Abstract
Expression of miR-137 is downregulated in brain tissue from patients with depression and suicidal behavior, and is also downregulated in peripheral blood from stroke patients. However, it is not yet known if miR-137 acts as a bridge between stroke and depression. To test this, we used middle cerebral artery occlusion and chronic mild stress to establish a post-stroke depression model in rats. Compared with controls, we found significantly lower miR-137 levels in the brain and peripheral blood from post-stroke depression rats. Injection of a miR-137 antagonist into the brain ventricles upregulated miR-137 levels, and improved behavioral changes in post-stroke depression rats. Luciferase assays showed miR-137 bound to the 3’UTR of Grin2A, regulating Grin2A expression in a neuronal cell line. Grin2A gene overexpression in the brain of post-stroke depression rats, noticeably suppressed the inhibitory effect of miR-137 on post-stroke depression. Overall, our results show that miR-137 suppresses Grin2A protein expression through binding to Grin2A mRNA, thereby exerting an inhibitory effect on post-stroke depression. Our results offer a new therapeutic direction for post-stroke depression.
Keywords: neural regeneration, brain injury, post-stroke depression, microRNA, cerebrovascular disease, Grin2A, miR-137, neuroregeneration
Research Highlights
(1) The aim of our study was to investigate the relationship between miR-137 and post-stroke depression.
(2) Our results demonstrate that in a rat model of post-stroke depression, miR-137 levels are significantly decreased in the brain and peripheral blood. Overexpression of miR-137 in the brain improves behavioral changes in post-stroke depression rats, by suppressing Grin2A expression at the post-transcriptional level.
INTRODUCTION
MicroRNAs (miRNAs) are a class of single-stranded small non-coding RNA molecules, between 18–25 nucleotides in length. MiRNAs bind to 3’untranslated regions (3’UTR) of mRNAs, regulating protein expression levels of target genes[1,2,3,4,5]. Recent research has suggested that miRNAs may be associated with depression. Smalheiser et al[6] examined brain tissue from 18 depressed patients with suicidal behavior, and found downregulated expression of 21 miRNAs, including miR-137. Comparing the spectrum of serum miRNAs in depressed patients before and after treatment, Bocchio-Chiavetto et al[7] showed that oral administration of the antidepressant drug citalopram induced an increase in levels of 28 serum miRNAs. Furthermore, Baudry et al[8] found that a selective serotonin reuptake inhibitor antidepressant drug, increases miR-16 levels and downregulates expression of the miR-16 target gene, a serotonin transporter, thereby exerting an antidepressive effect. Previous studies have also demonstrated that miRNAs likely play an important role in the occurrence and development of depression, and show potential as targets for treatment of clinical depression[9,10].
However, it is not yet known if miRNAs are associated with post-stroke depression. Recently, Scholars[11,12,13] showed altered expression of various miRNAs in peripheral blood, from acute stage stroke patients. A further study confirmed corresponding miRNA changes in the brain, in a mouse ischemia model. Interestingly, there are small overlaps between downregulated miRNA expression after cerebral ischemia, and in depression patients. Nevertheless, the effects of these overlapping miRNAs on the occurrence and development of post-stroke depression remains unclear. Therefore, in this study, we used a rat model of post-stroke depression to investigate the effects of miR-137 on behavior.
RESULTS
Quantitative analysis of experimental animals
A total of 43 rats were used. Six randomly selected rats were chosen as the control group. The remaining 37 rats were used to establish a model of post-stroke depression using middle cerebral artery occlusion and chronic mild stress. Two rats died from subarachnoid hemorrhaging, and five from severe cerebral edema. The remaining 30 rats were randomly assigned to five groups, specifically, model, agomir-137, agomir-NC (negative control), agomir-137 + Grin2A and agomir-137 + vector groups. The four agomir groups received an injection of agomir-137, a miR-137 antagonist into the left lateral ventricle 48 hours before model induction. Agomir-NC, LV-CMV-Grin2A and -control plasmids were also injected into the left lateral ventricle 48 hours before model induction. The control, model, agomir-137 and agomir-NC groups were used to investigate the effects of miR-137 on post-stroke depression. The agomir-137, agomir-NC, agomir-137 + Grin2A and agomir-137 + vector groups were used to investigate Grin2A involvement in the post-stroke depression effects of miR-137.
Downregulation of miR-137 levels in the brain of rats with post-stroke depression
Quantitative real-time PCR identified significantly lower miR-137 levels in brain and peripheral blood in our rat model of post-stroke depression, than in control rats (P < 0.05; Figure 1), suggesting that miR-137 is involved in the pathophysiology of post-stroke depression.
Figure 1.
