Abstract
In the present study, we investigated the effects of hypothyroidism on the morphology of astrocytes and microglia in the hippocampus of Zucker diabetic fatty rats and Zucker lean control rats. To induce hypothyroidism, Zucker lean control and Zucker diabetic fatty rats at 7 weeks of age orally received the vehicle or methimazole, an anti-thyroid drug, treatment for 5 weeks and were sacrificed at 12 weeks of age in all groups for blood chemistry and immunohistochemical staining. In the methimazole-treated Zucker lean control and Zucker diabetic fatty rats, the serum circulating thyronine (T3) and thyroxine (T4) levels were significantly decreased compared to levels observed in the vehicle-treated Zucker lean control or Zucker diabetic fatty rats. This reduction was more prominent in the methimazole-treated Zucker diabetic fatty group. Glial fibrillary acidic protein immunoreactive astrocytes and ionized calcium-binding adapter molecule 1 (Iba-1)-immunoreactive microglia in the Zucker lean control and Zucker diabetic fatty group were diffusely detected in the hippocampal CA1 region and dentate gyrus. There were no significant differences in the glial fibrillary acidic protein and Iba-1 immunoreactivity in the CA1 region and dentate gyrus between Zucker lean control and Zucker diabetic fatty groups. However, in the methimazole-treated Zucker lean control and Zucker diabetic fatty groups, the processes of glial fibrillary acidic protein tive astrocytes and Iba-1 immunoreactive microglia, were significantly decreased in both the CA1 region and dentate gyrus compared to that in the vehicle-treated Zucker lean control and Zucker diabetic fatty groups. These results suggest that diabetes has no effect on the morphology of astrocytes and microglia and that hypothyroidism during the onset of diabetes prominently reduces the processes of astrocytes and microglia.
Keywords: neural regeneration, astrocytes, microglia, diabetes, hypothyroidism, complications, thyroid hormone, obesity, methimazole, Zucker diabetic fatty rat, grants-supported paper, neuroregeneration
Research Highlights
(1) In the present study, we induced hypothyroidism during the onset of type 2 diabetes in Zucker diabetic fatty rats.
(2) The complex effects of type 2 diabetes and methimazole induced hypothyroidism were not fully confirmed.
(3) Hypothyroidism in type 2 diabetic patients affects the structure of hippocampal astrocytes and microglia.
INTRODUCTION
Thyroid hormones are essential for brain development[1,2]. Over the last few decades, great effort has been made to implement programs for the prevention of neonatal hypothyroidism because fetal and postnatal neuronal development is closely related to thyroid hormone action[3,4,5,6]. However, less attention has been focused on adult-onset hypothyroidism although it is a frequent condition in humans with the prevalence of increasing lifespan[7,8]. In rat, adult onset hypothyroidism induces amyloidogenic pathway and impaired hippocampal long-term potentiation and spatial memory performance[9,10]. In addition, thyroid hormone is a fundamental regulator of biological processes, including cell proliferation, differentiation, and metabolic balance[11,12,13]. Specifically, thyroid hormone affects the differentiation and maturation of different glial subtypes including astrocytes and microglia[12,13]. Astrocytes and microglia are prevalent neuroglia in the brain. The physiological role of astrocyte and microglia is now unveiling and participation in development, neuronal plasticity, and neuronal circuit are suggested[14,15,16]. Researches about the microglia and astrocytes in pathological states were conducted in plentiful studies[17,18,19,20,21,22]. However, the role of both glial cells is still controversial. Reactive gliosis by astrocytes and microglia is usually related with the pathological processes[23,24,25,26,27]. Additionally, the astrogliosis is considered as neuroprotective actions against neuronal damages[28,29]. Astroglial atrophy which results in malfunction of supportive role of astrocytes is thought as one of reason of early synaptic and cognitive impairment[30]. Hippocampal astrogliosis is reported in an animal model of hypothyroidism[31]. However, hypothyroidism delayed induction of reactive gliosis by brain injury like stabbing or ischemia[32,33]. Diabetes influences glial fibrillary acidic protein (GFAP) immunoreactivity in streptozotocin-induced rats[34] and also increases microglial proliferation in stroke patients[35]. Also in type 2 diabetes mice, impaired memory and coexistent astrogliosis and synaptotoxicity are attenuated by caffeine treatment[36]. Although the incidence of hypothyroidism is significantly increased (5.7%) in diabetic patients compared to the control population without diabetes (1.8%)[37], there were few reports regarding the effects of adult onset hypothyroidism on the astrocytes and microglia in type 2 diabetic animals.
Therefore, in the present study, we investigated the effects of hypothyroidism on astrocytes and microglia in the hippocampus during adult onset of diabetes using type 2 diabetic animals: Zucker diabetic fatty (Leprfa/fa) and its wild type, Zucker lean control (Lepr+/+) rats.
RESULTS
Quantitative analysis of animals
Ten Zucker diabetic fatty and 10 Zucker lean control rats were randomly divided into two groups with five rats in each group: vehicle-treated and methimazole-treated groups. Distilled water and methimazole was administered to Zucker lean control and Zucker diabetic fatty rats orally in drinking water for 5 weeks (7–12 weeks old). All Zucker diabetic fatty and Zucker lean control rats were used in the final analysis.
Effects of hypothyroidism on body weight, food intake, and blood glucose levels in rats with type 2 diabetes
In all groups, body weight was steadily increased with age. However, the body weight in vehicle-treated Zucker diabetic fatty rats was significantly higher (P < 0.05) compared to that in the vehicle-, methimazole-treated Zucker lean control rats as well as that in the methimazole-treated Zucker diabetic fatty rats at 9 weeks of age and this pattern was maintained until 12 weeks of age. Body weight was significantly decreased in the methimazole-treated Zucker lean control and Zucker diabetic fatty rats at 9 weeks of age compared to that in the vehicle-treated Zucker lean control and Zucker diabetic fatty rats, respectively. Especially, body weight was prominently lower in the methimazole-treated Zucker diabetic fatty rats compared to that in the vehicle-treated Zucker diabetic fatty rats (Figure 1).
Figure 1.
