Abstract
Atorvastatin decreases inflammation and thrombogenesis in patients with carotid artery plaque. Atorvastatin is administered to lower lipid levels, but its anti-inflammatory and anti-thrombogenic effects remain unclear. Eighty-nine patients from northeastern China with acute ischemic stroke caused by large-artery atherosclerosis were randomly divided into the study and control groups. All patients received routine treatment, including antiplatelet therapy, circulatory support, and symptomatic treatment. The study group (n = 43) also received daily atorvastatin 20 mg/d, and the control group (n = 46) received daily placebo pills containing glucose. After 4 weeks, the levels of C-reactive protein, fibrinogen, and D-dimer were significantly lower in the study group than in the control group. Decreases in the levels of C-reactive protein, fibrinogen, and D-dimer were not associated with decreases in the levels of triacylglycerol and low-density lipoprotein cholesterol. These results suggest that atorvastatin reduces inflammation and thrombogenesis independent of its lipid-lowering effects in patients with acute ischemic stroke caused by large-artery atherosclerosis.
Keywords: neural regeneration, brain injury, ischemic stroke, large-artery atherosclerosis, atorvastatin, C-reactive protein, fibrinogen, D-dimer, inflammation, thrombus, triacylglycerol, low-density lipo-protein cholesterol, grants-supported paper, neuroregeneration
Research Highlights
(1) The population of northeastern China has a high incidence of ischemic stroke. Previous studies have shown that intracranial large-artery atherosclerosis is one of the main causes of ischemic stroke, and that the mechanisms are related to inflammation and thrombosis of the affected arteries.
(2) This study of 89 patients from northeastern China with acute ischemic stroke caused by intracranial large-artery atherosclerosis evaluated the effects of atorvastatin treatment by measuring changes in the levels of markers of inflammation, thrombogenesis, and hyperlipidemia.
(3) Atorvastatin treatment decreased the levels of markers of inflammation, thrombogenesis, and hyperlipidemia.
(4) Most previous studies of patients with acute ischemic stroke caused by large-artery atherosclerosis focused mainly on measurement of the level of a single marker such as C-reactive protein, fibrinogen, or D-dimer. This study measured all three of these values to evaluate the effects of vastatin treatment, and expanded the range of atorvastatin available for the treatment of acute ischemic stroke.
INTRODUCTION
In China, the incidence of stroke has been increasing over recent years. The third survey of causes of death in the Chinese population by the Ministry of Health, published in 2008, showed that chronic noninfectious diseases caused the most deaths, and that mortality rates from infectious diseases, malnutrition, and mother-infant disease transmission were decreasing. Stroke is a leading cause of death and long-term disability, with a mortality rate of 22.45%[1]. Post-stroke disability severely affects the quality of life of many individuals in China. The incidence and causes of stroke in China vary by region[2]. The incidence of stroke is higher in northern China than in southern China, because of differences in environmental factors, eating habits, medical care, and sanitation. It is therefore particularly important to pay attention to the prevention and treatment of stroke in northern China.
Intracranial large-artery atherosclerosis is a major cause of ischemic stroke, elevates plasma cholesterol level and plays an important role in the pathogenesis of large-artery atherosclerosis[3]. Previous studies have investigated the pathophysiology of atherothrombosis by measuring the levels of various markers in plasma and urine[4]. In patients with large-artery atherosclerosis, three main mechanisms causing stroke are recognized: thrombogenic, inflammatory, and non-specific[5]. The thrombogenic mechanism is characterized by high levels of fibrinogen and D-dimer and a high D-dimer/fibrinogen ratio, and is a common cause of cardioembolic stroke. The inflammatory mechanism is characterized by high levels of fibrinogen and C-reactive protein and a high erythrocyte sedimentation rate, and causes atherothrombotic stroke. The non-specific mechanism is associated with other stroke types including lacunar stroke[5].
Statins are hydroxymethylglutaryl-CoA reductase inhibitors. Ischemic stroke is known to trigger an acute increase in plasma C-reactive protein level[6], and a high C-reactive protein level is associated with inflammation and progression of endothelial damage in atherosclerotic disease[7]. The fibrinogen level increases during both inflammatory and thrombotic processes. Thrombogenesis and inflammation are closely related, and both processes are present in all cases of ischemic stroke[5]. Isolated measurement of the fibrinogen level is insufficient to assess these processes. Weak associations have been reported among fibrinogen level (or other variations in the levels of thrombotic markers), carotid artery stenosis of ≥ 50%, and atherothrombotic and lacunar stroke[8,9]. SkoloudÍk et al[10] reported that D-dimer levels increased within 6 hours after the onset of stroke. D-dimer levels are much higher in patients with large-artery occlusion or cardioembolic stroke than in those with lacunar stroke or without arterial occlusion[10].
In addition to lowering cholesterol level, statins might have beneficial effects on inflammation[11,12,13]. Statins have also been reported to have vasodilatory, antithrombotic, antioxidant, and neuroprotective effects[14,15,16]. Walter et al[17] reported that atorvastatin appeared to influence thrombogenesis, fibrinolysis, and inflammation in patients with coronary artery disease. Colhoun et al[18] reported that atorvastatin 10 mg/d was safe and effective in reducing the risk of first cardiovascular events, including stroke, in patients with type 2 diabetes without elevated low-density lipoprotein cholesterol levels. Cortellaro et al[19] reported that atorvastatin reduced the inflammatory/thrombotic characteristics of carotid plaque.
It is currently unclear whether the lipid-lowering effects of atorvastatin are associated with anti-thrombogenic and anti-inflammatory effects. Few studies have focused on these parameters in patients with acute ischemic stroke caused by large-artery atherosclerosis, particularly in northeastern China.
This study had three aims. First, this study aimed to evaluate the effects of atorvastatin treatment on the levels of markers of in flammation (C-reactive protein), thrombogenesis (fibrinogen and D-dimer), and hyperlipidemia (total cholesterol, triglyceride, low-density lipoprotein cholesterol, and apolipoprotein B) in patients with acute ischemic stroke caused by large-artery atherosclerosis according to the Trial of Org 10172 in Acute Stroke Treatment (TOAST) criteria[20]. Second, this study aimed to investigate associations between changes in the levels of inflammatory and thrombogenic markers, and changes in hyperlipidemia markers, in the study group. Third, as most previous studies of patients with acute ischemic stroke caused by large-artery atherosclerosis focused mainly on measurement of the level of a single marker such as C-reactive protein, fibrinogen, or D-dimer, this study measured all three values to evaluate the effects of statin treatment, and expanded the range of statins available for the treatment of acute ischemic stroke.
