Abstract
A plasmid for cytoglobin expression, pAcGFP1-C1-cytoglobin, was transfected into SH-SY5Y cells. Cobalt chloride was used to establish a model of hypoxia. Western blotting indicated that cytoglobin was overexpressed and there was low expression of hypoxia-inducible factor-1α in SH-SY5Y cells after transfection. Following cobalt chloride-induced hypoxia, cytoglobin and hypoxia-inducible factor-1α expression gradually increased in SH-SY5Y cells. Flow cytometry showed that with increasing duration of hypoxia, the proportion of normal cells significantly diminished in the transfected and non-transfected groups. The proportion of cells in the early stages of apoptosis increased. However, the proportion of apoptotic cells was significantly lower in the transfected group compared with the non-transfected group. These results demonstrate that cytoglobin and hypoxia-inducible factor-1α are strongly up-regulated by hypoxia, and that there is a strong relationship between hypoxia-inducible factor-1α and cytoglobin during hypoxic injury.
Keywords: neural regeneration, cytoglobin, hypoxia, green fluorescent protein, transfection, SH-SY5Y cells, cobalt chloride, recombinant plasmid, neuroprotection, neuroregeneration
Research Highlights
(1) In this study, we aimed to determine whether cytoglobin could scavenge oxygen free radicals produced by hypoxia-induced oxidative stress and protect cells.
(2) Cytoglobin and hypoxia-inducible factor-1α expression was strongly upregulated by hypoxia. Overexpression of cytoglobin is a protective mechanism against hypoxia in SH-SY5Y cells. In comparison, the expression of hypoxia-inducible factor-1α was nearly undetectable in normal SH-SY5Y cells.
INTRODUCTION
Cytoglobin was initially identified as a protein belonging to the globin family of proteins by Burmester in 20021]. Cytoglobin differs from the better-known globins, hemoglobin, myoglobin and neuroglobin. Cytoglobin is widely expressed in many different tissues at different stages and is mainly present in the cytoplasm or nucleolus[2]. Cytoglobin is expressed in retinal tissue, including retinal ganglion cells, the inner nuclear layer and the inner and outer plexiform layers. The specific kinetics of this hexacoordinate globin is indicative of its role as a temporary oxygen reservoir, which might provide a minimal, but continuous supply of intracellular oxygen during ischemic and anoxic conditions[3,4]. The specific localization of cytoglobin might be suggestive of an important role in oxygen homeostasis and ischemia-induced cell signaling in human retinas and optic nerves[5,6,7].
Cytoglobin expression increases in response to various stress conditions, including hypoxia, oxidative stress and fibrotic stimulation[6]. Cytoglobin is strongly up-regulated by hypoxia, which suggests that its expression is induced by hypoxia and that it promotes cell survival[8,9,10,11]. Fordel et al[8] found that cytoglobin mRNA expression increased in neuroblastoma N2a cells under oxidative stress. Moreover, resistance of cells to H2O2 decreased and cellular necrosis increased after deletion of the cytoglobin gene. Exposure of SH-SY5Y cells to paraquat or H2O2 results in a concentration-dependent and time-dependent induction of apoptotic and necrotic cell death. These findings suggest that cytoglobin protects SH-SY5Y cells against oxidative stress-induced cell death[9]. Hypoxia elevates expression of cytoglobin, which can enhance neuronal tolerance to hypoxia[10].
The discovery of hypoxia-inducible factor-1 and the subsequent identification of other members of the hypoxia-inducible factor-1 family of transcriptional activators has provided insight into the molecular underpinnings of oxygen homeostasis. Hypoxia-inducible factor-1α expression mainly depends on intracellular oxygen supply, and it is a multifunctional transcription factor that participates in the response to cell and tissue hypoxia[3,4,12,13,14]. Numerous studies have shown that expression of hypoxia-inducible factor-1α is low in brain tissues with normal oxygen content, but rapidly increases after acute hypoxia[15,16,17]. Wystub et al[7] showed that cytoglobin contains both hypoxia response elements, which can bind hypoxia-inducible factor-1, suggesting that cytoglobin expression is regulated by the transcription factor.
In this study, we transfected the cytoglobin gene into SH-SY5Y cells using Lipofectamine 2000 to examine the expression of cytoglobin and hypoxia-inducible factor-1α, and the neuroprotective effect of cytoglobin in SH-SY5Y cells following cobalt chloride-induced hypoxia.