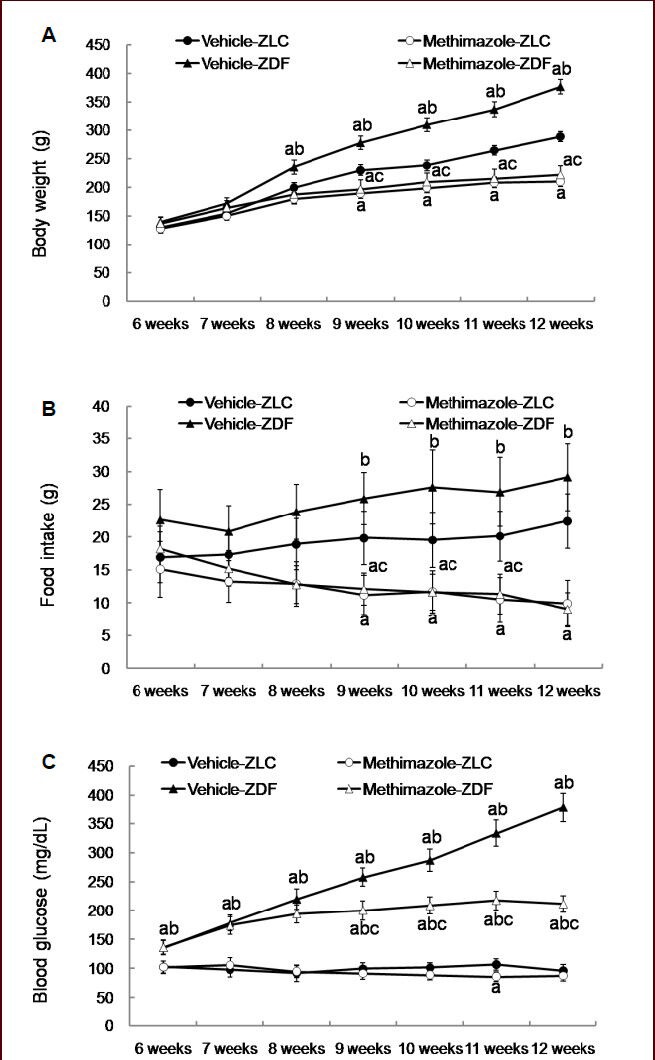
Changes in body weight (A), food intake (B) and blood glucose levels (C) in vehicle-treated Zucker lean control (vehicle-ZLC), methimazole-treated ZLC (methimazole-ZLC), vehicle-treated Zucker diabetic fatty (vehicle-ZDF), and methimazole-treated Zucker diabetic fatty (methimazole-ZDF) rats.
Differences between the means were analyzed using two-way analysis of variance (ANOVA) followed by Bonferroni's post-tests (n = 5 per group; aP < 0.05, vs. ZLC group; bP < 0.05, vs. ZDF group; cP < 0.05, vs. ZLC-methi group). The bars indicate mean ± standard error (SE).
Similarly, food intake was high in the vehicle-treated Zucker diabetic fatty rats compared to that in the vehicle-treated Zucker lean control rats although the significance was not detected. Food intake was significantly decreased at 9 weeks of age in both methimazole-treated Zucker lean control and Zucker diabetic fatty rats compared to that in the vehicle-treated Zucker lean control and Zucker diabetic fatty rats, respectively (P < 0.05; Figure 1).
Blood glucose levels was increased in vehicle-treated Zucker diabetic fatty rats with age, but blood glucose levels in methimazole-treated Zucker diabetic fatty rats increased at early stage (until 8 weeks of age) and was significantly lower from 9 to 12 weeks of age compared to that in the vehicle-treated Zucker diabetic fatty rats (Figure 1).
Effects of hypothyroidism on circulating free tri-iodothyronin (T3), and thyroxine (T4) levels in serum in rats with type 2 diabetes
At 12 weeks of age, in the methimazole-treated Zucker lean control group, the average circulating T3 and T4 levels were significantly decreased compared to that observed in vehicle-treated Zucker lean control group, respectively (P < 0.05). In the methimazole-treated Zucker diabetic fatty group, the average circulating T3 and T4 levels were significantly decreased compared to that in the vehicle-treated Zucker diabetic fatty group (P < 0.05). In methimazole-treated Zucker diabetic fatty group, the average circulating T3 and T4 levels were low compared to that observed in the methimazole-treated Zucker lean control group (Figure 2).
Figure 2.
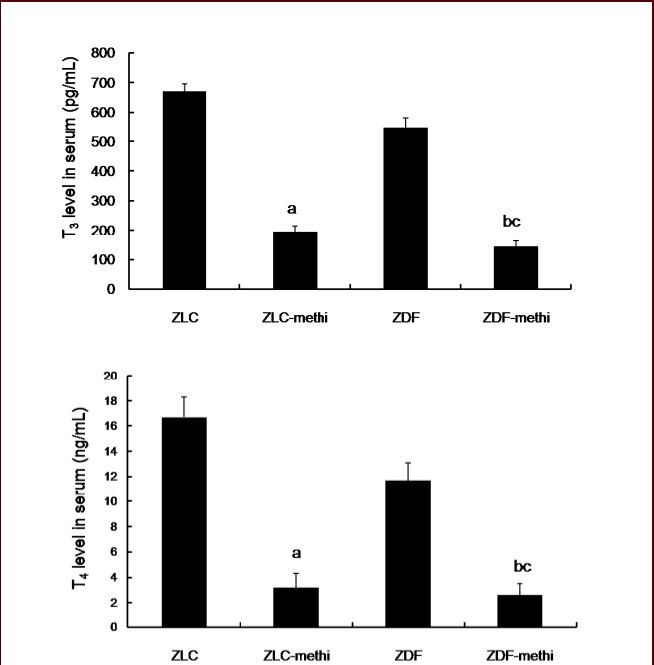
Changes in serum circulating free tri-iodothyronin (T3), and thyroxine (T4) levels in the vehicle-treated Zucker lean control (ZLC), methimazole-treated ZLC (ZLC-methi), vehicle-treated Zucker diabetic fatty (ZDF), and methimazole-treated ZDF (ZDF-methi) rats.
Differences between the means were analyzed using two-way analysis of variance (ANOVA) followed by Bonferroni's post-tests (n = 5 per group; aP < 0.05, vs. ZLC group; bP < 0.05, vs. ZDF group; cP < 0.05, vs. ZLC-methi group). The bars indicate mean ± standard error (SE).