RESULTS
Quantitative analysis and baseline data of participants
Eighty-nine patients who were treated for ischemic stroke caused by large-artery atherosclerosis in Liaoning Province, China from March 2009 to February 2010 were enrolled. Patients were randomly divided into the study and control groups in a double-blind manner, using a randomization algorithm. All patients received antiplatelet therapy, circulatory support, and symptomatic treatment. Patients in the study group (n = 43) received oral atorvastatin, and patients in the control group (n = 46) received placebo pills containing glucose. All 89 patients were included in the final analysis. Figure 1 shows a flow chart of patient selection.
Figure 1.
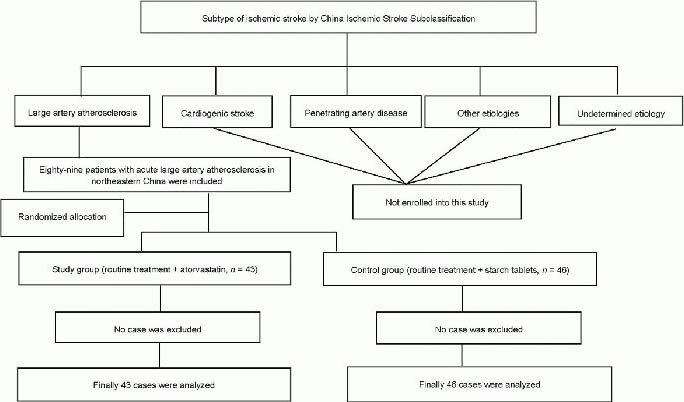
Flowchart of patient selection.
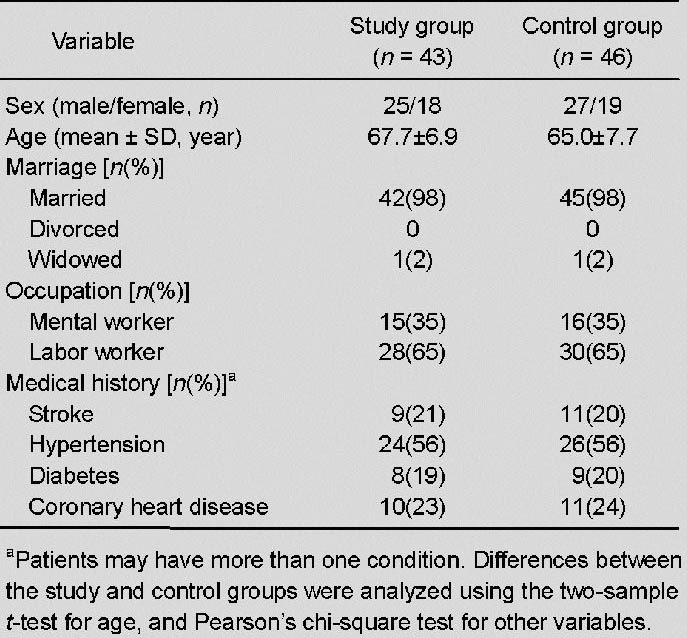
There were no significant differences in sex, age, marital status, occupation, or medical history between the two groups (all P > 0.05; Table 1).
Table 1.
Baseline characteristics of the study patients
Changes in the levels of markers of inflammation, thrombogenesis, and hyperlipidemia
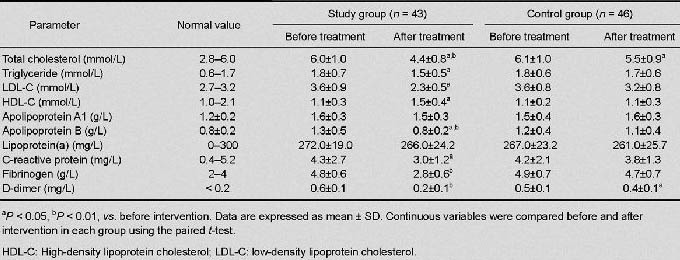
The Kolmogorov-Smirnov test results showed that the distributions of data were normal, with equal variances in the two groups. There were no significant differences in the levels of the markers before treatment between the two groups. After treatment, the levels of total cholesterol, triglyceride, low-density lipoprotein, cholesterol, and apolipoprotein B were significantly lower in the study group than in the control group (all P < 0.05), and the level of high-density lipoprotein cholesterol was significantly higher in the study group than in the control group (P < 0.05).
The levels of C-reactive protein, fibrinogen, and D-dimer were also significantly lower in the study group than in the control group (P < 0.05, P < 0.01, and P < 0.01, respectively). In the study group, the levels of total cholesterol, triglyceride, low-density lipoprotein cholesterol, and apolipoprotein B were significantly lower after 4 weeks of treatment than before treatment (P < 0.01, P < 0.05, P < 0.05, and P < 0.01, respectively), the level of high-density lipoprotein cholesterol was significantly higher after 4 weeks of treatment than before treatment (both P < 0.05), and the levels of C-reactive protein, fibrinogen, and D-dimer were significantly lower after 4 weeks of treatment than before treatment (P < 0.05, P < 0.01, and P < 0.01, respectively). In the control group, the levels of total cholesterol and D-dimer were significantly lower after 4 weeks of treatment than before treatment (both P < 0.05; Table 2).
Table 2.
Comparisons of laboratory findings in the study and control groups
Correlations between decreased levels of markers of inflammation and thrombogenesis, and decreased levels of markers of hyperlipidemia
In the study group, correlation analysis showed that decreases in the levels of C-reactive protein, fibrinogen, and D-dimer after 4 weeks of treatment were not correlated with decreases in the levels of low-density lipoprotein cholesterol and total cholesterol (all P > 0.05; Table 3, Figure 2).
Table 3.