RESULTS
Generation of stably-transfected SH-SY5Y-green fluorescent protein (GFP)-cytoglobin cell lines
Fluorescence microscopy showed GFP expression after SH-SY5Y-GFP-cytoglobin transfection. Under an excitation wavelength of 488 nm, green fluorescence was emitted from the SH-SY5Y cells (Figure 1).
Figure 1.
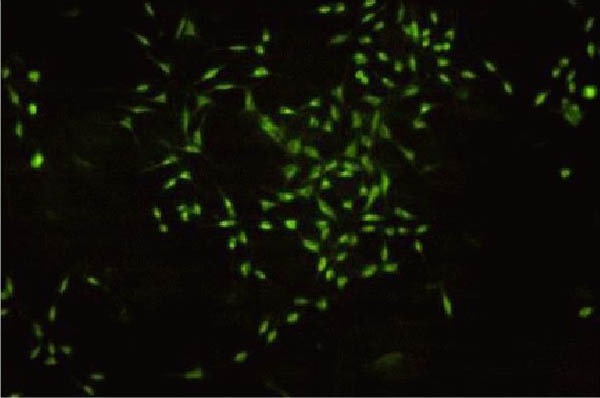
Transfected SH-SY5Y-GFP-cytoglobin cells (fluorescence microscopy, × 100).
SH-SY5Y cells were transfected with pAcGFP1-C1-cytoglobin using Lipofectamine 2000. The successfully-transfected cells showed green fluorescence.
GFP: Green fluorescent protein.
Changes in cytoglobin and hypoxia-inducible factor-1α protein expression in SH-SY5Y cells after hypoxic injury
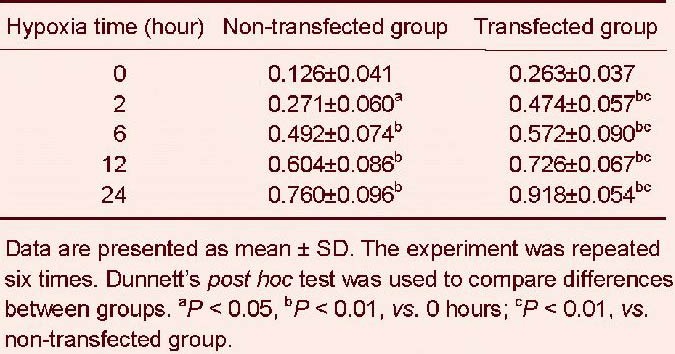
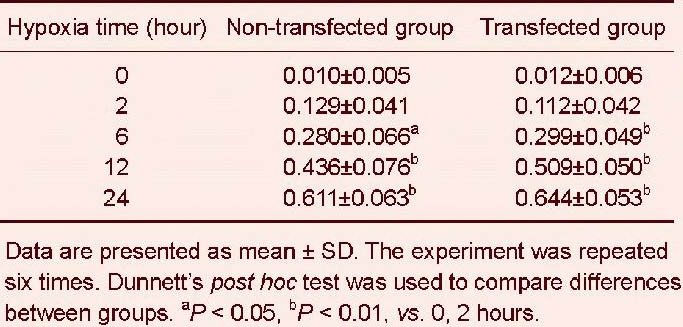
Western blotting revealed that expression of cytoglobin at 0 hours was low in the non-transfected group, while in the transfected group, the expression of cytoglobin was upregulated significantly. Compared to the non- transfected group, the expression of cytoglobin increased in the transfected group in a time-dependent manner (P < 0.01, P < 0.05; Figure 2, Table 1).
Figure 2.
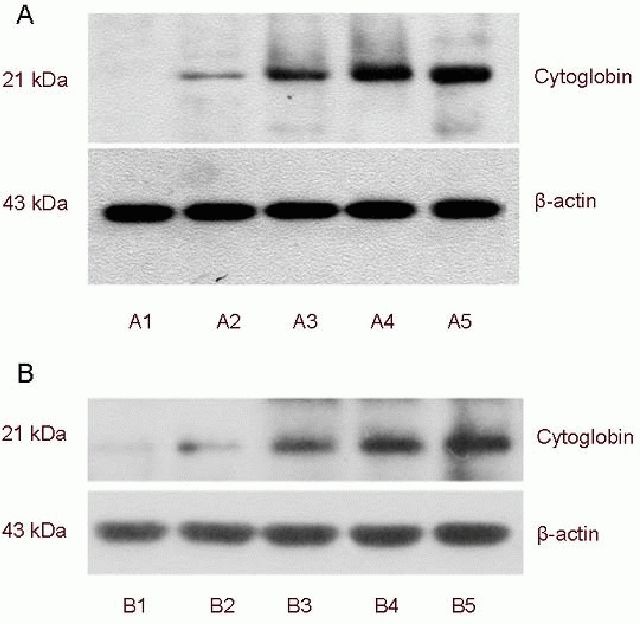
Cytoglobin expression in SH-SY5Y cells after hypoxia (western blot analysis).