Effects of hypothyroidism on astrocytes in the hippocampus in rats with type 2 diabetes
At 12 weeks of age, the GFAP immunoreactive astrocytes in vehicle-treated Zucker lean control group were found in the polymorphic and molecular layer of the dentate gyrus and in the strata oriens and radiatum of the hippocampal CA1 region (Figure 3A, B). In this group, the GFAP immunoreactive astrocytes had a small cytoplasm with long processes. In the methimazole-treated Zucker lean control group, the overall morphology and the number of GFAP immunoreactive astrocytes was similar to vehicle-treated Zucker lean control group in the dentate gyrus and hippocampal CA1 region (Figure 3C, D, J). However, some GFAP immunoreactive astrocytes had poorly developed processes in the polymorphic layer of the dentate gyrus (Figure 3C) and strata oriens and radiatum of the CA1 region (Figure 3D), respectively. In methimazole-treated Zucker lean control group, the GFAP immunoreactivity in the dentate gyrus and CA1 region was significantly decreased compared to those observed in the vehicle-treated Zucker lean control group, respectively (Figure 3I). In the vehicle-treated Zucker diabetic fatty group, the GFAP immunoreactive astrocytes demonstrated a similar morphology and the number in the dentate gyrus and hippocampal CA1 region (Figure 3E, F, J). In addition, the GFAP immunoreactivity in the dentate gyrus and CA1 region was similar between the vehicle-treated Zucker lean control and vehicle-treated Zucker diabetic fatty group, respectively (Figure 3I). In the methimazole-treated Zucker diabetic fatty group, the GFAP immunoreactive astrocytes had a small cytoplasm with poorly developed processes in the polymorphic layer of the dentate gyrus (Figure 3G) and strata oriens and radiatum of the CA1 region (Figure 3H). In methimazole-treated Zucker diabetic fatty group, the GFAP immunoreactivity in the dentate gyrus and CA1 region was significantly decreased compared to those observed in the methimazole-treated Zucker lean control group as well as vehicle-treated Zucker diabetic fatty group, respectively (Figure 3I). However, the number of GFAP immunoreactive astrocytes was not changed (Figure 3J).
Figure 3.
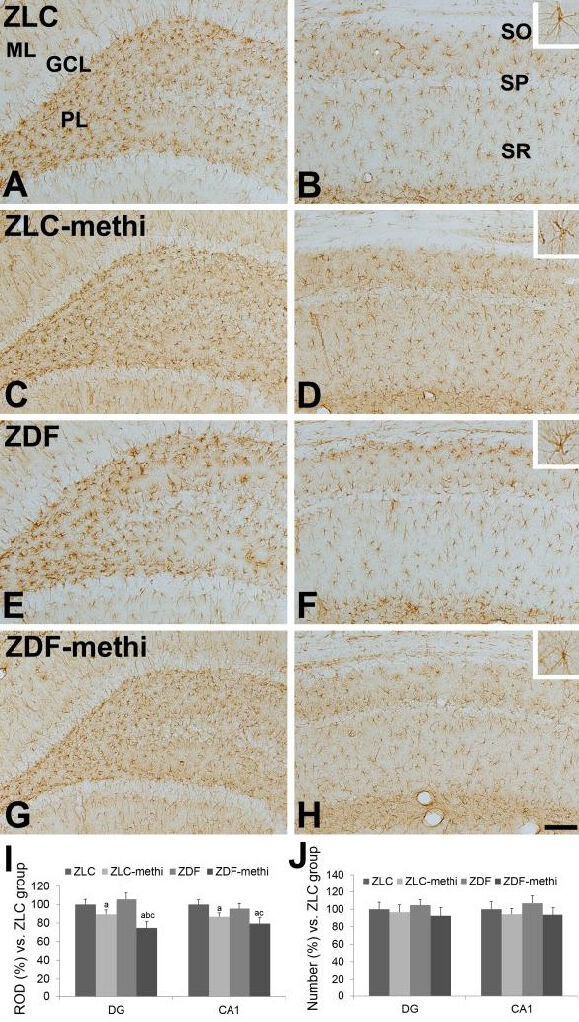
Glial fibrillary acidic protein (GFAP) immunoreactivity in the dentate gyrus (A, C, E, and G), and CA1 region (B, D, F, and H) of vehicle-treated Zucker lean control (ZLC; A, B), methimazole-treated ZLC (ZLC-methi; C, D), vehicle-treated Zucker diabetic fatty (ZDF; E, F), and methimazole-treated ZDF (ZDF-methi; G, H) rats.
GFAP immunoreactivity is mainly detected in the polymorphic layer (PL) and molecular layer (ML) of the dentate gyrus and stratum oriens (SO) and radiatum (SR) of CA1 region. GFAP immunoreactive astrocytes have retracted processes in the ZLC-methi and ZDF-methi groups compared to those observed in the ZLC or ZDF group. These phenomena are prominent in the ZDF-methi group compared to that in the ZLC-methi group. In contrast, the number of GFAP immunoreactive cells is not significantly different between groups. GCL: Granule cell layer; SP: stratum pyramidale. Scale bar: 100 μm.
The relative optical densities (ROD, I) and the relative number (J), expressed as a percentage of the value in the ZLC group of GFAP immunoreactivity in the dentate gyrus and hippocampal CA1 region per section of the ZLC, ZLC-methi, ZDF, and ZDF-methi groups. Differences between the means were analyzed using two-way analysis of variance (ANOVA) followed by Bonferroni's post-tests (10 sections per animal and 5 animals per group. aP < 0.05, vs. ZLC group; bP < 0.05, vs. ZDF group; cP < 0.05, vs. ZLC-methi group). The bars indicate mean ± standard error (SE).
Effects of hypothyroidism on microglia in the hippocampus
At 12 weeks of age, ionized calcium-binding adapter molecule 1 (Iba-1) immunoreactive microglia in the vehicle-treated Zucker lean control group were found in the polymorphic and molecular layer of the dentate gyrus and in the strata oriens and radiatum of the hippocampal CA1 region (Figure 4A, B). In this group, Iba-1 immunoreactive microglia had a round cytoplasm with ramified processes. In the methimazole-treated Zucker lean control group, Iba-1 immunoreactive microglia showed morphological changes; the processes were retracted in the dentate gyrus and hippocampal CA1 region (Figure 4C, D).
Figure 4.