Correlations between decreased levels of markers of inflammation (C-reactive protein) and markers of thrombogenesis (fibrinogen, D-dimer), and decreased levels of markers of hyperlipidemia (total cholesterol, LDL-C) in patients with acute ischemic stroke caused by large artery atherosclerosis, after 4 weeks of treatment with atorvastatin
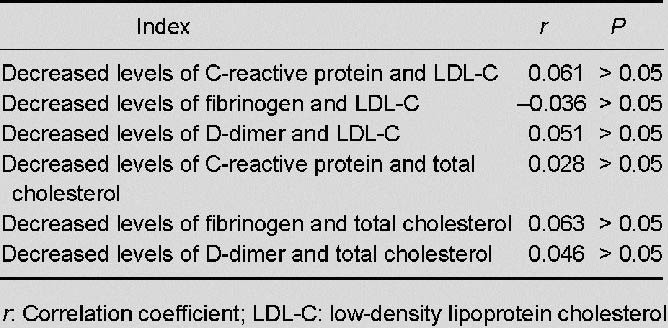
Figure 2.
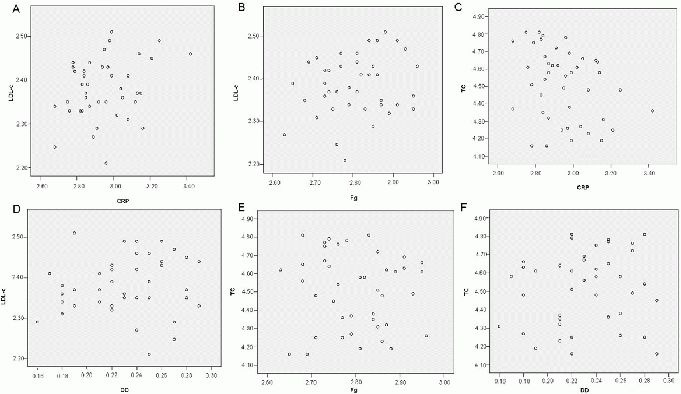
Scatter diagram analyses of the relationships between decreased levels of C-reactive protein (CRP), fibrinogen (Fg), and D-dimer (DD); and decreased levels of low-density lipoprotein cholesterol (LDL-C) and total cholesterol (TC) in the study group after 4 weeks of treatment with atorvastatin.
There were no significant correlations between decreased levels of markers of inflammation and thrombogenesis, and decreased levels of markers of hyperlipidemia (A–F; rank correlation test).
DISCUSSION
Atherosclerosis is a systemic disease with multifactorial etiology, and is considered to be the primary cause of morbidity and mortality in developing countries. Large-vessel atherothrombosis and arterial or cardiac thromboembolism cause approximately 80% of cases of acute ischemic stroke[21]. In individuals with intracranial atherosclerosis, the risk of stroke increases with the degree of arterial stenosis. Elevated plasma cholesterol level is a pathogenic factor in atherosclerosis[22,23,24,25,26], and plasma lipid profiles are useful for assessment of atherosclerotic risk. There is also increasing evidence that inflammation plays an important role in the progression of acute ischemic stroke. Atherosclerosis is considered to result from a systemic inflammatory process involving complex interactions between circulating blood cells and the cells of the arterial walls, leading to stenosis/occlusion of the arterial lumen[3,27]. The first stage of atherosclerosis is characterized by endothelial cell dysfunction, and progression of atherosclerosis is characterized by inflammatory processes in the plaque mediated by cytokines and leukocyte recruitment[28,29].
The inflammation in atherosclerotic lesions is influenced by many factors such as cigarette smoking, insulin resistance/diabetes, and hypertension (especially hypertension mediated by the renin-angiotensin-aldosterone system)30]. It therefore seems that the inflammatory pathways of both the innate and adaptive immune responses influence the risk of atherosclerosis[31]. High-sensitivity C-reactive protein is an acute phase reactant synthesized by the liver that is considered to be an indicator of systemic inflammation[32]. C-reactive protein has recently been identified as a potential marker of increased atherosclerotic risk as well as a potential therapeutic target for the prevention of atherosclerotic cerebrovascular disease[33]. It is possible that high-sensitivity-C-reactive protein plays a role in both the early and late stages of atherogenesis. In the late phase, high-sensitivity-C-reactive protein may induce matrix metalloproteinase expression and collagenase activity in monocytes/macrophages[34,35]. The results of studies using cultured human vascular smooth muscle cells and human C-reactive protein-overexpressing transgenic mice suggest that high-sensitivity-C-reactive protein may induce exaggerated vascular remodeling[35,36]. Inflammation as assessed by C-reactive protein level is significantly positively correlated with infarct volume, and may influence the vascular protective effects of statin therapy[37].
This study included patients with acute ischemic stroke caused by large-artery atherosclerosis according to the TOAST criteria. The patients had significantly elevated C-reactive protein levels at the time of enrollment. Activated macrophages secrete cytokines, which induce liver cells to produce large amounts of C-reactive protein after the onset of stroke.
High C-reactive protein levels reflect endothelial cell damage, inflammatory cytokine activation, vascular lesions, and thrombosis, indicating a marked inflammatory process[38]. The levels of total cholesterol, triglyceride, low-density lipoprotein cholesterol, and apolipoprotein B were significantly lower after 4 weeks of atorvastatin treatment than before treatment. The C-reactive protein level in the study group after 4 weeks of treatment was significantly lower than the C-reactive protein level in the study group before treatment, and significantly lower than the C-reactive protein level in the control group after 4 weeks of placebo administration. Correlation analysis showed no correlations between decreased C-reactive protein level and decreased low-density lipoprotein cholesterol and total cholesterol levels in patients who received atorvastatin. These results suggest that atorvastatin treatment results in decreased levels of markers of inflammation in patients with acute ischemic stroke caused by large-artery atherosclerosis, independent of its lipid-lowering effects.