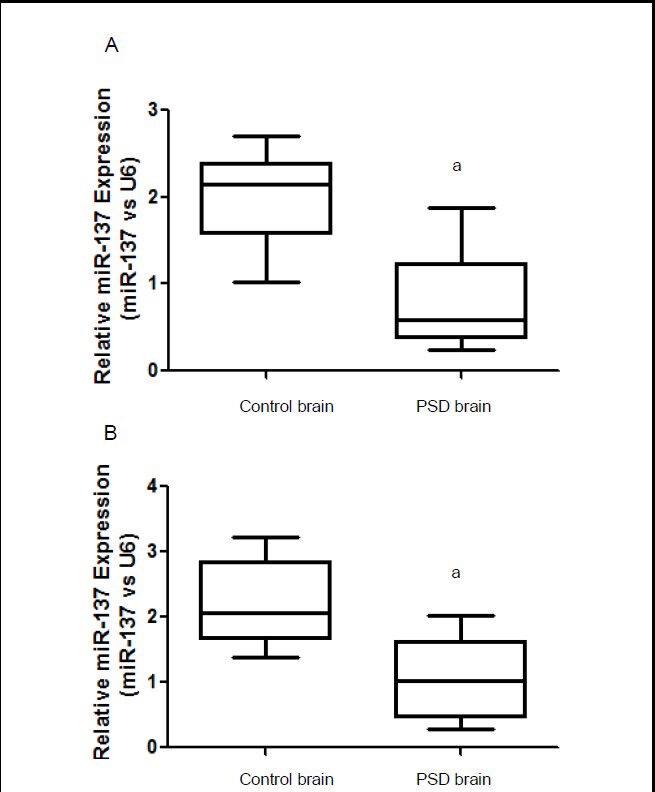
MiR-137 levels analyzed using real-time PCR in rats with post-stroke depression (PSD).
(A) MiR-137 expression levels in brain.
(B) MiR-137 expression levels in peripheral blood.
Data are expressed as mean ± SD. Six rats were used in each group. Intergroup comparisons were performed using one-way analysis of variance. aP < 0.05, vs. control rats.
MiR-137 improved behavioral changes in rats with post-stroke depression
Using the open field test at 3 weeks post-stroke, we found significantly higher rearing and locomotor activities in post-stroke depression rats injected with agomir-137, than rats injected with agomir-NC and untreated post-stroke depression rats (P < 0.05; Figure 2A). In addition, at 14 days after cerebral ischemia, the sucrose preference test showed significantly higher sucrose consumption in the agomir-137 group, than in agomir-NC and untreated post-stoke depression rats (P < 0.05; Figure 2B).
Figure 2.
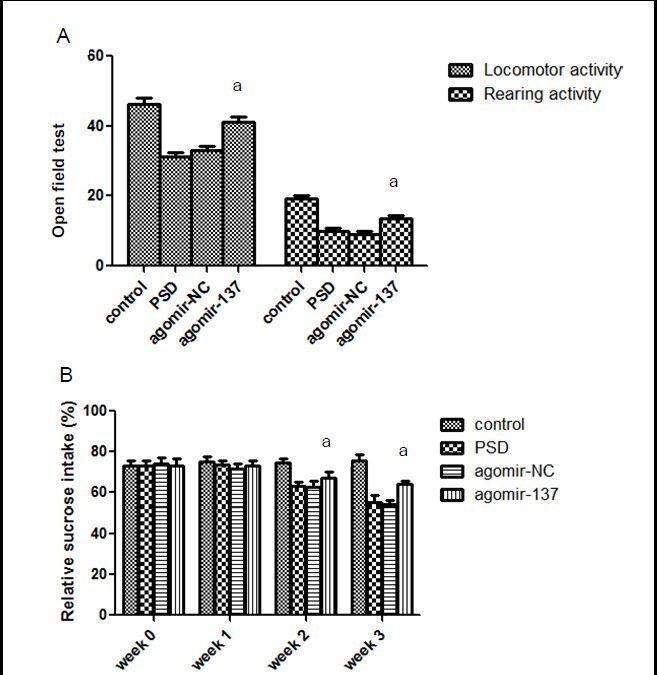
Effects of miR-137 on behavior in rats.
(A) Open field test. The frequency of crossing the square was used to determine locomotor activity. The frequency of rearing determined rearing activity. (B) Sucrose consumption. Sucrose consumption percentage (%) = sucrose consumption/ (sucrose + plain water consumption) × 100%.
Data are expressed as mean ± SD. Six rats were used in each group. Intergroup comparisons were performed using one-way analysis of variance. aP < 0.05, vs. agomir-negative control (NC) group. PSD: Post-stroke depression.
MiR-137 bound to the 3’UTR of Grin2A mRNA and downregulated Grin2A protein expression
Luciferase assays found significantly lower fluorescence intensities in HEK-293 cells transfected with Grin2A-3’UTR-wt, compared with negative controls (miR-con) (P < 0.05). However, no significant difference was detected between HEK-293 cells transfected with Grin2A-3’UTR-mut or empty vector (P > 0.05; Figure 3B). This suggests miR-137 binds to the 3’UTR of Grin2A mRNA, and regulates its translation. Moreover, western blot assays showed that in PC12 cells, miR-137 mimic (mimics of synthesized mir-137, showing similar effects to natural mir-137) significantly reduced (P < 0.05), but miR-137 inhibitor significantly increased (P < 0.05), Grin2A levels (Figure 3C). These results suggest that miR-137 binds to the 3’UTR of Grin2A mRNA and regulates Grin2A protein expression.
Figure 3.