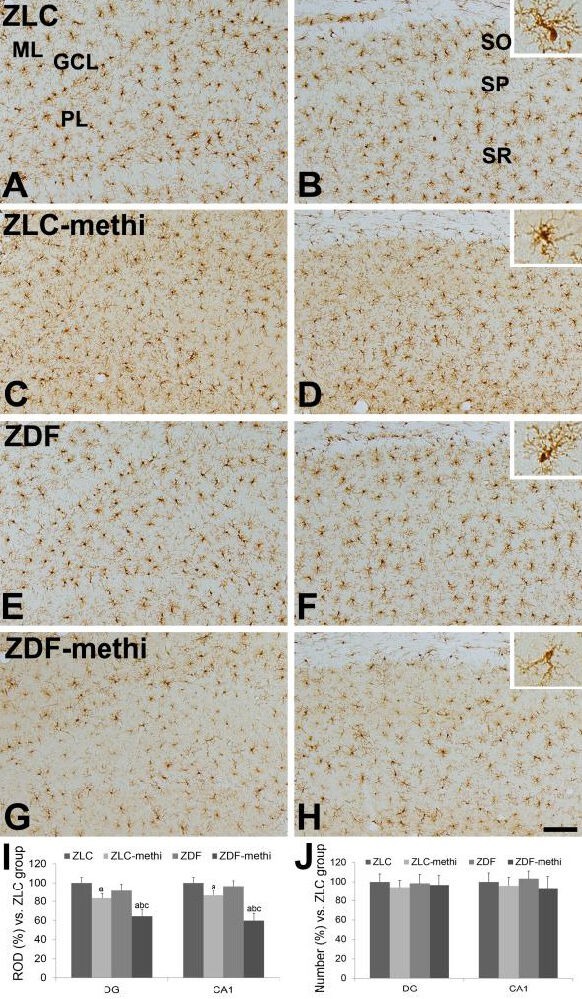
Ionized calcium-binding adapter molecule 1 (Iba-1) immunoreactivity in the dentate gyrus (A, C, E, and G), and CA1 region (B, D, F, and H) of vehicle-treated Zucker lean control (ZLC; A, B), methimazole-treated ZLC (ZLC-methi; C, D), vehicle-treated Zucker diabetic fatty (ZDF; E, F), and methimazole-treated ZDF (ZDF-methi; G, H) rats.
Iba-1 immunoreactivity is mainly detected in the polymorphic layer (PL) and molecular layer (ML) of the dentate gyrus and stratum oriens (SO) and radiatum (SR) of the CA1 region. The Iba-1 immunoreactive microglias in the ZLC-methi and ZDF-methi group have less ramified processes compared to those identified in the ZLC or ZDF group. These morphological changes are prominent in the ZDF-methi group compared to those in the ZLC-methi group. However, there are no significant differences in the number of Iba-1 immunoreactive cells between groups. GCL: Granule cell layer; SP: stratum pyramidale. Scale bar: 100 μm.
The relative optical densities (ROD; I) and the relative number (J), expressed as a percentage of the value in the vehicle-treated ZLC group of Iba-1 immunoreactivity in the dentate gyrus and hippocampal CA1 region per section of the ZLC, ZLC-methi, ZDF, and ZDF-methi groups. Differences between the means were analyzed using two-way analysis of variance (ANOVA) followed by Bonferroni's post-tests (10 sections per animal and 5 animals per group. aP < 0.05, vs. ZLC group; bP < 0.05, vs. ZDF group; cP < 0.05, vs. ZLC-methi group). The bars indicate mean ± standard error (SE).
However, the number of Iba-1 immunoreactive microglia was similar between vehicle-treated and methimazole-treated groups (Figure 4J). In the vehicle-treated Zucker diabetic fatty group, the distribution pattern and morphology of Iba-1 immunoreactive microglia were similar to those observed in the Zucker lean control group (Figure 4E, F). In addition, the Iba-1 immunoreactivity and number in the dentate gyrus and CA1 region of this group was similar to those identified in the Zucker lean control group (Figure 4I). In the methimazole-treated Zucker diabetic fatty group, the Iba-1 immunoreactive microglias were markedly decreased in the polymorphic and molecular layer of the dentate gyrus and in the strata oriens and radiatum of the hippocampal CA1 region (Figure 4G, H). Specifically, the Iba-1 immunoreactive microglia in this group had poorly ramified processes. In addition, the Iba-1 immunoreactivity in this group was significantly decreased in the dentate gyrus and CA1 region compared to that observed in the methimazole-treated Zucker lean control group and vehicle-treated Zucker diabetic fatty group (Figure 4I). However, the number of Iba-1 immunoreactive microglia was not changed (Figure 4J).
DISCUSSION
Hypothyroidism is a prevalent thyroid disorder in both the elderly population and in type 2 diabetic patients[38,39,40]. In the present study, we induced hypothyroidism during the onset of type 2 diabetes in Zucker diabetic fatty rats. The circulating serum T3 and T4 levels were significantly decreased in the vehicle-treated Zucker diabetic fatty rats compared to that observed in the vehicle-treated Zucker lean control rats. This result was supported by previous studies which reported the significantly low levels of plasma T3 in the obese animals as compared to their lean (euthyroid) control littermates[41,42,43].
Similarly, the serum T3 level was significantly lower in diabetic patients as compared to normal subjects[44]. The administration of methimazole to Zucker lean control and Zucker diabetic fatty rats significantly reduced the serum T3 levels as reported previously[45].
Next, we made the observation that type 2 diabetes did not demonstrate any differences in the distribution pattern and morphology of GFAP immunoreactive astrocytes and Iba-1 immunoreactive microglia in the hippocampus. This result was contradictory to the streptozotocin-induced type 1 diabetic model where a reduced GFAP-positive cell count was found on day 3 when these cells were significantly decreased in size and less arborized with respect to the control[34]. This observation was reversed on day 7 when the GFAP-positive cells increased in both number and size in addition to becoming more ramified[34]. In contrast, increase of astrocytes was observed in type 2 diabetic mice after 4 months duration of diabetic state[36]. In addition, microglial proliferation was more prominent in the peri-infarct region and in the non-lesional hemisphere in the presence of diabetes mellitus than in the absence of diabetes mellitus in stroke patients[35]. This discrepancy may be associated with the potency and duration of diabetes.
In the present study, we observed that hypothyroidism significantly decreased the processes of GFAP immunoreactive astrocytes in the hippocampus of Zucker lean control and Zucker diabetic fatty rats although the number of GFAP immunoreactive astrocytes was not changed. This result was supported by previous studies that a reduction in the number of mature astrocytes was prominent in the brain of hypothyroid animals within the white matter tracts[46,47]. In addition, in hypothyroid neonatal rats there was a reduction in the GFAP content in the hippocampus and basal forebrain[48]. In the previous study, we reported that methimazole significantly alleviated the reduction of cell proliferation in the subgranular zone of the dentate gyrus compared to that observed in the vehicle-treated Zucker diabetic fatty rats[49]. The reduction of GFAP immunoreactivity in the present study may be related to the decreased maturation of astrocytes and/or the damage to mature astrocytes in the hippocampus. Additionally, thyroid hormone regulates the morphological maturation of astrocytes (transition from radial glia to GFAP positive cell) and the expression of GFAP[50].