Acute infectious disease, acute stroke, and myocardial infarction are associated with increased plasma fibrinogen levels[39]. Fibrinogen is a soluble 340 kDa dimeric glycoprotein that is synthesized in the liver and secreted into the plasma, where it activates signals via receptors on cells of the hematopoietic, immune, and nervous systems[40,41]. Fibrinogen is a prominent acute-phase reactant that has a pivotal role in thrombogenesis, inflammation, immune responses, and atherogenesis. In this study, the fibrinogen level decreased in patients with acute ischemic stroke caused by large-artery atherosclerosis after 4 weeks of atorvastatin treatment, whereas the fibrinogen level did not change significantly in the control group, which is consistent with previously reported findings[42]. A decrease in fibrinogen level may reduce atherosclerotic thrombosis. Ernst and Resch[43] reported that increased fibrinogen levels were associated with a hypercoagulable state, resulting in thrombosis around atherosclerotic plaques, erythrocyte aggregation, increased blood viscosity, activation of coagulation factor VII, increased blood coagulation, increased platelet aggregation, activation of plasmin inhibitor, and abnormal fibrinolysis. Another study reported that the reduction in fibrinogen level caused by statins was related to increased transcription of the endothelial nitric oxide synthase gene and increased production of endothelial nitric oxide synthase[44]. Statins influence erythrocyte deformability, reduce the levels of plasminogen activator inhibitor and fibrinogen, reduce smooth muscle cell proliferation and oxidized low-density lipoprotein-induced macrophage proliferation, and reduce blood lymphocyte and monocyte proliferation, resulting in inhibition of thrombosis and blood coagulation and reduction of the high risk of ischemic stroke associated with an elevated fibrinogen level[45].
D-dimer is a product of plasmin-mediated cross-linked fibrin degradation, and an elevated D-dimer level is a marker of vessel occlusion. When the coagulation and fibrinolytic systems are activated, plasmin splits fibrin into fibrin degradation products and D-dimer[46]. The D-dimer level is elevated in thrombogenic disorders, especially disorders that affect the venous system[5]. Barber et al[47] reported that measurement of the D-dimer level could help physicians to target interventions for prevention of early neurological deterioration after acute ischemic stroke. Higher D-dimer levels might reflect greater atherosclerotic plaque remodeling or atherosclerotic disease burden. Statins improve atherosclerotic plaque stability and restore endothelial function[48]. In this study, the D-dimer level was above the normal range in both groups within 72 hours after the onset of stroke. After 4 weeks of atorvastatin treatment, the D-dimer level was significantly lower in the study group than in the control group, indicating that atorvastatin treatment decreases the D-dimer level in patients with acute ischemic stroke caused by large-artery atherosclerosis. This may be because atorvastatin activates the fibrinolytic system resulting in degradation of circulating fibrin.
Inflammation is an important factor in atherothrombosis. Agents with both anti-inflammatory and anti-thrombogenic properties may help to reduce the high morbidity and mortality rates associated with atherothrombotic vascular disease[49]. Atorvastatin targets inflammatory chemokines of the endocytic pathway, and may therefore have anti-inflammatory effects on endothelial cell function[50]. Treatment with statins results in reduced infarct size and a more favorable outcome in patients with ischemic stroke, irrespective of stroke etiology[51]. Better outcomes were reported after treatment with high-dose statins (≥ 60 mg) than with low-dose statins (≤ 60 mg) in all types of acute ischemic stroke[52]. However, another study suggested that high-dose statins should only be used in patients with atherosclerotic stroke[53]. Combination of the various effects of statins may be particularly important in the first 3–7 days after stroke[54].
International guidelines advise objective assessment of cardiovascular risk to determine the appropriateness of statins for primary prevention, and near-universal use of statins for secondary prevention after the acute phase of ischemic stroke. There is no consensus regarding the choice of agent, timing of initiation, or dose and duration of therapy. Some guidelines advocate high-dose atorvastatin, while others suggest use of simvastatin because of its generic availability and low cost[55].
It remains unclear whether C-reactive protein is a useful therapeutic target. Although the results of animal studies suggest that C-reactive protein may play a role in the development of atherosclerosis, recent Mendelian randomization studies failed to demonstrate a causal role between C-reactive protein level and atherosclerosis, suggesting that C-reactive protein is more likely to be a marker of atherosclerosis than a cause[34]. Multiple studies reported that an elevated high-sensitivity-C-reactive protein level was associated with increased cardiovascular risk. Although there is increasing evidence that C-reactive protein may be directly involved in the pathogenesis of atherosclerosis, the question of whether reduction in the C-reactive protein level or its associated downstream effects will provide novel therapeutic avenues for reducing cardiovascular risk requires further investigation[33].
The present study focused on the effects of statins on the levels of inflammatory and thrombogenic biomarkers in patients with acute ischemic stroke caused by large-artery atherosclerosis. Measurement of the markers studied is inexpensive and widely available, and may provide information about pathophysiology in stroke patients without severe systemic disease. It is possible that several markers will prove to be useful in evaluating the efficacy of therapy or in predicting specific patient groups that are likely to benefit from targeted intervention. It is probable that no single marker will provide appropriate information for all clinical settings. Several chemokines including CXCL1/growth-related oncogene-a, CXCL8/interleukin-8, and CCL2/monocyte chemoattractant protein-1 are important in the pathogenesis of atherosclerosis, and can be influenced by statin treatment. Assessment using multiple markers should therefore be further evaluated. Future studies of plasma markers should investigate pathophysiological pathways as well as clinical applications[4].
This study has some limitations. First, the sample size was relatively small. Second, the severity of stroke (National Institutes of Health Stroke Scale[56] or discharge modified Rankin scale[57] was not taken into account. Baseline comorbidities[58] were not recorded, and outcomes were not assessed according to different C-reactive protein levels[59] or atorvastatin doses. Finally, patients were not followed up after the study period.
Further studies are needed to confirm the associations reported in this study. Studies with larger sample sizes are needed to definitively establish the outcomes after statin treatment in patients with stroke.
In conclusion, atorvastatin treatment decreased the levels of total cholesterol, triglyceride, low-density lipoprotein cholesterol, apolipoprotein B, C-reactive protein, fibrinogen, and D-dimer, and increased the level of high-density lipoprotein cholesterol, in patients with acute ischemic stroke caused by large-artery atherosclerosis. Decreases in the levels of C-reactive protein, fibrinogen, and D-dimer were not correlated with decreases in the levels of low-density lipoprotein cholesterol and total cholesterol, suggesting that atorvastatin has anti-inflammatory effects that are independent of its lipid-lowering effects. The levels of total cholesterol and D-dimer also decreased significantly after 4 weeks in the control group.
SUBJECTS AND METHODS
Design
A randomized, double-blind, placebo-controlled, hospital-based study.
Time and setting
This study was performed at Department of Neurology, First Affiliated Hospital of Liaoning Medical University, China, from March 2009 to February 2010.
