Mental Disorders, Version IV, and received no medication within one month prior to survey; (2) aged 18–49 years; (3) males, right-handed; (4) all subjects volunteered to participate in this study, were informed of experimental objects, methods, processes, possible discomfort and risks, and gave informed consent.
Exclusion criteria for control group
(1) Cases with or previous history of severe physical or nervous system disease; (2) cases with or previous history of mental disorder according to the Diagnostic and Statistical Manual of Mental Disorders, Version IV; (3) first-degree relatives have severe mental or nervous system disease; (4) patients with metal implants.
During the first survey in 2005, patients definitely diagnosed with PTSD received amitriptyline (25 mg/tablet, 100 tablets) from our research group as a gift, but they cannot recall the accurate period and time of administering drugs. In the subsequent follow-ups, subjects refused medication. According to the Treatment Guidelines for PTSD Veterans formulated by the United States Department of Veterans Affairs and the Department of Defense, the drug treatment effect of PTSD is very limited. Furthermore, the drug must be given in the maximum dose that can be tolerated, and last for at least 12 weeks. For example, the amitriptyline therapeutic dose ranged from 150–300 mg per day for 12 successive weeks[42]. In the present study, the effect of drug treatment can be ignored after 2-year follow-up if amitriptyline was given at the minimum therapeutic dose (150 mg per day) for 16 days. Therefore, all the subjects involved in this study are considered at the natural course of 2 years.
Methods
Demographic and psychological assessment
General information: name, gender, age, education level, marital status, downhole length of service, underground location when mine disaster occurred, past medical history, personal history, family history, and presence or absence of metal implants and dentures. All information were inquired and filled in by the investigators.
Structural Clinical Interview for the Diagnostic and Statistical Manual of Mental Disorders, Version IV, Axis I Disorders[44]: the screening was performed by two psychiatrists. The tested survivors were included in the imaging data research if they met the diagnostic criteria for PTSD and inclusion criteria.
State-Trait Anxiety Inventory consisted of the Instruction and two subscales[45]. Items 1–20 are State-Trait Anxiety Inventory-states, and are used to assess the fear, tension, anxiety, and neurotic experiences or feelings immediately or within recent period. Items 21–40 are State-Trait Anxiety Inventory-traits, and are used to assess regular emotional experience. The subjects chose the scores based on their own experiences or feelings. The subscale score has a range from 20–80 points, and reflects the severity of state or trait anxiety[35]; higher scores indicate a more severe anxiety condition. The accumulated scores of each subscale in State-Trait Anxiety Inventory were calculated.
The CAPS[36], developed by the U.S. National Research Center for PTSD in 1990, can be used to assess PTSD symptoms severity through interviews and questionnaires[46]. This scale comprises three groups of symptoms: re-experience symptoms, avoidance and numbing symptoms, and high vigilance symptoms. PTSD can be clearly diagnosed upon one item in re-experience symptoms, three items in avoidance and numbing symptoms, and two items in high vigilance symptoms[47]. The CAPS total score ranges from 0–136 points in five levels: 0–19, asymptomatic/few symptoms; 20–39, mild PTSD symptom/minimum (subthreshold); 40–59, moderate PTSD symptom/beginning; 60–79, severe PTSD symptom; > 80, extremely severe PTSD symptom. The higher scores indicate more severe symptoms[47].
Brain imaging assessment
MRI data were collected using Signa 1.5 T TwinSpeed Magnetic Resonance System (GE, Milwaukee, WI, USA) in the MRI Room, Department of Radiology, the Second Xiangya Hospital, Central South University, China. The subjects were forced to maintain their body stationary in the supine position during scanning, and the head was fixed in a foam pad.
Three-dimensional scanning parameters: Images were collected based on the T1-weighted image using a three-dimensional gradient echo imaging sequence. Scanning parameters: repetition time = 12 ms, echo time = 4.2 ms, flip angle 15°, field of view = 24 × 24 cm, matrix = 512 × 512, slice thickness = 1.8 mm, no intervals. Scanning layer: 172 layers, scanning time: 16 minutes.