In addition, we observed that hypothyroidism in Zucker lean control and Zucker diabetic fatty rats significantly decreased the ramified processes of Iba-1 immunoreactive microglia in the hippocampus although the number of Iba-1 immunoreactive microglia was not changed. Purified amoeboid-shaped microglia respond to T3 exposure by increasing the extension of cell processes, an important step in the acquisition of a ramified phenotype[12]. In the present study, the significant reduction of T3 may be associated with the decrease of ramified processes of the microglia. This result was supported by a previous study which demonstrated a diminished number of cell bodies and a decreased density of abundant microglial process in the cortical forebrain of the hypothyroid neonatal rat[12,51]. Conversely, T3 favored the survival of microglial cells in vitro and may have induced the extension of their processes[12,51].
Diabetes is reported to exacerbate ischemic brain damage associated with higher morbidity and mortality[52,53,54,55]. In streptozotocin-induced rats, Cu or Ag intoxication exhibits enhanced neurotoxicity and exacerbation of sensory, motor and cognitive function as compared to normal animals[56]. In the present study, the decrease of processes in GFAP immunoreactive astrocytes and Iba-1 immunoreactive microglia was prominent in the methimazole-treated Zucker diabetic fatty rats compared to that in the methimazole-treated Zucker lean control rats. This result suggests that the diabetes exacerbates hypothyroidism-induced damage in astrocytes and microglia.
In conclusion, adult onset of hypothyroidism significantly decreases the processes of astrocytes and microglia without any changes in number of astrocytes and microglia. In addition, diabetes aggravates hypothyroidism-induced dysfunction of astrocytes and microglia in the hippocampus.
MATERIALS AND METHODS
Design
A randomized, controlled, animal experiment.
Time and setting
This study was performed at the Department of Anatomy and Cell Biology, College of Veterinary Medicine, and Research Institute for Veterinary Science, Seoul National University, Seoul, South Korea from 2009 to 2011.
Materials
Male and female heterozygous type (Leprfa/+) of Zucker diabetic fatty rats were acquired from Genetic Models (Indianapolis, ME) and mated to get homozygous 10 male Zucker diabetic fatty and Zucker lean control rats each. The animals were housed in a conventional state under adequate temperature (23°C) and humidity (60%) control with a 12-hour light-dark cycle, and were fed tap water and Purina 5008 diet ad libitum as recommended by Genetic Models Co. (Purina, St. Louis, MO, USA). The procedures for handling and caring of animals follow the Guide for the Care and Use of Laboratory Animals issued by Institute of Laboratory Animal Resources, U.S.A., 1996. All of the experiments were conducted to minimize the number of animals utilized and the suffering caused by the procedures of the present study.
Methods
Genotyping of Leprfa gene and experimental design
Genotype of Leprfa gene herein was determined with the process described in our previous study[57]. For effects of 2-mercapto-1-methyl-imidazole (methimazole) induced hypothyroidism on astrocytes and microglia in the hippocampus during onset of diabetes, Zucker lean control and Zucker diabetic fatty rats were randomly divided into vehicle- and methimazole-treated groups (n = 5 in each group). At 7 weeks of age, 0.03% methimazole (300 mg per 1 000 mL distilled water; Sigma, St. Louis, MO, USA) was administered to Zucker lean control and Zucker diabetic fatty groups orally in drinking water for 5 weeks because Zucker diabetic fatty rats show insulin insufficiency from 7 or 8 weeks of ages and impairment in glucose disposal and hepatic glucose suppression[58]. Methimazole was used to prevent thyroid hormone synthesis by inhibiting coupling and iodination[59].
Food intake, body weight, and blood glucose sampling
Total food/water intake and body weight over the course of the study (between 6 and 12 weeks of age) were also determined for each animal by summing the weekly averages. To measure blood glucose concentration, blood was sampled each morning (9:00 a.m.) by “tail nick” using a 27 G needle and analyzed by using a blood glucose monitor (Ascensia Elite XL Blood Glucose Meter, Bayer, Toronto, ON, Canada).
Serum levels of thyroid hormones
To confirm the hypothyroid state, blood samples collected from left ventricle of heart in the morning (9:00–11:00 a.m.) were drawn from the euthyroid and hypothyroid rats upon killing at the age of 12 weeks for analysis of serum circulating free tri-iodothyronin (T3), and thyroxine (T4) levels to determine thyroid function in these rats using commercial assay kits (Monobind, Inc., Lake Forest, CA, USA).
Immunohistochemistry for GFAP and Iba-1
Animals in each group were anesthetized with an intraperitoneal injection of 30 mg/kg Zoletil 50 (Virbac, Carros, France) and perfused transcardially with 0.1 mol/L phosphate-buffered saline (PBS; pH 7.4) followed by 4% paraformaldehyde in 0.1 mol/L phosphate buffer (PB; pH 7.4). The brains were removed and postfixed in the same fixative for 6 hours. The brain tissues were cryoprotected by infiltration with 30% sucrose overnight. The 30-μm-thick brain sections in the coronal plane were serially cut using a cryostat (Leica, Wetzlar, Germany). The sections were collected into 6-well plates containing PBS for further processing.
To obtain the accurate data for immunohistochemistry, the free-floating sections were carefully processed under the same conditions. The tissue sections were selected between –3.00 and –4.08 mm to the bregma in reference to the rat atlas[60] for each animal. Ten sections were in 90 μm apart from each other, and the sections were sequentially treated with 0.3% hydrogen peroxide (H2O2) in PBS for 30 minutes and 10% normal goat serum in 0.05 mol/L PBS for 30 minutes. They were then incubated with diluted rabbit anti-GFAP antibody (diluted 1:1 000; Chemicon International, Temecula, CA, USA) and rabbit anti-Iba-1 antibody (1:500; Wako, Osaka, Japan) overnight at room temperature and subsequently exposed to biotinylated goat anti-rabbit IgG and streptavidin peroxidase complex (1:200; Vector, Burlingame, CA, USA). They were then visualized by reaction to 3,3’-diaminobenzidine tetrachloride (Sigma) in 0.1 mol/L Tris-HCl buffer (pH 7.2) and mounted on gelatin-coated slides. The sections were mounted in Canada Balsam (Kanto, Tokyo, Japan) following dehydration.