Subjects
The study group included 25 males (58%) and 18 females (42%) with a mean age of 67.7 ± 6.9 years. The control group included 27 males (59%) and 19 females (41%) with a mean age of 65.0 ± 7.7 years. The baseline medications taken by patients were similar in both groups. All patients gave informed consent for inclusion in the study, and the study protocol was approved by the State Council of China[60], according to the Administrative Regulations on Medical Institutions.
Diagnostic criteria
Acute ischemic stroke was defined as a sudden onset of nonconvulsive, focal neurological deficit persisting for longer than 24 hours. The diagnosis was based on clinical findings and was confirmed by brain CT or MRI findings. The etiology of stroke was determined according to the TOAST criteria[20]. Large-artery atherosclerosis was defined as a ≥ 50% diameter stenosis of the carotid artery on ultrasonography, or a ≥ 50% diameter stenosis of the carotid artery, anterior cerebral artery, middle cerebral artery, posterior cerebral artery, or vertebrobasilar artery on digital subtraction angiography or magnetic resonance angiography[61]. Digital subtraction angiography examinations included intracranial and extracranial portions of the bilateral carotid and vertebrobasilar systems. Magnetic resonance angiography was performed in patients with suspected artery dissection or intracranial stenosis based on ultrasonography findings. All angiography findings were reviewed by one neuroradiologist and one neurologist, and the degree of stenosis of the extracranial vessels (common, internal, and external carotid arteries and extracranial vertebral arteries) and intracranial vessels (middle, anterior, and posterior cerebral arteries and intracranial vertebrobasilar arteries) was calculated according to the North American Symptomatic Carotid Endarterectomy Trial criteria[62].
Inclusion criteria
The inclusion criteria were: (1) hospitalized for the treatment of acute ischemic stroke in the Department of Neurology, First Affiliated Hospital of Liaoning Medical University, China from March 2009 to February 2010; (2) age ≥ 50 years; (3) CT or MRI findings indicating acute ischemic stroke; (4) diagnosis with 72 hours of the onset of symptoms; (5) diagnosis of large-artery atherosclerosis based on the TOAST criteria; (6) not taking anti-thrombogenic medication; (7) patient able and willing to give informed consent, or informed consent obtained from patient's family; and (8) any hypertension and diabetes stable or controlled by medication for at least 3 months[63].
Exclusion criteria
The exclusion criteria were: (1) other subtypes of ischemic stroke; (2) previous treatment with statins; and (3) acute illness or poorly controlled chronic disease such as acute coronary syndrome, atrial fibrillation, bicuspid aortic valve, rheumatic valve disease, infection, fever, cancer, left ventricular ejection fraction < 60%, more than mild aortic regurgitation, creatinine level > 15 μg/L in males or > 89 μg/L in females, high aminotransferase levels, creatinine kinase level > 190 U/L in males or > 170 U/L in females, or C-reactive protein level > 10 mg/L.
Methods
Intervention
All patients were instructed not to smoke, drink excessive amounts of alcohol, or ingest a high-fat diet. All patients received routine treatment including antiplatelet medication, circulatory support, and symptomatic treatment for 2 weeks. Patients in the study group also received oral atorvastatin 20 mg/d (Pfizer Pharmaceuticals Co., Ltd., USA, lot No. 085837032) in the evening for 4 weeks. Patients in the control group received a placebo pill containing glucose for 4 weeks. The placebo pills had the same appearance, taste, and smell as the atorvastatin pills, and were produced by the same company. The method of drug administration was the same in both groups. Symptomatic treatment was given if patients experienced digestive symptoms or slight changes in liver function. Patients with hypertension, coronary artery disease, or diabetes mellitus continued taking their regular medications.
Laboratory tests
Fasting morning venous blood samples were collected from an antecubital vein before the onset of the study intervention and 4 weeks after the onset of intervention.
To measure markers of hyperlipidemia, 2 mL of blood was collected and was stored without agitation for 30 minutes, and then centrifuged at 1 600 × g at 4°C for 15 minutes to separate the serum, which was stored at –20°C. Serum levels of triglyceride, total cholesterol, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, apolipoprotein A1, apolipoprotein B, and lipoprotein were measured using an automatic biochemical analyzer (Model 7170; Hitachi, Tokyo, Japan).
To measure the C-reactive protein level, 2 mL of blood was collected and stored without agitation for 30 minutes, and then centrifuged at 3 500 r/min at 4°C for 15 minutes to separate the serum, which was stored at –20°C. C-reactive protein was assayed by rate immunoturbidimetry (Array360 System turbidimeter; Beckman, Brea, CA, USA).
To measure the fibrinogen level, 2 mL of blood was collected in a sodium citrate tube, and was centrifuged at 3 000 r/min at 4°C for 10 minutes to separate the plasma, which was stored at –20°C. The fibrinogen level was measured using the Clauss method, with a normal range of 2–4 g/L (reagents: Biopool AB, Ventura, CA, USA; automated coagulation analyzer: Thrombolyzer Compact XR; BE company, Germany).
To measure the D-dimer level, 2 mL of blood was collected and stored without agitation for 30 minutes, and was then centrifuged at 3 000 r/min at 4°C for 10 minutes to separate the plasma, which was stored at –20°C. The D-dimer level was measured by enzyme-linked immunosorbent assay (reagents: Sunbio Co., Ltd., Shanghai, China). Glucose and electrolyte levels and liver, kidney, and coagulation function were measured in all patients.
Statistical analysis
Categorical variables were expressed as number and percentage, and continuous variables were expressed as mean ± SD. Analyses were performed using SPSS 17.0 statistical software (SPSS, Chicago, IL, USA). Continuous variables were compared between the two groups using the two-sample t-test, and were compared before and after intervention within each group using the paired t-test. Categorical variables were compared using the chi-square test. The relationships between variables were analyzed using the rank correlation test. A value of P < 0.05 was considered statistically significant.
Footnotes
Funding: This project was supported by the Natural Science Foundation of Liaoning Province in China, No. 20092192; and the National Natural Science Foundation of China, No. 81071058.
Conflicts of interest: None declared.
Ethical approval: The study protocol was approved by the Ethics Committee of First Affiliated Hospital of Liaoning Medical University, China.