Three-dimensional data processing: The brain structure data obtained were converted into img format using the MRIconvert software (http://lcni.uoregon.edu/~jolinda/MRIConvert/), and processed using voxel-based morphometry software in SPM8 (Wellcome Trust Center for Neuroimaging, University College London, UK) on Matlab7.6 (R 2008a), to perform data standardization, segmentation, and smoothing. The resulting images in img format were normalized to 1 × 1 × 1 mm voxels, according to the standard template in SPM8 software package (Montreal Neurological Institute, Canada). The images were then divided into segments and each voxel was classified into gray matter, white matter, and cerebrospinal fluid using segmentation algorithm in cluster analysis. Finally, the divided images were subject to space smoothing with full width at half maximum Gaussian filter (8 mm) to improve the signal-to-noise ratio.
Statistical analysis
Data were analyzed using SPSS 13.0 (SPSS, Chicago, IL, USA). Demographic and psychological assessment data among three groups were compared using one-way analysis of variance and post-hoc test. The predictors of the CAPS total scores were analyzed using a logistic regression. Image data comparisons were carried out with a one-way analysis of variance and post-hoc test to compare the difference between two groups. The results of analysis of variance were preserved to restrict two-sample t-test results. The brain areas with differences were subject to Alphasim correction. The brain areas with significant differences were regarded as the region of interest. The signal in the region of interest was extracted using REST software (http://resting-fmri.sourceforge.net), and analyzed by Pearson correlation with psychological assessment data. P < 0.05 was considered statistically significant.
Research background: The published data on the alterations in brain structures in patients with post-traumatic stress disorder over the course of the disease remain contradictory.
Research frontiers: There is no description of the post-traumatic stress disorder symptoms with respect to brain imaging of post-traumatic stress disorder patients. Understanding the brain area that is responsible for the improvement of post-traumatic stress disorder symptoms will guide the prognosis of patients.
Clinical significance: Voxel-based morphometry can be used to analyze brain structural characteristics of post-traumatic stress disorder patients over different periods. We explored the correlation between brain structure and post-traumatic stress disorder pathogenesis to examine the pathophysiological mechanism underlying post-traumatic stress disorder.
Academic terminology: Voxel-based morphometry, a measurement method for brain morphometry in neuropsychiatric diseases, can normalize brain MRI gradient echo T1-weighted images to an identical three-dimensional space, obtain brain structure images with high resolution, high definition and high gray matter-white matter contrast, and measure gray matter, white matter, and cerebrospinal fluid with the voxel as the basic unit. Additionally, each voxel in the structural image is compared to determine the difference between gray matter, white matter, and cerebrospinal fluid.
Peer review: The changes in the morphology of brain areas associated with post-traumatic stress disorder were investigated using the voxel-based morphometry method. This is the first morphological analysis of post-traumatic stress disorder.
Acknowledgments:
We thank all staff from a Coal Mine in the Hunan Province, China for their support and participation.
Footnotes
Funding: Key Program for Guangming Lu, No. BWS11J063 and No.10z026.
Conflicts of interest: None declared.
Ethical approval: This studied was approved by the Ethics Committee of Second Xiangya Hospital, Central South University, China.