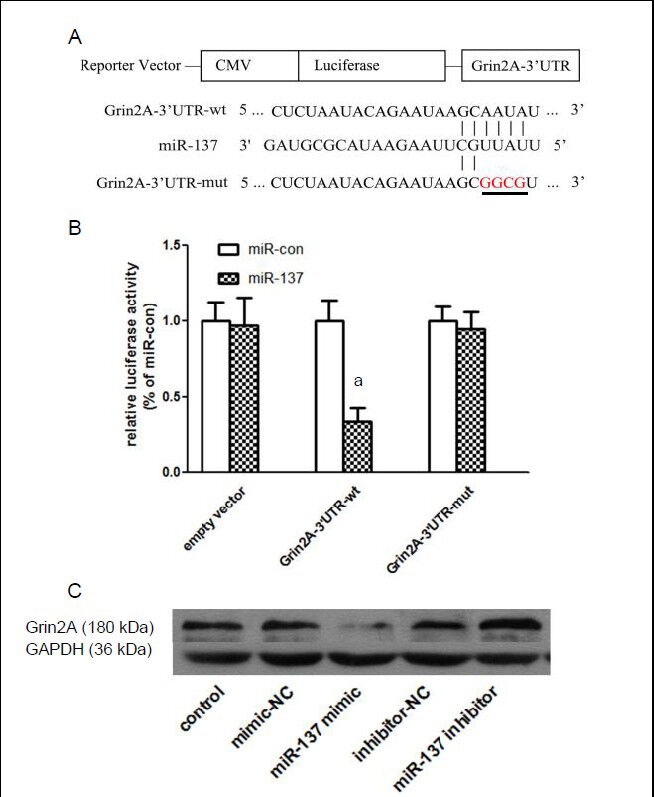
MiR-137 downregulates Grin2A expression by binding to the 3’UTR of Grin2A mRNA.
(A) MiR-137 binding (Grin2A-3’UTR-wt) and mutant (Grin2A-3’UTR-mut) sequences, predicted by Targetscan and miRBase, were cloned into Luciferase reporter vectors.
(B) Luciferase assays showed that following miR-137 transfection, fluorescence intensities were significantly lower in the Grin2A-3’UTR-wt group, than in negative controls (miR-con). No significant difference was detected between the Grin2A-3’UTR-mut or empty vector groups and negative controls. Data are expressed as mean ± SD. Intergroup comparisons were performed using one-way analysis of variance. aP < 0.05, vs. control.
(C) Western blot analysis in PC12 cells revealed that miR-137 mimic significantly reduced, and miR-137 inhibitor significantly increased Grin2A levels. NC: Negative control.
Grin2A overexpression prevented miR-137-induced behavioral improvements in post-stroke depression rats
To determine if Grin2A participates in miR-137-induced behavioral improvements in post-stroke depression rats, we injected a plasmid containing the Grin2A gene into the brain of post-stroke depression rats, via the brain ventricles. We found significantly lower locomotor and rearing activities in the agomir-137 + Grin2A group than in the negative control (agomir-137 + vector) and agomir-137 groups (P < 0.05; Figure 4A). Moreover, the sucrose consumption percentage was significantly lower in the agomir-137 + Grin2A group than in the negative control and agomir-137 groups (P < 0.05; Figure 4B). These results suggest that Grin2A overexpression prevents the improved behavioral effects of miR-137 in rats with post-stroke depression, and confirms the involvement of Grin2A in the miR-137 therapeutic effect in post-stroke depression rats.
Figure 4.
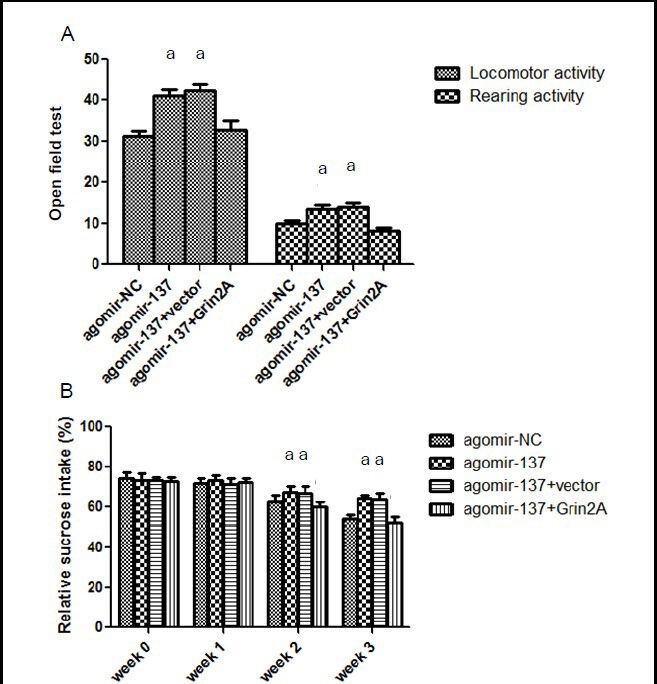
Grin2A prevents miR-137 behavioral effects in rats with post-stroke depression.
(A) Open field test. Locomotor and rearing activities in 3 minutes were significantly lower in the agomir-137 + Grin2A group, than in the agomir-137 + vector and agomir-137 groups.
(B) Sucrose consumption percentage was significantly lower in the agomir-137 + Grin2A group, than in the agomir-137 + vector and agomir-137 groups. Sucrose consumption percentage (%) = sucrose consumption/ (sucrose + plain water consumption) × 100%.
Data are expressed as mean ± SD. Six rats were used in each group. Intergroup comparisons were performed using one-way analysis of variance. aP < 0.05, vs. agomir-137 + Grin2A group.
DISCUSSION
Recent studies have suggested that miR-137 is strongly associated with neuropsychological diseases.