The number of GFAP immunoreactive astrocytes and Iba-1 immunoreactive microglia in the dentate gyrus and hippocampal CA1 region was counted using an image analyzing system equipped with a computer-based CCD camera (Optimas 6.5 software, CyberMetrics, Scottsdale, AZ, USA). Analysis of a region of interest in the hippocampal CA1 region or dentate gyrus was performed using an image analysis system. Images were calibrated into an array of 512 × 512 pixels corresponding to a tissue area of 140 μm × 140 μm (40 × primary magnification). Each pixel resolution was 256 gray levels. The intensity of GFAP and Iba-1 immunoreactivity was evaluated by means of a relative optical density (ROD), which was obtained after transformation of the mean gray level using the formula: ROD = log (256/mean gray level). ROD of background was determined in unlabeled portions and the value subtracted for correction, yielding high ROD values in the presence of preserved structures and low values after structural loss using NIH Image 1.59 software (National Institutes of Health, Bethesda, MD, USA). A ratio of the ROD was calibrated as percentage.
Statistical analysis
The data shown here represent the mean ± standard error (SE) of experiments performed for each experimental region. Differences among the means were statistically analyzed by two-way analysis of variance followed by Bonferroni's post-tests in order to elucidate differences among the groups using GraphPad Prism 5.0 software (GraphPad Software Inc., La Jolla, CA, USA).
Research background: Thyroid hormone is essential for brain development. It is worth mentioning that thyroid hormone also affects the differentiation and maturation of astrocytes and microglia.
Research frontiers: At present, there are many studies regarding hypothyroidism in neonates, but few studies were about hypothyroidism in adults. Studies regarding the effects of adult onset hypothyroidism on astrocytes and microglia in animals with type 2 diabetes are hardly reported.
Clinical significance: Maintaining a constant level of thyroid hormone is of important significance for preventing against cognitive dysfunction and hippocampal abnormality in diabetic patients.
Academic terminology: Zucker diabetic fatty rats are selected from Zucker (fa/fa) rats with diabetic phenotype. Zucker diabetic fatty rats are characterized by typical obesity, hyperinsulinemia, hyperglycemia, hyperlipemia, peripheral insulin resistance and hypertension and therefore are ideal animal models of type 2 diabetes.
Peer review: Cognitive dysfunction and hippocampal abnormality are the major diabetes-related complications. It is important whether hypothyroidsm contributes to these complications in diabetes.
Footnotes
Funding: This work was supported by the National Research Foundation of Korea Grant funded by the Korean Government (MEST), Republic of Korea, No. 2010-0007712.
Conflicts of interest: None declared.
Ethical approval: The protocol for handling and caring of animals was approved by the Institutional Animal Care and Use Committee (IACUC) of Seoul National University, South Korea.
(Reviewed by Choi JI, Zhang N, Wang LS)
(Edited by Li CH, Song LP, Liu WJ, Zhao M)
REFERENCES
- 1.Patel J, Landers K, Li H, et al. Mortimer RH, Richard K. Thyroid hormones and fetal neurological development. J Endocrinol. 2011;209:1–8. doi: 10.1530/JOE-10-0444. [DOI] [PubMed] [Google Scholar]
- 2.Horn S, Heuer H. Thyroid hormone action during brain development: more questions than answers. Mol Cell Endocrinol. 2010;315:19–26. doi: 10.1016/j.mce.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 3.Anderson GW, Schoonover CM, Jones SA. Control of thyroid hormone action in the developing rat brain. Thyroid. 2003;13:1039–1056. doi: 10.1089/105072503770867219. [DOI] [PubMed] [Google Scholar]
- 4.Gilbert ME, Sui L, Walker MJ, et al. Thyroid hormone insufficiency during brain development reduces parvalbumin immunoreactivity and inhibitory function in the hippocampus. Endocrinology. 2007;148:92–102. doi: 10.1210/en.2006-0164. [DOI] [PubMed] [Google Scholar]
- 5.Lasley SM, Gilbert ME. Developmental thyroid hormone insufficiency reduces expression of brain-derived neurotrophic factor (BDNF) in adults but not in neonates. Neurotoxicol Teratol. 2011;33:464–472. doi: 10.1016/j.ntt.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 6.Williams GR. Neurodevelopmental and neurophysiological actions of thyroid hormone. J Neuroendocrinol. 2008;20:784–794. doi: 10.1111/j.1365-2826.2008.01733.x. [DOI] [PubMed] [Google Scholar]
- 7.Roberts CGP, Ladenson PW. Hypothyroidism. Lancet. 2004;363:793–803. doi: 10.1016/S0140-6736(04)15696-1. [DOI] [PubMed] [Google Scholar]
- 8.Vanderpump MP, Tunbridge WM. Epidemiology and prevention of clinical and subclinical hypothyroidism. Thyroid. 2002;12:839–847. doi: 10.1089/105072502761016458. [DOI] [PubMed] [Google Scholar]
- 9.Ghenimi N, Alfos S, Redonnet A, et al. Adult-onset hypothyroidism induces the amyloidogenic pathway of amyloid precursor protein processing in the rat hippocampus. J Neuroendocrinol. 2010;22:951–959. doi: 10.1111/j.1365-2826.2010.02002.x. [DOI] [PubMed] [Google Scholar]
- 10.Artis AS, Bitiktas S, Taşkın E, et al. Experimental hypothyroidism delays field excitatory post-synaptic potentials and disrupts hippocampal long-term potentiation in the dentate gyrus of hippocampal formation and Y-maze performance in adult rats. J Neuroendocrinol. 2012;24:422–433. doi: 10.1111/j.1365-2826.2011.02253.x. [DOI] [PubMed] [Google Scholar]
- 11.Larsen PR, Davies TF, Hay ID. The thyroid gland. In: Wilson JD, Foster DW, Kronenberg HM, Larsen PR, editors. Williams Textbook of Endocrinology. 9th ed. Philadelphia: W.B. Sanunders Co; 1998. [Google Scholar]