(Reviewed by Dean J, Raye W, Cao XH, Sun CR)
(Edited by Yang Y, Li CH, Song LP, Liu WJ, Zhao M)
REFERENCES
- 1.Hao W. 5th ed. Beijing: People's Medical Publishing House; 2004. Psychiatry. [Google Scholar]
- 2.Zhang YL. Changsha: Central South University Press; 2007. Advanced Psychiatry. [Google Scholar]
- 3.Kessler RC, Sonnega A, Bromet E, et al. Posttraumatic stress disorder in the National Comorbidity Survey. Arch Gen Psychiatry. 1995;52:1048–1060. doi: 10.1001/archpsyc.1995.03950240066012. [DOI] [PubMed] [Google Scholar]
- 4.4th ed. Washington, DC: American Psychiatric Press; 2000. American Psychiatric Association. Diagnostic and Statistical Manual for Mental Disorders. [Google Scholar]
- 5.Sher L. Neurobiology of suicidal behavior in post-traumatic stress disorder. Expert Rev Neurother. 2010;10:1233–1235. doi: 10.1586/ern.10.114. [DOI] [PubMed] [Google Scholar]
- 6.Sareen J, Cox BJ, Stein MB, et al. Physical and mental comorbidity, disability, and suicidal behavior associated with posttraumatic stress disorder in a large community sample. Psychosom Med. 2007;69:242–248. doi: 10.1097/PSY.0b013e31803146d8. [DOI] [PubMed] [Google Scholar]
- 7.Bremner JD. Neuroimaging in posttraumatic stress disorder and other stress-related disorders. Neuroimaging Clin N Am. 2007;17:523–538. doi: 10.1016/j.nic.2007.07.003. ix. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Francati V, Vermetten E, Bremner JD. Functional neuroimaging studies in posttraumatic stress disorder: review of current methods and findings. Depress Anxiety. 2007;24:202–218. doi: 10.1002/da.20208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bremner JD, Randall P, Scott TM, et al. MRI-based measurement of hippocampal volume in patients with combat-related posttraumatic stress disorder. Am J Psychiatry. 1995;152:973–981. doi: 10.1176/ajp.152.7.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bing X, Ming-Guo Q, Ye Z, et al. Alterations in the cortical thickness and the amplitude of low-frequency fluctuation in patients with post-traumatic stress disorder. Brain Res. 2013;1490:225–232. doi: 10.1016/j.brainres.2012.10.048. [DOI] [PubMed] [Google Scholar]
- 11.Liu Y, Li YJ, Luo EP, et al. Cortical thinning in patients with recent onset post-traumatic stress disorder after a single prolonged trauma exposure. PLoS One. 2012;7(6):e39025. doi: 10.1371/journal.pone.0039025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen Y, Fu K, Feng C, et al. Different regional gray matter loss in recent onset PTSD and non PTSD after a single prolonged trauma exposure. PLoS One. 2012;7:e48298. doi: 10.1371/journal.pone.0048298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lyoo IK, Kim JE, Yoon SJ, et al. The neurobiological role of the dorsolateral prefrontal cortex in recovery from trauma. Longitudinal brain imaging study among survivors of the South Korean subway disaster. Arch Gen Psychiatry. 2011;68:701–713. doi: 10.1001/archgenpsychiatry.2011.70. [DOI] [PubMed] [Google Scholar]
- 14.Zhang L, Zhang Y, Li L, et al. Different white matter abnormalities between the first-episode, treatment-naive patients with posttraumatic stress disorder and generalized anxiety disorder without comorbid conditions. J Affect Disord. 2011;133:294–299. doi: 10.1016/j.jad.2011.03.040. [DOI] [PubMed] [Google Scholar]
- 15.Woodward SH, Schaer M, Kaloupek DG, et al. Smaller global and regional cortical volume in combat-related posttraumatic stress disorder. Arch Gen Psychiatry. 2009;66:1373–1382. doi: 10.1001/archgenpsychiatry.2009.160. [DOI] [PubMed] [Google Scholar]