Geekiyanage et al[14] identified significantly lower miR-137 levels in the brain and serum from Alzheimer's patients, than normal persons, and miR-137 expression was inversely proportional to serine palmitoyltransferase and β-amyloid levels. Thus, it is possible that miR-137 has anti-Alzheimer's disease effects via regulation of serine palmitoyltransferase. Furthermore, an in vitro study detected high miR-137 expression in neural progenitors during differentiation[15], finding miR-137 promotes neural cell maturation and differentiation by suppressing expression of the Mind bomb-1 and histone lysine specific demethylase 1 proteins. A genomic study found miR-137 mutation leads to schizophrenia and affective disorder, suggesting miR-137 is potentially associated with emotional function[16]. A further study showed miR-137 exerted its effects through downregulation of certain schizophrenia-related genes, for example, CSMD1, C10orf26, CACNA1C and TCF4[17]. Downregulation of miR-137 levels in the brain and peripheral blood of rat models of post-stroke depression suggests miR-137 is likely associated with the pathogenesis of post-stroke depression. Notably, miR-137 levels are downregulated in both stroke and depression patients[6,12]. Combined with our results, we can conclude that miR-137 may be a bridge between stroke and depression.
To verify miR-137 effects on post-stroke depression, we established a rat model of post-stroke depression, and injected the miR-137 antagonist, agomir-137, into the brain of rats with post-stroke depression, via the brain ventricles. Our results demonstrate that increased miR-137 levels significantly increase open field test scores and sucrose consumption, suggesting miR-137 can counteract the effects of post-stroke depression. However, the molecular biological basis of miR-137 in post-stroke depression is still not clear. To address this problem, we searched the Targetscan and miRBase databases for potential miR-137 targets, identifying a 7-mer sequence at the 3’UTR of Grin2A mRNA, as a potential miR-137 binding site, suggesting Grin2A is a downstream target gene for miR-137 effects.
Grin2A protein is an important component of the N-methyl-D-aspartate (NMDA) receptor that is extensively distributed within the central nervous system, and considered to play an important role in memory, emotion and learning[18,19,20,21,22]. NMDA receptors belong to the family of ionotropic glutamate receptors and are composed of two Grin1, and two Grin2 (Grin2A and Grin2B), subunits[23,24,25]. The NMDA receptor has been associated with depression. The binding ability of the NMDA receptor is significantly higher in depressed patients compared with normal controls, and in animal models, blocking NMDA receptors causes antidepressive effects[26,27]. Following cerebral ischemia, stress induces abundant glutamate production, excessively activating NMDA receptors and through a cascade reaction, results in abnormal hypothalamic-pituitary-adrenal activation and subsequent involvement in the occurrence of post-stroke depression[28,29,30,31,32,33,34]. Numerous studies have verified NMDA receptor antagonists as an effective treatment for depression and post-stroke depression[35,36,37,38,39,40]. However, because of severe complications, for example, muscle relaxation, ataxia, and learning and memory impairments, the use of common anti-NMDA receptor drugs is restricted clinically. Thus, more and more researchers are investigating NMDA receptor antagonists that selectively affect its subunits, such as Grin2A or Grin2B. Our luciferase assay results show that transfection of miR-137 mimic causes decreased fluorescence intensities in 3’UTR segments containing wild-type Grin2A, but was not affected by 3’UTRs containing mutant miR-137 binding sites. Moreover, in PC12 cells, western blots show decreased Grin2A protein levels with miR-137 overexpression, but increased levels with inhibition of miR-137 expression, suggesting miR-137 suppresses mRNA translation by binding to the 3’UTR of Grin2A. Overall, our results suggest that miR-137 inhibits Grin2A protein synthesis at the post-transcriptional level, and also that miR-137 could be used as a selective NMDA receptor antagonist for the treatment of depression or post-stroke depression.
Grin2A is a downstream target of miR-137, but its involvement in the antidepressive effect of miR-137 required further confirmation. Therefore, we injected both Grin2A and agomir-137 into brain ventricles of rats with post-stroke depression to investigate the relationship between Grin2A and agomir-137 in the pathogenesis of post-stroke depression. Our results show significantly lower rearing and locomotor activities in rats with Grin2A and agomir-137 overexpression than in negative control (agomir-137 + vector) and agomir-137 groups. This suggests that Grin2A overexpression prevents the antidepressive effect of miR-137, and indirectly confirms Grin2A as an important downstream target in mediating miR-137 antidepressive effects.
In summary, miR-137 levels are noticeably downregulated in peripheral blood and brain in a rat model of post-stroke depression. In addition, miR-137 overexpression in the brain improves behavioral changes in post-stroke depression rats. Furthermore, we show that miR-137 exerts its anti-post-stroke depression effect by suppressing Grin2A expression at the post-transcriptional level. Our study provides a potential therapeutic target for post-stroke depression, and increases our knowledge of its pathogenesis.
MATERIALS AND METHODS
Design
A randomized, controlled molecular biology study.
Time and setting
Experiments were performed at the Laboratory of Pathology, Shandong University, China from April to September 2012.
Materials
Animals
A total of 31 pathogen-free healthy male Sprague-Dawley rats aged 8–10 weeks and weighing 280–340 g were supplied by the Animal Experiment Center of Shandong University in China (Animal license No. SCXK (Lu) 2003-0002). The animals were housed in separate cages at 21 ± 2°C with relative humidity of 30–35% in a 12-hour light/dark cycle, and allowed free access to full-nutrient food and water. No difference in exposure factors including weight, age and feeding were observed in any group. Protocols were conducted in accordance with the Guidance Suggestions for the Care and Use of Laboratory Animals, published by the Ministry of Science and Technology of China[41].
Cells and plasmids
HEK-293 (Invitrogen, Carlsbad, CA, USA) and PC12 (Boster, Wuhan, Hubei Province, China) cells were obtained.