- 12.Lima FRS, Gervais A, Colin C, et al. Regulation of microglial development: a novel role for thyroid hormone. J Neurosci. 2001;21:2028–2038. doi: 10.1523/JNEUROSCI.21-06-02028.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Trentin AG. Thyroid hormone and astrocyte morphogenesis. J Endocrinol. 2006;189:189–197. doi: 10.1677/joe.1.06680. [DOI] [PubMed] [Google Scholar]
- 14.Pont-Lezica L, Béchade C, Belarif-Cantaut Y, et al. Physiological roles of microglia during development. J Neurochem. 2011;119:901–908. doi: 10.1111/j.1471-4159.2011.07504.x. [DOI] [PubMed] [Google Scholar]
- 15.Tremblay MÈ, Stevens B, Sierra A, et al. The role of microglia in the healthy brain. J Neurosci. 2011;31:16064–16069. doi: 10.1523/JNEUROSCI.4158-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pascual O, Ben Achour S, Rostaing P, et al. Microglia activation triggers astrocyte-mediated modulation of excitatory neurotransmission. Proc Natl Acad Sci U S A. 2012;109:E197–E205. doi: 10.1073/pnas.1111098109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Serrano-Pozo A, Muzikansky A, Gómez-Isla T, et al. Differential relationships of reactive astrocytes and microglia to fibrillar amyloid deposits in Alzheimer disease. J Neuropathol Exp Neurol. 2013;72:462–471. doi: 10.1097/NEN.0b013e3182933788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Skaper SD. Ion channels on microglia: therapeutic targets for neuroprotection. CNS Neurol Disord Drug Targets. 2011;10:44–56. doi: 10.2174/187152711794488638. [DOI] [PubMed] [Google Scholar]
- 19.Tanaka Y, Matsuwaki T, Yamanouchi K, et al. Exacerbated inflammatory responses related to activated microglia after traumatic brain injury in progranulin-deficient mice. Neuroscience. 2013;231:49–60. doi: 10.1016/j.neuroscience.2012.11.032. [DOI] [PubMed] [Google Scholar]
- 20.McGann JC, Lioy DT, Mandel G. Astrocytes conspire with neurons during progression of neurological disease. Curr Opin Neurobiol. 2012;22:850–858. doi: 10.1016/j.conb.2012.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Singh S, Swarnkar S, Goswami P, et al. Astrocytes and microglia: responses to neuropathological conditions. Int J Neurosci. 2011;121:589–597. doi: 10.3109/00207454.2011.598981. [DOI] [PubMed] [Google Scholar]
- 22.Liu W, Tang Y, Feng J. Cross talk between activation of microglia and astrocytes in pathological conditions in the central nervous system. Life Sci. 2011;89:141–146. doi: 10.1016/j.lfs.2011.05.011. [DOI] [PubMed] [Google Scholar]
- 23.Croisier E, Graeber MB. Glial degeneration and reactive gliosis in alpha-synucleinopathies: the emerging concept of primary gliodegeneration. Acta Neuropathol. 2006;112:517–530. doi: 10.1007/s00401-006-0119-z. [DOI] [PubMed] [Google Scholar]
- 24.Tanuma N, Sakuma H, Sasaki A, et al. Chemokine expression by astrocytes plays a role in microglia/macro-phage activation and subsequent neurodegeneration in secondary progressive multiple sclerosis. Acta Neuropathol. 2006;112:195–204. doi: 10.1007/s00401-006-0083-7. [DOI] [PubMed] [Google Scholar]
- 25.Neary JT, Kang Y. Signaling from P2 nucleotide receptors to protein kinase cascades induced by CNS injury: implications for reactive gliosis and neurodegeneration. Mol Neurobiol. 2005;31:95–103. doi: 10.1385/MN:31:1-3:095. [DOI] [PubMed] [Google Scholar]
- 26.Pekny M, Nilsson M. Astrocyte activation and reactive gliosis. Glia. 2005;50:427–434. doi: 10.1002/glia.20207. [DOI] [PubMed] [Google Scholar]
- 27.Veiga S, Azcoitia I, Garcia-Segura LM. Ro5-4864, a peripheral benzodiazepine receptor ligand, reduces reactive gliosis and protects hippocampal hilar neurons from kainic acid excitotoxicity. J Neurosci Res. 2005;80:129–137. doi: 10.1002/jnr.20430. [DOI] [PubMed] [Google Scholar]
- 28.Kraft AW, Hu X, Yoon H, et al. Attenuating astrocyte activation accelerates plaque pathogenesis in APP/PS1 mice. FASEB J. 2013;27:187–198. doi: 10.1096/fj.12-208660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Duan YL, Wang SY, Zeng QW, et al. Astroglial reaction to delta opioid peptide [D-Ala2, D-Leu5] enkephalin confers neuroprotection against global ischemia in the adult rat hippocampus. Neuroscience. 2011;192:81–90. doi: 10.1016/j.neuroscience.2011.06.067. [DOI] [PubMed] [Google Scholar]
- 30.Rodríguez JJ, Olabarria M, Chvatal A, et al. Astroglia in dementia and Alzheimer's disease. Cell Death Differ. 2009;16:378–385. doi: 10.1038/cdd.2008.172. [DOI] [PubMed] [Google Scholar]
- 31.Cortés C, Eugenin E, Aliaga E, et al. Hypothyroidism in the adult rat causes incremental changes in brain-derived neurotrophic factor, neuronal and astrocyte apoptosis, gliosis, and deterioration of postsynaptic density. Thyroid. 2012;22:951–963. doi: 10.1089/thy.2010.0400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miyake T, Imamura Y, Fukuda M, et al. Delay of astrocyte reaction in the injured cerebral cortex of hypothyroid mouse. Brain Res. 1989;493:376–379. doi: 10.1016/0006-8993(89)91174-8. [DOI] [PubMed] [Google Scholar]
- 33.Lee CH, Yoo KY, Hwang IK, et al. Hypothyroid state does not protect but delays neuronal death in the hippocampal CA1 region following transient cerebral ischemia: focus on oxidative stress and gliosis. J Neurosci Res. 2010;88:2661–2668. doi: 10.1002/jnr.22436. [DOI] [PubMed] [Google Scholar]
- 34.Lebed YV, Orlovsky MA, Nikonenko AG, et al. Early reaction of astroglial cells in rat hippocampus to streptozotocin-induced diabetes. Neurosci Lett. 2008;444:181–185. doi: 10.1016/j.neulet.2008.07.094. [DOI] [PubMed] [Google Scholar]
- 35.Li G, Xu X, Wang D, et al. Microglial activation during acute cerebral infarction in the presence of diabetes mellitus. Neurol Sci. 2011;32:1075–1079. doi: 10.1007/s10072-011-0632-2. [DOI] [PubMed] [Google Scholar]