- 16.Geuze E, Westenberg HG, Heinecke A, et al. Thinner prefrontal cortex in veterans with posttraumatic stress disorder. Neuroimage. 2008;41:675–681. doi: 10.1016/j.neuroimage.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 17.Rauch SL, Shin LM, Segal E, et al. Selectively reduced regional cortical volumes in post-traumatic stress disorder. Neuroreport. 2003;14:913–916. doi: 10.1097/01.wnr.0000071767.24455.10. [DOI] [PubMed] [Google Scholar]
- 18.Woodward SH, Kaloupek DG, Streeter CC, et al. Decreased anterior cingulate volume in combat-related PTSD. Biol Psychiatry. 2006;59:582–587. doi: 10.1016/j.biopsych.2005.07.033. [DOI] [PubMed] [Google Scholar]
- 19.Kitayama N, Quinn S, Bremner JD. Smaller volume of anterior cingulate cortex in abuse-related posttraumatic stress disorder. J Affect Disord. 2006;90:171–174. doi: 10.1016/j.jad.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kühn S, Gallinat J. Gray matter correlates of posttraumatic stress disorder: a quantitative meta-analysis. Biol Psychiatry. 2013;73:70–74. doi: 10.1016/j.biopsych.2012.06.029. [DOI] [PubMed] [Google Scholar]
- 21.De Bellis M, Hall J, Boring A, et al. A pilot longitudinal study of hippocampal volumes in pediatric maltreatment-related posttraumatic stress disorder. Biol Psychiatry. 2001;50:305–309. doi: 10.1016/s0006-3223(01)01105-2. [DOI] [PubMed] [Google Scholar]
- 22.Hakamata Y, Matsuoka Y, Inagaki M, et al. Structure of orbitofrontal cortex and its longitudinal course in cancer-related post-traumatic stress disorder. Neurosci Res. 2007;59:383–389. doi: 10.1016/j.neures.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 23.Bonne O, Brandes D, Gilboa A, et al. Longitudinal MRI study of hippocampal volume in trauma survivors with PTSD. Am J Psychiatry. 2001;158:1248–1251. doi: 10.1176/appi.ajp.158.8.1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dickie EW, Brunet A, Akerib V, et al. Anterior cingulate cortical thickness is a stable predictor of recovery from post-traumatic stress disorder. Psychol Med. 2013;43:645–653. doi: 10.1017/S0033291712001328. [DOI] [PubMed] [Google Scholar]
- 25.Dickie EW, Brunet A, Akerib V, et al. Neural correlates of recovery from post-traumatic stress disorder: a longitudinal fMRI investigation of memory encoding. Neuropsychologia. 2011;49:1771–1778. doi: 10.1016/j.neuropsychologia.2011.02.055. [DOI] [PubMed] [Google Scholar]
- 26.Cardenas VA, Samuelson K, Lenoci M, et al. Changes in brain anatomy during the course of posttraumatic stress disorder. Psychiatry Res. 2011;193:93–100. doi: 10.1016/j.pscychresns.2011.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wright IC, McGuire PK, Poline JB, et al. A voxel-based method for the statistical analysis of gray and white matter density applied to schizophrenia. Neuroimage. 1995;2:244–252. doi: 10.1006/nimg.1995.1032. [DOI] [PubMed] [Google Scholar]
- 28.Qin JB. Voxel-based morphometry in the application of cognitive dysfunction. Zhongnan Daxue Xuebao: Yixue Ban. 2010;29:477–480. [Google Scholar]
- 29.Ashburner J, Friston KJ. Voxel-based morphometry--the methods. Neuroimage. 2000;11:805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- 30.Eckart C, Stoppel C, Kaufmann J, et al. Structural alterations in lateral prefrontal, parietal and posterior midline regions of men with chronic posttraumatic stress disorder. J Psychiatry Neurosci. 2011;36:176–186. doi: 10.1503/jpn.100010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nardo D, Högberg G, Lanius RA, et al. Gray matter volume alterations related to trait dissociation in PTSD and traumatized controls. Acta Psychiatr Scand. doi: 10.1111/acps.12026. in press. [DOI] [PubMed] [Google Scholar]