Agomir-137, Agomir-NC, miR-137 mimic and miR-137 inhibitor were from Ribobio (Guangzhou, Guangdon Province, China. Lentiviral-CMV-Grin2A and -control plasmids were synthesized by Genechem (Shanghai, China).
Methods
Establishment of a rat model of post-stroke depression
Stroke was established by middle cerebral artery occlusion[42]. Rats were subcutaneously anesthetized with ketamine (100 mg/kg), xylazine (2.5 mg/kg) and acepromazine (2.5 mg/kg) (Jinan Wanxingda Chemical Co., Ltd., Jinan, Shandong Province, China), and fixed in the supine position. Incisions were made in the neck, and the left common carotid, external carotid and internal carotid arteries were dissociated. Small incisions were made in the proximal end of the common carotid artery using eye scissors, and a 0.26 mm-diameter fishing line with a blunt end inserted, reaching the origin of the middle cerebral artery through the internal carotid artery. Blood flow through the middle cerebral artery was blocked for 2 hours. The fishing line was then removed, the external carotid artery ligated, and the incision sutured. After regaining consciousness, rats were housed at 20–25°C and allowed free access to food and water.
Two days after model establishment, rats that scored ≥ 1 and < 4 according to Longa's criteria[43] were selected. In accordance with the method of Willner[44], rats underwent chronic mild stress to induce post-stroke depression. Rats received tail-clamping for 1 minute, water deprivation for 24 hours, fasting for 24 hours, a high temperature environment for 5 minutes (45°C), swimming in ice water for 5 minutes (4°C), day and night inversion (24 hours) and weaving for 30 minutes at 160 times/min. The seven stimuli were given respectively from Monday to Sunday in order, for a total of 3 weeks i.e., each stimulus was given three times during induction. All rats were housed in separate cages.
Lentivirus plasmid injection into brain ventricles
Following the method as described previously[20], lentivirus plasmid was injected into brain ventricles 48 hours prior to establishment of the stroke model. After disinfection with 75% alcohol, a 2.5 cm-long sagittal incision was made along the midline to expose the skull. The syringe insertion coordinate was labeled on the skull (0.3 mm anterior, 3.6 mm ventral and 1.1 mm left of the anterior fontanelle[45]), followed by drilling. Cerebral dura mater was pierced with a needle and a cannula inserted into the left lateral ventricle. Using a microsyringe pump, appropriate concentrations of reagents were injected, specifically, 10 nmol agomir-137 or agomir-NC (dissolved in 10 μL PBS), and 10 μL 108 TU/mL LV-CMV-Grin2A or LV-CMV-control plasmids. In the agomir-137 + Grin2A and agomir-137 + vector groups, plasmids were injected first, followed by agomir. Head skin was sutured, and rats were kept in an incubator at 37°C for 60 minutes. Subsequently, rats were maintained at 22°C in individual cages with free access to food and water.
Behavioral tests
Open field test: in accordance with Wang et al[46], rats were placed in an open-field box under quiet conditions. The frequency of crossing the square was used to determine locomotor activity. Four claws entering the square scored 1. The frequency of rearing determined rearing activity. Two forelimbs from the ground once scored 1. Each rat was measured three times for 3 minutes each time and the average value calculated.
Sucrose preference test: after 20 hours of water deprivation and fasting, all rats were administered with bottles of 1% sucrose water and plain water. The sucrose consumption percentage within 1 hour was calculated as: sucrose consumption percentage (%) = sucrose consumption / (sucrose + plain water consumption) × 100%.
Quantitative real-time PCR
In accordance with Duan et al[47], PCR was performed to determine miR-137 expression in the brain and peripheral blood of rats with post-stroke depression. Total RNA was extracted using Trizol (Sigma-Aldrich, St. Louis, MO, USA), and cDNA synthesized using EasyScript First- Strand cDNA Synthesis SuperMix (TransGen Biotech, Beijing, China). In brief, 2.5 μL cDNA and 1 μL specific primer (RiboBio) were added to TransStart™ SYBR Green qPCR Supermix (TransGen Biotech). Quantitative real-time PCR was performed to determine miR-137 levels. PCR Detector (ABI, Beijing, China) was used to measure SYBR Green fluorescence intensity. The miRNA molecule, U6, served as an internal reference.
Luciferase assay
To investigate the mechanism underlying miR-137 behavioral improvements in rats with post-stroke depression, potential miR-137 targets were identified using the databases, Targetscan (http://www.targetscan.org/) and miRBase (www.mirbase.org). To determine if the identified sequence downregulates Grin2A mRNA translation following miR-137 binding, Grin2A 3’UTRs containing the target site (Grin2A-3’UTR-wt) or a mutated site (Grin2A-3’UTR-mut), were constructed in a luciferase reporter system. Subsequently, miR-137 and either luciferase empty vector or Grin2A-3’UTR-wt or Grin2A-3’UTR-mut plasmids were co-transfected into HEK-293 cells[47]. HEK-293 cells were incubated in RPMI 1640 medium (Gibco, Carlsbad, CA, USA) containing 10% fetal bovine serum (Thermo, Waltham, MA, USA) at 37°C in an incubator containing 5% CO2. Full-length Grin2A 3’UTR containing the miR-137 binding site, was PCR amplified and cloned into HindIII and SacI sites of the pMIR-REPORT miRNA expression reporter vector (Ambion, Inc., Shanghai, China). The resulting vector was named Grin2A-3’UTR-wt. A point mutation of Grin2A-3’UTR-wt was identified using the Easy Mutagenesis System (TransGen Biotech) and subcloned also, with the resulting vector named Grin2A-3’UTR-mut. HEK 293T cells were seeded into 24-well plates and divided into three groups. Empty vector (400 ng) was added to the control group, Grin2A-3’UTR-wt plasmid (400 ng) to the wild-type group, and Grin2A-3’UTR-mut plasmid (400 ng) to the mutation group. Plasmids and miR-137 were transfected using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA), and 20 ng pRL-TK (RiboBio) was added as an internal reference. Within each group, parallel wells were used for the addition of mimic-NC (RiboBio), a negative control for miR-137 mimic (miR-con). After 36 hours of transfection, the fluorescence intensity of cells was measured using the Dual-Luciferase Reporter Assay System (Promega, Madison, WI, USA).