- 36.Duarte JM, Agostinho PM, Carvalho RA, et al. Caffeine consumption prevents diabetes-induced memory impairment and synaptotoxicity in the hippocampus of NONcZNO10/LTJ mice. PLoS One. 2012;7:e21899. doi: 10.1371/journal.pone.0021899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tamez-Pérez HE, Martínez E, Quintanilla-Flores DL, et al. The rate of primary hypothyroidism in diabetic patients is greater than in the non-diabetic population. An observational study. Med Clin (Barc) 2012;138:475–477. doi: 10.1016/j.medcli.2011.08.009. [DOI] [PubMed] [Google Scholar]
- 38.Díez JJ, Sánchez P, Iglesias P. Prevalence of thyroid dysfunction in patients with type 2 diabetes. Exp Clin Endocrinol Diabetes. 2011;119:201–207. doi: 10.1055/s-0031-1271691. [DOI] [PubMed] [Google Scholar]
- 39.Ishay A, Chertok-Shaham I, Lavi I, et al. Prevalence of subclinical hypothyroidism in women with type 2 diabetes. Med Sci Monit. 2009;15:CR151–CR155. [PubMed] [Google Scholar]
- 40.Sathyapalan T, Manuchehri AM, Rigby AS, et al. Subclinical hypothyroidism is associated with reduced all- cause mortality in patients with type 2 diabetes. Diabetes Care. 2010;33:e37. doi: 10.2337/dc09-1555. [DOI] [PubMed] [Google Scholar]
- 41.Jensen MD, Johnson CM, Cryer PE, et al. Thermogenesis after a mixed meal: role of leg and splanchnic tissues in men and women. Am J Physiol. 1995;268:E433–E438. doi: 10.1152/ajpendo.1995.268.3.E433. [DOI] [PubMed] [Google Scholar]
- 42.Katzeff HL, Selgrad C. Impaired peripheral thyroid hormone metabolism in genetic obesity. Endocrinology. 1993;132:989–995. doi: 10.1210/endo.132.3.8440199. [DOI] [PubMed] [Google Scholar]
- 43.Torrance CJ, Devente JE, Jones JP, et al. Effects of thyroid hormone on GLUT4 glucose transporter gene expression and NIDDM in rats. Endocrinology. 1997;138:1204–1214. doi: 10.1210/endo.138.3.4981. [DOI] [PubMed] [Google Scholar]
- 44.Kabadi UM. Serum T3 and reverse T3 concentrations: indices of metabolic control in diabetes mellitus. Diabetes Res. 1986;3:417–421. [PubMed] [Google Scholar]
- 45.Hwang IK, Kim IY, Kim YN, et al. Effects of methimazole on the onset of type 2 diabetes in leptin receptor-deficient rats. J Vet Med Sci. 2009;71:275–280. doi: 10.1292/jvms.71.275. [DOI] [PubMed] [Google Scholar]
- 46.Martínez-Galán JR, Pedraza P, Santacana M, et al. Early effects of iodine deficiency on radial glial cells of the hippocampus of the rat fetus. A model of neurological cretinism. J Clin Invest. 1997;99:2701–2709. doi: 10.1172/JCI119459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schoonover CM, Seibel MM, Jolson DM, et al. Thyroid hormone regulates oligodendrocyte accumulation in developing rat brain white matter tracts. Endocrinology. 2004;145:5013–5020. doi: 10.1210/en.2004-0065. [DOI] [PubMed] [Google Scholar]
- 48.Faivre-Sarrailh C, Rami A, Fages C, et al. Effect of thyroid deficiency on glial fibrillary acidic protein (GFAP) and GFAP-mRNA in the cerebellum and hippocampal formation of the developing rat. Glia. 1991;4:276–284. doi: 10.1002/glia.440040305. [DOI] [PubMed] [Google Scholar]
- 49.Yi SS, Hwang IK, Choi JW, et al. Effects of hypothyroidism on cell proliferation and neuroblasts in the hippocampal dentate gyrus in a rat model of type 2 diabetes. Anat Cell Biol. 2010;43:185–193. doi: 10.5115/acb.2010.43.3.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dezonne RS, Stipursky J, Gomes FC. Effect of thyroid hormone depletion on cultured murine cerebral cortex astrocytes. Neurosci Lett. 2009;467:58–62. doi: 10.1016/j.neulet.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 51.Mallat M, Lima FRS, Gervais A, et al. New insights into the role of thyroid hormone in the CNS: the microglial track. Mol Psychiatry. 2002;7:7–8. doi: 10.1038/sj.mp.4000988. [DOI] [PubMed] [Google Scholar]
- 52.Srinivasan K, Sharma SS. Augmentation of endoplasmic reticulum stress in cerebral ischemia/reperfusion injury associated with comorbid type 2 diabetes. Neurol Res. 2011;33:858–865. doi: 10.1179/1743132811Y.0000000015. [DOI] [PubMed] [Google Scholar]
- 53.Dave KR, Tamariz J, Desai KM, et al. Recurrent hypoglycemia exacerbates cerebral ischemic damage in streptozotocin-induced diabetic rats. Stroke. 2011;42:1404–1411. doi: 10.1161/STROKEAHA.110.594937. [DOI] [PubMed] [Google Scholar]
- 54.Tureyen K, Bowen K, Liang J, et al. Exacerbated brain damage, edema and inflammation in type-2 diabetic mice subjected to focal ischemia. J Neurochem. 2011;116:499–507. doi: 10.1111/j.1471-4159.2010.07127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bruno A, Liebeskind D, Hao Q, et al. Diabetes mellitus, acute hyperglycemia, and ischemic stroke. Curr Treat Options Neurol. 2010;12:492–503. doi: 10.1007/s11940-010-0093-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sharma HS, Patnaik R, Sharma A. Diabetes aggravates nanoparticles induced breakdown of the blood-brain barrier permeability, brain edema formation, alterations in cerebral blood flow and neuronal injury. An experimental study using physiological and morphological investigations in the rat. J Nanosci Nanotechnol. 2010;10:7931–7945. doi: 10.1166/jnn.2010.3616. [DOI] [PubMed] [Google Scholar]
- 57.Hwang IK, Yi SS, Kim YN, et al. Reduced hippocampal cell differentiation in the subgranular zone of the dentate gyrus in a rat model of type II diabetes. Neurochem Res. 2008;33:394–400. doi: 10.1007/s11064-007-9440-8. [DOI] [PubMed] [Google Scholar]
- 58.Etgen GJ, Oldham BA. Profiling of Zucker diabetic fatty rats in their progression to the overt diabetic state. Metabolism. 2000;49:684–688. doi: 10.1016/s0026-0495(00)80049-9. [DOI] [PubMed] [Google Scholar]
- 59.Cooper DS. Antithyroid drugs. N Engl J Med. 1984;311:1353–1362. doi: 10.1056/NEJM198411223112106. [DOI] [PubMed] [Google Scholar]
- 60.Paxinos G, Watson C. 6th ed. Amsterdam: Elsevier Academic Press; 2007. The Rat Brain in Stereotaxic Coordinates. [Google Scholar]