- 32.Geuze E, Westenberg HG, Heinecke A, et al. Thinner prefrontal cortex in veterans with posttraumatic stress disorder. Neuroimage. 2008;41:675–681. doi: 10.1016/j.neuroimage.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 33.Hakamata Y, Matsuoka Y, Inagaki M, et al. Structure of orbitofrontal cortex and its longitudinal course in cancer-related post-traumatic stress disorder. Neurosci Res. 2007;59:383–389. doi: 10.1016/j.neures.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 34.Carrion VG, Weems CF, Richert K, et al. Decreased prefrontal cortical volume associated with increased bedtime cortisol in traumatized youth. Biol Psychiatry. 2010;68:491–493. doi: 10.1016/j.biopsych.2010.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chao LL, Lenoci M, Neylan TC. Effects of post-traumatic stress disorder on occipital lobe function and structure. Neuroreport. 2012;23:412–419. doi: 10.1097/WNR.0b013e328352025e. [DOI] [PubMed] [Google Scholar]
- 36.Tavanti M, Battaglini M, Borgogni F, et al. Evidence of diffuse damage in frontal and occipital cortex in the brain of patients with post-traumatic stress disorder. Neurol Sci. 2012;33:59–68. doi: 10.1007/s10072-011-0659-4. [DOI] [PubMed] [Google Scholar]
- 37.Fennema-Notestine C, Stein MB, Kennedy CM, et al. Brain morphometry in female victims of intimate partner violence with and without posttraumatic stress disorder. Biol Psychiatry. 2002;52:1089–1101. doi: 10.1016/s0006-3223(02)01413-0. [DOI] [PubMed] [Google Scholar]
- 38.Breslau N. Outcomes of posttraumatic stress disorder. J Clin Psychiatry. 2001;62(Suppl 17):55–59. [PubMed] [Google Scholar]
- 39.Yehuda R, LeDoux J. Response variation following trauma: a translational neuroscience approach to understanding PTSD. Neuron. 2007;56:19–32. doi: 10.1016/j.neuron.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 40.Shucard JL, Cox J, Shucard DW, et al. Symptoms of posttraumatic stress disorder and exposure to traumatic stressors are related to brain structural volumes and behavioral measures of affective stimulus processing in police officers. Psychiatry Res. 2012;204:25–31. doi: 10.1016/j.pscychresns.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 41.Lindauer RJ, Vlieger EJ, Jalink M, et al. Smaller hippocampal volume in Dutch police officers with posttraumatic stress disorder. Biol Psychiatry. 2004;56:356–363. doi: 10.1016/j.biopsych.2004.05.021. [DOI] [PubMed] [Google Scholar]
- 42.Kiliç EZ, Kiliç C, Yilmaz S. Is anxiety sensitivity a predictor of PTSD in children and adolescents? J Psychosom Res. 2008;65:81–86. doi: 10.1016/j.jpsychores.2008.02.013. [DOI] [PubMed] [Google Scholar]
- 43.Kroes MC, Whalley MG, Rugg MD, et al. Association between flashbacks and structural brain abnormalities in posttraumatic stress disorder. Eur Psychiatry. 2011;26:525–531. doi: 10.1016/j.eurpsy.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 44.First MB, Spitzer RL, Gibbon M, et al. Patient Edition (SCID-I/P) New York: Biometrics Research, New York State Psychiatric Institute; 2002. Structured Clinical Interview for DSM-IV-TR Axis I Disorders. Research Version. [Google Scholar]
- 45.Wang XD, Wang XL, MA H. Revised and enlarged edition. Beijing: Press of Chinese Mental Health Journal; 1999. Rating scales for mental health. [Google Scholar]
- 46.Blake DD, Weathers FW, Nagy LM, et al. The development of a Clinician-Administered PTSD Scale. J Trauma Stress. 1995;8:75–90. doi: 10.1007/BF02105408. [DOI] [PubMed] [Google Scholar]
- 47.Hou CL, Li LJ, Jia FJ, et al. Clinician-administered post-traumatic stress disorder. Zhongguo Xingwei Yixue Zazhi. 2008;17:851–852. [Google Scholar]