Western blot assay
PC12 cells were seeded into 24-well plates and divided into five groups. The control group received conventional culture. MiR-137 mimic was added to the miR-137 transfection and negative control (mimic-NC) groups. MiR-137 inhibitor was added to the miR-137 inhibitor and inhibitor negative control (inhibitor-NC) groups. Following 48 hours of transfection, PC12 cells were collected, centrifuged and lysed with lysis buffer for 30 minutes. Protein concentrations were measured using the BCA method[48]. Each lysate underwent sodium dodecyl sulphate-poly-acrylamide gel electropho-resis on 10% discontinuous gels (Boster), and then wet transferred onto the membrane. Membranes were blocked with 5% skimmed milk for 2 hours, washed six times in Tris-buffered saline with Tween-20 (Boster) (10 minutes each wash), and incubated with rabbit anti-Grin2A or glyceraldehyde-3-phosphate dehydrogenase (GAPDH) polyclonal antibody IgG (1:200; Santa Cruz Biotechnology, Santa Cruz, CA, USA) at 4°C overnight. The next day, membranes were rinsed six times in Tris-buffered saline with Tween-20 (10 minutes each wash), incubated with horseradish peroxidase-labeled goat anti-rabbit polyclonal antibody IgM (1:2 000; Santa Cruz Biotechnology) at room temperature for 2 hours, followed by enhanced chemiluminescence. GAPDH served as an internal reference.
Statistical analysis
Data were analyzed using SPSS 17.0 for Windows (SPSS, Chicago, IL, USA), and expressed as mean ± SD. Differences among multiple groups were compared using analysis of variance. Differences between two groups were compared using two-sample t-tests (α = 0.05).
Research background: MiRNAs likely play an important role in the occurrence and development of depression, and can be used as potential targets for treatment of depression.
Research frontiers: Studies have shown that miR-137 expression is downregulated in the brain from depression patients with suicidal behavior. Moreover, miR-137 expression is also downregulated in peripheral blood from stroke patients.
Clinical significance: Detection of a gene involved in a rat model of post-stroke depression identifies a potential therapeutic target for post-stroke depression in the clinic.
Academic terminology: MicroRNAs (miRNA) are a class of single-stranded small non-coding RNA molecules that bind to 3’UTR regions, suppressing their translation and resulting in biological effects.
Peer review: There are many studies concerning post-stroke depression in rats, but few at the gene level. Our study verifies that miR-137 reflects a regulatory direction of post-stroke depression by inhibiting Grin2A expression.
Footnotes
Conflicts of interest: None declared.
Ethical approval: This study was approved by the Animal Ethics Committee, Shandong Provincial Hospital in China.
(Reviewed by James R, Robens J, Pan JY, Kang ZC)
(Edited by Yu J, Qiu Y, Li CH, Song LP, Liu WJ, Zhao M)
REFERENCES
- 1.Van Wynsberghe PM, Chan SP, Slack FJ, et al. Analysis of microRNA expression and function. Methods Cell Biol. 2011;106:219–252. doi: 10.1016/B978-0-12-544172-8.00008-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berezikov E. Evolution of microRNA diversity and regulation in animals. Nat Rev Genet. 2011;12(12):846–860. doi: 10.1038/nrg3079. [DOI] [PubMed] [Google Scholar]
- 3.Bilsland AE, Revie J, Keith W. MicroRNA and senescence: the senectome, integration and distributed control. Crit Rev Oncog. 2013;18(4):373–390. doi: 10.1615/critrevoncog.2013007197. [DOI] [PubMed] [Google Scholar]
- 4.De Cecco L, Dugo M, Canevari S, et al. Measuring microRNA expression levels in oncology: from samples to data analysis. Crit Rev Oncog. 2013;18(4):273–287. doi: 10.1615/critrevoncog.2013007207. [DOI] [PubMed] [Google Scholar]
- 5.Xu L, Yang BF, Ai J. MicroRNA transport: a new way in cell communication. J Cell Physiol. 2013;228(8):1713–1719. doi: 10.1002/jcp.24344. [DOI] [PubMed] [Google Scholar]
- 6.Smalheiser NR, Lugli G, Rizavi HS, et al. MicroRNA expression is down-regulated and reorganized in prefrontal cortex of depressed suicide subjects. PLoS One. 2012;7(3):e33201. doi: 10.1371/journal.pone.0033201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bocchio-Chiavetto L, Maffioletti E, Bettinsoli P, et al. Blood microRNA changes in depressed patients during antidepressant treatment. Eur Neuropsychopharmacol. 2013;23(7):602–611. doi: 10.1016/j.euroneuro.2012.06.013. [DOI] [PubMed] [Google Scholar]
- 8.Baudry A, Mouillet-Richard S, Schneider B, et al. miR-16 targets the serotonin transporter: a new facet for adaptive responses to antidepressants. Science. 2010;329(5998):1537–1541. doi: 10.1126/science.1193692. [DOI] [PubMed] [Google Scholar]