(Reviewed by Elgin M, Rave W, Wang JT, Shi TS)
(Edited by Wang J, Qiu Y, Li CH, Song LP, Liu WJ, Zhao M)
REFERENCES
- [1].Liu L, Wang D, Wong KS, et al. Stroke and stroke care in China: huge burden, significant workload, and a national priority. Stroke. 2011;42(12):3651–3654. doi: 10.1161/STROKEAHA.111.635755. [DOI] [PubMed] [Google Scholar]
- [2].Jia Q, Liu LP, Wang YJ. Stroke in China. Clin Exp Pharmacol Physiol. 2010;37(2):259–264. doi: 10.1111/j.1440-1681.2009.05290.x. [DOI] [PubMed] [Google Scholar]
- [3].Gorelick PB, Wong KS, Bae HJ, et al. Large artery intracranial occlusive disease: a large worldwide burden but a relatively neglected frontier. Stroke. 2008;39(8):2396–2399. doi: 10.1161/STROKEAHA.107.505776. [DOI] [PubMed] [Google Scholar]
- [4].Ridker PM, Brown NJ, Vaughan DE, et al. Established and emerging plasma biomarkers in the prediction of first atherothrombotic events. Circulation. 2004;109(25 Suppl 1):IV6–19. doi: 10.1161/01.CIR.0000133444.17867.56. [DOI] [PubMed] [Google Scholar]
- [5].Alvarez-Perez FJ, Castelo-Branco M, Alvarez-Sabin J. Usefulness of measurement of fibrinogen, D-dimer, D-dimer/fibrinogen ratio, C reactive protein and erythrocyte sedimentation rate to assess the pathophysiology and mechanism of ischaemic stroke. J Neurol Neurosurg Psychiatry. 2011;82(9):986–992. doi: 10.1136/jnnp.2010.230870. [DOI] [PubMed] [Google Scholar]
- [6].Rajeshwar K, Kaul S, Al-Hazzani A, et al. C-reactive protein and nitric oxide levels in ischemic stroke and its subtypes: correlation with clinical outcome. Inflammation. 2012;35(3):978–984. doi: 10.1007/s10753-011-9401-x. [DOI] [PubMed] [Google Scholar]
- [7].Kusche-Vihrog K, Urbanova K, Blanqué A, et al. C-reactive protein makes human endothelium stiff and tight. Hypertension. 2011;57(2):231–237. doi: 10.1161/HYPERTENSIONAHA.110.163444. [DOI] [PubMed] [Google Scholar]
- [8].Van Goor MP, Gomez-Garcia EB, Leebeek FW, et al. Thee148 C/T fibrinogen gene polymorphism and fibrinogen levels in ischaemic stroke: a caseecontrol study. J Neurol Neurosurg Psychiatry. 2005;76(1):121–123. doi: 10.1136/jnnp.2004.038414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Kofoed SC, Wittrup HH, Sillesen H, et al. Fibrinogen predicts ischaemic stroke and advanced atherosclerosis but not echolucent, rupture-prone carotid plaques: the copenhagen city heart study. Eur Heart J. 2003;24(6):567–576. doi: 10.1016/s0195-668x(02)00467-0. [DOI] [PubMed] [Google Scholar]
- [10].Skoloudík D, Bar M, Sanák D, et al. D-dimers increase in acute ischemic stroke patients with the large artery occlusion, but do not depend on the time of artery recanalization. J Thromb Thrombolysis. 2010;29(4):477–482. doi: 10.1007/s11239-009-0372-9. [DOI] [PubMed] [Google Scholar]
- [11].Hol J, Otterdal K, Breland UM, et al. Statins affect the presentation of endothelial chemokines by targeting to multivesicular bodies. PLoS One. 2012;7(7):e40673. doi: 10.1371/journal.pone.0040673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Esposito E, Rinaldi B, Mazzon E, et al. Anti-inflammatory effect of simvastatin in an experimental model of spinal cord trauma: involvement of PPAR-α. J Neuroinflammation. 2012;9:81. doi: 10.1186/1742-2094-9-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Sellner J, Weber MS, Vollmar P, et al. The combination of interferon-Beta and HMG-CoA reductase inhibition in multiple sclerosis: enthusiasm lost too soon? CNS Neurosci Ther. 2010;16(6):362–373. doi: 10.1111/j.1755-5949.2010.00179.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Cheng G, Wei L, Zhi-Dan S, et al. Atorvastatin ameliorates cerebral vasospasm and early brain injury after subarachnoid hemorrhage and inhibits caspase-dependent apoptosis pathway. BMC Neurosci. 2009;10:7. doi: 10.1186/1471-2202-10-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Berger C, Xia F, Maurer MH, et al. Neuroprotection by pravastatin in acute ischemic stroke in rats. Brain Res Rev. 2007;58(1):48–56. doi: 10.1016/j.brainresrev.2007.10.010. [DOI] [PubMed] [Google Scholar]
- [16].Tanaka N, Katayama Y, Katsumata T, et al. Effects of long-term administration of HMG-CoA reductase inhibitor, atorvastatin, on stroke events and local cerebral blood flow in stroke-prone spontaneously hypertensive rats. Brain Res. 2007;1169:125–132. doi: 10.1016/j.brainres.2007.07.012. [DOI] [PubMed] [Google Scholar]
- [17].Walter T, Szabo S, Suselbeck T, et al. Effect of atorvastatin on haemostasis, fibrinolysis and inflammation in normocholesterolaemic patients with coronary artery disease: a post hoc analysis of data from a prospective, randomized, double-blind study. Clin Drug Investig. 2010;30(7):453–460. doi: 10.2165/11536270-000000000-00000. [DOI] [PubMed] [Google Scholar]
- [18].Colhoun HM, Betteridge DJ, Durrington PN, et al. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet. 2004;364(9435):685–696. doi: 10.1016/S0140-6736(04)16895-5. [DOI] [PubMed] [Google Scholar]
- [19].Cortellaro M, Cofrancesco E, Arbustini E, et al. Atorvastatin and thrombogenicity of the carotid atherosclerotic plaque: the ATROCAP study. Thromb Haemost. 2002;88(1):41–47. [PubMed] [Google Scholar]
- [20].Adams HP, Jr, Bendixen BH, Kappelle LJ, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. Toast. Trial of org 10172 in acute stroke treatment. Stroke. 1993;24(1):35–41. doi: 10.1161/01.str.24.1.35. [DOI] [PubMed] [Google Scholar]
- [21].Mohr JP, Caplan LR, Melski JW, et al. Harvard Cooperative Stroke Registry: a prospective registry. Neurology. 1978;28(8):754–762. doi: 10.1212/wnl.28.8.754. [DOI] [PubMed] [Google Scholar]
- [22].Steinberg D. Thematic review series: the pathogenesis of atherosclerosis. An interpretive history of the cholesterol controversy, part I. J Lipid Res. 2004;45(9):1583–1593. doi: 10.1194/jlr.R400003-JLR200. [DOI] [PubMed] [Google Scholar]
- [23].Steinberg D. Thematic review series: the pathogenesis of atherosclerosis. An interpretive history of the cholesterol controversy: part II: the early evidence linking hypercholesterolemia to coronary disease in humans. J Lipid Res. 2005;46(2):179–190. doi: 10.1194/jlr.R400012-JLR200. [DOI] [PubMed] [Google Scholar]
- [24].Steinberg D. Thematic review series: the pathogenesis of atherosclerosis. An interpretive history of the cholesterol controversy, part III: mechanistically defining the role of hyperlipidemia. J Lipid Res. 2005;46(10):2037–2051. doi: 10.1194/jlr.R500010-JLR200. [DOI] [PubMed] [Google Scholar]
- [25].Steinberg D. The pathogenesis of atherosclerosis. An interpretive history of the cholesterol controversy, part IV: the 1984 coronary primary prevention trial ends it-almost. J Lipid Res. 2006;47(1):1–14. doi: 10.1194/jlr.R500014-JLR200. [DOI] [PubMed] [Google Scholar]
- [26].Steinberg D. Thematic review series: the pathogenesis of atherosclerosis. An interpretive history of the cholesterol controversy, part V: the discovery of the statins and the end of the controversy. J Lipid Res. 2006;47(7):1339–1351. doi: 10.1194/jlr.R600009-JLR200. [DOI] [PubMed] [Google Scholar]
- [27].Kim OJ, Hong SH, Oh SH, et al. Association between VEGF polymorphisms and homocysteine levels in patients with ischemic stroke and silent brain infarction. Stroke. 2011;42(9):2393–2402. doi: 10.1161/STROKEAHA.110.607739. [DOI] [PubMed] [Google Scholar]
- [28].Guiraud V, Amor MB, Mas JL, et al. Triggers of ischemic stroke: a systematic review. Stroke. 2010;41(11):2669–2677. doi: 10.1161/STROKEAHA.110.597443. [DOI] [PubMed] [Google Scholar]
- [29].Wu T, Trevisan M, Genco RJ, et al. Periodontal disease and risk of cerebrovascular disease: the first national health and nutrition examination survey and its follow-up study. Arch Intern Med. 2000;160(18):2749–2755. doi: 10.1001/archinte.160.18.2749. [DOI] [PubMed] [Google Scholar]
- [30].Libby P, Aikawa M. Stabilization of atherosclerotic plaques: new mechanisms and clinical targets. Nat Med. 2002;8(11):1257–1262. doi: 10.1038/nm1102-1257. [DOI] [PubMed] [Google Scholar]
- [31].Packard RR, Libby P. Inflammation in atherosclerosis: from vascular biology to biomarker discovery and risk prediction. Clin Chem. 2008;54(1):24–38. doi: 10.1373/clinchem.2007.097360. [DOI] [PubMed] [Google Scholar]
- [32].Heart Protection Study Collaborative Group. Jonathan E, Derrick B, et al. C-reactive protein concentration and the vascular benefits of statin therapy: an analysis of 20,536 patients in the Heart Protection Study. Lancet. 2011;377(9764):469–476. doi: 10.1016/S0140-6736(10)62174-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Oh J, Teoh H, Leiter LA. Should C-reactive protein be a target of therapy? Diabetes Care. 2011;34(Suppl 2):S155–160. doi: 10.2337/dc11-s211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Li L, Roumeliotis N, Sawamura T, et al. C-reactive protein enhances LOX-1 expression in human aortic endothelial cells: relevance of LOX-1 to C-reactive protein-induced endothelial dysfunction. Circ Res. 2004;95(9):877–883. doi: 10.1161/01.RES.0000147309.54227.42. [DOI] [PubMed] [Google Scholar]
- [35].Wang CH, Li SH, Weisel RD, et al. C-reactive protein upregulates angiotensin type 1 receptors in vascular smoothmuscle. Circulation. 2003;107(13):1783–1790. doi: 10.1161/01.CIR.0000061916.95736.E5. [DOI] [PubMed] [Google Scholar]
- [36].Teoh H, Quan A, Lovren F, et al. Impaired endothelial function in C-reactive protein overexpressing mice. Atherosclerosis. 2008;201(2):318–325. doi: 10.1016/j.atherosclerosis.2008.02.034. [DOI] [PubMed] [Google Scholar]
- [37].Ormstad H, Aass HC, Lund-Sørensen N, et al. Serum levels of cytokines and C-reactive protein in acute ischemic stroke patients, and their relationship to stroke lateralization, type, and infarct volume. J Neurol. 2011;258(4):677–685. doi: 10.1007/s00415-011-6006-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Terruzzi A, Valente L, Mariani R, et al. C-reactive protein and aetiological subtypes of cerebral infarction. Neurol Sci. 2008;29(4):245–249. doi: 10.1007/s10072-008-0975-5. [DOI] [PubMed] [Google Scholar]
- [39].Ehling R, Pauli FD, Lackner P, et al. Fibrinogen is not elevated in the cerebrospinal fluid of patients with multiple sclerosis. Fluids Barriers CNS. 2011;8:25. doi: 10.1186/2045-8118-8-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Doolittle RF, Spraggon G, Everse SJ. Three-dimensional structural studies on fragments of fibrinogen and fibrin. Curr Opin Struct Biol. 1998;8(6):792–798. doi: 10.1016/s0959-440x(98)80100-0. [DOI] [PubMed] [Google Scholar]
- [41].Haidaris PJ, Francis CW, Sporn LA, et al. Megakaryocyte and hepatocyte origins of human fibrinogen biosynthesis exhibit hepatocyte-specific expression of gamma chain-variant polypeptides. Blood. 1989;74(2):743–750. [PubMed] [Google Scholar]