- 9.Mouillet-Richard S, Baudry A, Launay JM, et al. MicroRNAs and depression. Neurobiol Dis. 2012;46(2):272–278. doi: 10.1016/j.nbd.2011.12.035. [DOI] [PubMed] [Google Scholar]
- 10.O’Connor RM, Dinan TG, Cryan JF. Little things on which happiness depends: microRNAs as novel therapeutic targets for the treatment of anxiety and depression. Mol Psychiatry. 2012;17(4):359–376. doi: 10.1038/mp.2011.162. [DOI] [PubMed] [Google Scholar]
- 11.Tan KS, Armugam A, Sepramaniam S, et al. Expression profile of MicroRNAs in young stroke patients. PLoS One. 2009;4(11):e7689. doi: 10.1371/journal.pone.0007689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jeyaseelan K, Lim KY, Armugam A. MicroRNA expression in the blood and brain of rats subjected to transient focal ischemia by middle cerebral artery occlusion. Stroke. 2008;39(3):959–966. doi: 10.1161/STROKEAHA.107.500736. [DOI] [PubMed] [Google Scholar]
- 13.Dharap A, Vemuganti R. Ischemic pre-conditioning alters cerebral microRNAs that are upstream to neuroprotective signaling pathways. J Neurochem. 2010;113(6):1685–1691. doi: 10.1111/j.1471-4159.2010.06735.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Geekiyanage H, Jicha GA, Nelson PT, et al. Blood serum miRNA: non-invasive biomarkers for Alzheimer's disease. Exp Neurol. 2012;235(2):491–496. doi: 10.1016/j.expneurol.2011.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smrt RD, Szulwach KE, Pfeiffer RL, et al. MicroRNA miR-137 regulates neuronal maturation by targeting ubiquitin ligase mind bomb-1. Stem Cells. 2010;28(6):1060–1070. doi: 10.1002/stem.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cummings E, Donohoe G, Hargreaves A, et al. Mood congruent psychotic symptoms and specific cognitive deficits in carriers of the novel schizophrenia risk variant at MIR-137. Neurosci Lett. 2013;532:33–38. doi: 10.1016/j.neulet.2012.08.065. [DOI] [PubMed] [Google Scholar]
- 17.Kwon E, Wang W, Tsai LH. Validation of schizophrenia-associated genes CSMD1, C10orf26, CACNA1C and TCF4 as miR-137 targets. Mol Psychiatry. 2013;18(1):11–12. doi: 10.1038/mp.2011.170. [DOI] [PubMed] [Google Scholar]
- 18.Mozhui K, Karlsson RM, Kash TL, et al. Strain differences in stress responsivity are associated with divergent amygdala gene expression and glutamate-mediated neuronal excitability. J Neurosci. 2010;30(15):5357–5367. doi: 10.1523/JNEUROSCI.5017-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reutlinger C, Helbig I, Gawelczyk B, et al. Deletions in 16p13 including GRIN2A in patients with intellectual disability, various dysmorphic features, and seizure disorders of the rolandic region. Epilepsia. 2010;51(9):1870–1873. doi: 10.1111/j.1528-1167.2010.02555.x. [DOI] [PubMed] [Google Scholar]
- 20.Cherlyn SY, Woon PS, Liu JJ, et al. Genetic association studies of glutamate, GABA and related genes in schizophrenia and bipolar disorder: a decade of advance. Neurosci Biobehav Rev. 2010;34(6):958–977. doi: 10.1016/j.neubiorev.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 21.Sanhueza M, Lisman J. The CaMKII/NMDAR complex as a molecular memory. Mol Brain. 2013;6:10. doi: 10.1186/1756-6606-6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bliss TV, Collingridge GL. Expression of NMDA receptor-dependent LTP in the hippocampus: bridging the divide. Mol Brain. 2013;6:5. doi: 10.1186/1756-6606-6-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paoletti P. Molecular basis of NMDA receptor functional diversity. Eur J Neurosci. 2011;33(8):1351–1365. doi: 10.1111/j.1460-9568.2011.07628.x. [DOI] [PubMed] [Google Scholar]
- 24.Spalloni A, Nutini M, Longone P. Role of the N-methyl-D-aspartate receptors complex in amyotrophic lateral sclerosis. Biochim Biophys Acta. 2013;1832(2):312–322. doi: 10.1016/j.bbadis.2012.11.013. [DOI] [PubMed] [Google Scholar]
- 25.Santangelo RM, Acker TM, Zimmerman SS, et al. Novel NMDA receptor modulators: an update. Expert Opin Ther Pat. 2012;22(11):1337–1352. doi: 10.1517/13543776.2012.728587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Szakacs R, Janka Z, Kalman J. The “blue” side of glutamatergic neurotransmission: NMDA receptor antagonists as possible novel therapeutics for major depression. Neuropsychopharmacol Hung. 2012;14(1):29–40. [PubMed] [Google Scholar]
- 27.Autry AE, Adachi M, Nosyreva E, et al. NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature. 2011;475(7354):91–95. doi: 10.1038/nature10130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Castillo CS, Schultz SK, Robinson RG. Clinical correlates of early-onset and late-onset poststroke generalized anxiety. Am J Psychiatry. 1995;152(8):1174–1179. doi: 10.1176/ajp.152.8.1174. [DOI] [PubMed] [Google Scholar]