- [42].Undas A, Brummel KE, Musial J, et al. Simvastatin depresses blood clotting by inhibiting activation of prothrombin, factor V, and factor XIII and by enhancing factor Va inactivation. Circulation. 2001;103(18):2248–2253. doi: 10.1161/01.cir.103.18.2248. [DOI] [PubMed] [Google Scholar]
- [43].Ernst E, Resch KL. Fibrinogen as a cardiovascular risk factor: a meta-analyses and review of the literature. Ann Intern Med. 1993;118(12):956–963. doi: 10.7326/0003-4819-118-12-199306150-00008. [DOI] [PubMed] [Google Scholar]
- [44].Ridker PM, Rifai N, Pfeffer MA, et al. Long-term effects of pravastatin on plasma concentration of C-reactive protein. The Cholesterol and Recurrent Events (CARE) Investigators. Circulation. 1999;100(3):230–235. doi: 10.1161/01.cir.100.3.230. [DOI] [PubMed] [Google Scholar]
- [45].Undas A, Brummel KE, Musial J, et al. Simvastatin depresses blood clotting by inhibiting activation of prothrombin, factor V, and factor XIII and by enhancing factor Va inactivation. Circulation. 2001;103(18):2248–2253. doi: 10.1161/01.cir.103.18.2248. [DOI] [PubMed] [Google Scholar]
- [46].Park YW, Koh EJ, Choi HY. Correlation between Serum D-Dimer Level and Volume in Acute Ischemic Stroke. J Korean Neurosurg Soc. 2011;50(2):89–94. doi: 10.3340/jkns.2011.50.2.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Barber M, Langhorne P, Rumley A, et al. Hemostatic function and progressing ischemic stroke: D-dimer predicts early clinical progression. Stroke. 2004;35(6):1421–1425. doi: 10.1161/01.STR.0000126890.63512.41. [DOI] [PubMed] [Google Scholar]
- [48].Biffi A, Devan WJ, Anderson CD, et al. Statin treatment and functional outcome after ischemic stroke: case-control and meta-analysis. Stroke. 2011;42(5):1314–1319. doi: 10.1161/STROKEAHA.110.605923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Vidula H, Tian L, Liu K, et al. Comparison of effects of statin use on mortality in patients with peripheral arterial disease with versus without elevated C-reactive protein and d-dimer levels. Am J Cardiol. 2010;105(9):1348–1352. doi: 10.1016/j.amjcard.2009.12.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Hol J, Otterdal K, Breland UM, et al. Statins affect the presentation of endothelial chemokines by targeting to multivesicular bodies. PLoS One. 2012;7(7):e40673. doi: 10.1371/journal.pone.0040673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Birnbaum Y, Lin Y, Ye Y, et al. Pretreatment with high dose statins, but not low dose statin, ezetimibe or the combination of low dose statin and ezetimibe, limit infarct size in rats. J Cardiovasc Pharmacol Ther. 2008;13:72–79. doi: 10.1177/1074248407312839. [DOI] [PubMed] [Google Scholar]
- [52].Flint AC, Kamel H, Navi BB, et al. Statin use during ischemic stroke hospitalization is strongly associated with improved poststroke survival. Stroke. 2012;43(1):147–154. doi: 10.1161/STROKEAHA.111.627729. [DOI] [PubMed] [Google Scholar]
- [53].Furie KL. High-dose statins should only be used in atherosclerotic strokes. Stroke. 2012;43(7):1994–1995. doi: 10.1161/STROKEAHA.111.633339. [DOI] [PubMed] [Google Scholar]
- [54].Moonis M. High-dose statins should be used in all acute ischemic strokes. Stroke. 2012;43(7):1992–1993. doi: 10.1161/STROKEAHA.111.633354. [DOI] [PubMed] [Google Scholar]
- [55].Sett AK, Robinson TG, Mistri AK. Current status of statin therapy for stroke prevention. Expert Rev Cardiovasc Ther. 2011;9(10):1305–1314. doi: 10.1586/erc.11.106. [DOI] [PubMed] [Google Scholar]
- [56].Goldstein LB, Bertels C, Davis JN. Interrater reliability of the NIH stroke scale. Arch Neurol. 1989;46(6):660–662. doi: 10.1001/archneur.1989.00520420080026. [DOI] [PubMed] [Google Scholar]
- [57].Nedeltchev K, Der Maur TA, Georgiadis D, et al. Ischaemic stroke in young adults: predictors of outcome and recurrence. J Neurol Neurosurg Psychiatry. 2005;76(2):191–195. doi: 10.1136/jnnp.2004.040543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Solomon A, Dobranici L, Kåreholt I, et al. Comorbidity and the rate of cognitive decline in patients with Alzheimer dementia. Int J Geriatr Psychiatry. 2011;26(12):1244–1251. doi: 10.1002/gps.2670. [DOI] [PubMed] [Google Scholar]
- [59].Ladenvall C, Jood K, Blomstrand C, et al. Serum C-reactive protein concentration and genotype in relation to ischemic stroke subtype. Stroke. 2006;37(8):2018–2023. doi: 10.1161/01.STR.0000231872.86071.68. [DOI] [PubMed] [Google Scholar]
- [60].Administrative Regulations on Medical Institution; 1994. State Council of the People's Republic of China. [Google Scholar]
- [61].Liu HM, Tu YK, Yip PK, et al. Evaluation of intracranial and extracranial carotid steno-occlusive diseases in Taiwan Chinese patients with MR angiography: preliminary experience. Stroke. 1996;27(4):650–653. doi: 10.1161/01.str.27.4.650. [DOI] [PubMed] [Google Scholar]
- [62].N Engl J Med. 7. Vol. 325. Hamilton, ON, Canada: Department of Clinical Epidemiology and Biostatistics, McMaster University; 1991. Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. North American Symptomatic Carotid Endarterectomy Trial Collaborators; pp. 445–453. [DOI] [PubMed] [Google Scholar]
- [63].Wilkinson D, Doody R, Helme R, et al. Donepezil in vascular dementia: a randomised, placebo-controlled study. Neurology. 2003;61(4):479–486. doi: 10.1212/01.wnl.0000078943.50032.fc. [DOI] [PubMed] [Google Scholar]