- 29.Isaev NK, Stelmashook EV, Plotnikov EY, et al. Role of acidosis, NMDA receptors, and acid-sensitive ion channel 1a (ASIC1a) in neuronal death induced by ischemia. Biochemistry (Mosc) 2008;73(11):1171–1175. doi: 10.1134/s0006297908110011. [DOI] [PubMed] [Google Scholar]
- 30.Marini AM, Popolo M, Pan H, et al. Brain adaptation to stressful stimuli: a new perspective on potential therapeutic approaches based on BDNF and NMDA receptors. CNS Neurol Disord Drug Targets. 2008;7(4):382–390. doi: 10.2174/187152708786441849. [DOI] [PubMed] [Google Scholar]
- 31.Hei MY, Kuang SJ. N-methyl-D-aspartate receptor-1 activation and neonatal brain injury. Zhongguo Dang Dai Er Ke Za Zhi. 2008;10(3):431–434. [PubMed] [Google Scholar]
- 32.Benquet P, Gee CE, Gerber U. Transient brain ischemia: NMDA receptor modulation and delayed neuronal death. Med Sci (Paris) 2008;24(2):185–190. doi: 10.1051/medsci/2008242185. [DOI] [PubMed] [Google Scholar]
- 33.Villmann C, Becker CM. On the hypes and falls in neuroprotection: targeting the NMDA receptor. Neuroscientist. 2007;13(6):594–615. doi: 10.1177/1073858406296259. [DOI] [PubMed] [Google Scholar]
- 34.Verkhratsky A, Kirchhoff F. NMDA Receptors in glia. Neuroscientist. 2007;13(1):28–37. doi: 10.1177/1073858406294270. [DOI] [PubMed] [Google Scholar]
- 35.Cam E, Yulug B, Ozan E. MK 801: a possible neuroprotective agent by poststroke depression? J Neuropsychiatry Clin Neurosci. 2008;20(3):367–368. doi: 10.1176/jnp.2008.20.3.367. [DOI] [PubMed] [Google Scholar]
- 36.Robinson RG, Jorge RE, Clarence-Smith K, et al. Double-blind treatment of apathy in patients with poststroke depression using nefiracetam. J Neuropsychiatry Clin Neurosci. 2009;21(2):144–151. doi: 10.1176/jnp.2009.21.2.144. [DOI] [PubMed] [Google Scholar]
- 37.Cho SI, Park UJ, Chung JM, et al. Neu2000, an NR2B-selective, moderate NMDA receptor antagonist and potent spin trapping molecule for stroke. Drug News Perspect. 2010;23(9):549–556. doi: 10.1358/dnp.2010.23.9.1513493. [DOI] [PubMed] [Google Scholar]
- 38.Mehta SL, Manhas N, Raghubir R. Molecular targets in cerebral ischemia for developing novel therapeutics. Brain Res Rev. 2007;54(1):34–66. doi: 10.1016/j.brainresrev.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 39.Catarzi D, Colotta V, Varano F. Competitive Gly/NMDA receptor antagonists. Curr Top Med Chem. 2006;6(8):809–821. doi: 10.2174/156802606777057544. [DOI] [PubMed] [Google Scholar]
- 40.Gogas KR. Glutamate-based therapeutic approaches: NR2B receptor antagonists. Curr Opin Pharmacol. 2006;6(1):68–74. doi: 10.1016/j.coph.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 41.The Ministry of Science and Technology of the People's Republic of China. Guidance Suggestions for the Care and Use of Laboratory Animals. 2006 Sep 30; [Google Scholar]
- 42.Hahn CD, Manlhiot C, Schmidt MR, et al. Remote ischemic per-conditioning: a novel therapy for acute stroke? Stroke. 2011;42(10):2960–2962. doi: 10.1161/STROKEAHA.111.622340. [DOI] [PubMed] [Google Scholar]
- 43.Longa EZ, Weinstein PR, Carlson S, et al. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20(1):84–91. doi: 10.1161/01.str.20.1.84. [DOI] [PubMed] [Google Scholar]
- 44.Willner P. Chronic mild stress (CMS) revisited: consistency and behavioural-neurobiological concordance in the effects of CMS. Neuropsychobiology. 2005;52(2):90–110. doi: 10.1159/000087097. [DOI] [PubMed] [Google Scholar]
- 45.Nishida F, Morel GR, Hereñú CB, et al. Restorative effect of intracerebroventricular insulin-like growth factor-I gene therapy on motor performance in aging rats. Neuroscience. 2011;177:195–206. doi: 10.1016/j.neuroscience.2011.01.013. [DOI] [PubMed] [Google Scholar]
- 46.Wang SH, Zhang ZJ, Guo YJ, et al. Decreased expression of serotonin 1A receptor in the dentate gyrus in association with chronic mild stress: a rat model of post-stroke depression. Psychiatry Res. 2009;170(2-3):245–251. doi: 10.1016/j.psychres.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 47.Duan Q, Wang X, Gong W, et al. ER stress negatively modulates the expression of the miR-199a/214 cluster to regulates tumor survival and progression in human hepatocellular cancer. PLoS One. 2012;7(2):e31518. doi: 10.1371/journal.pone.0031518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang SH, Wang CJ, Shi L, et al. High Expression of FLOT1 Is Associated with Progression and Poor Prognosis in Hepatocellular Carcinoma. PLoS One. 2013;8(6):e64709. doi: 10.1371/journal.pone.0064709. [DOI] [PMC free article] [PubMed] [Google Scholar]