(A1–5) After 0, 2, 6, 12 and 24 hours of hypoxia in the transfected group; (B1–5) after 0, 2, 6, 12 and 24 hours of hypoxia in the non-transfected group.
Table 1.
Relative protein expression of cytoglobin (absorbance ratio to β-actin) in SH-SY5Y cells after hypoxia
Hypoxia-inducible factor-1α expression was almost undetectable in the transfected and non-transfected groups. With increasing duration of hypoxia, the expression of hypoxia-inducible factor-1α increased significantly in both groups (P < 0.01, P < 0.05; Figure 3, Table 2).
Figure 3.
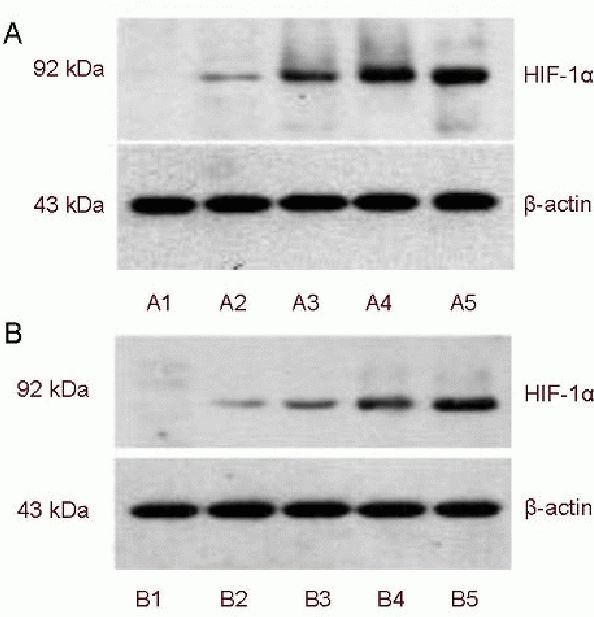
Hypoxia-inducible factor-1α (HIF-1α) expression in SH-SY5Y cells after hypoxia (western blot analysis).
(A1–5) After 0, 2, 6, 12 and 24 hours of hypoxia in the transfected group; (B1–5) after 0, 2, 6, 12 and 24 hours of hypoxia in the non-transfected group.
Table 2.
Relative protein expression of hypoxia-inducible factor-1α (absorbance ratio to β-actin) in SH-SY5Y cells after hypoxia
Cytoglobin overexpression reduced apoptosis of SH-SY5Y cells induced by hypoxia
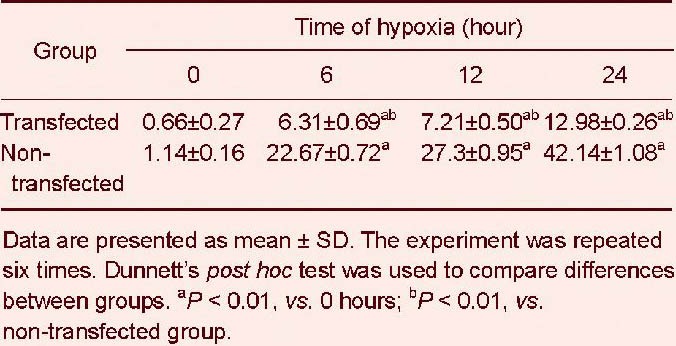
Flow cytometry demonstrated that the proportion of cells in the early stages of apoptosis was significantly decreased in transfected cells compared with non-transfected cells at the various time points of hypoxic treatment (P < 0.01). Furthermore, cell viability was significantly increased. The proportion of non-transfected cells continued to decrease, and the proportion of apoptotic cells steadily rose with increasing duration of hypoxia. These results suggest that cytoglobin protects cells and decreases hypoxia-induced cell death (Figure 4, Tables 3, 4).
Figure 4.
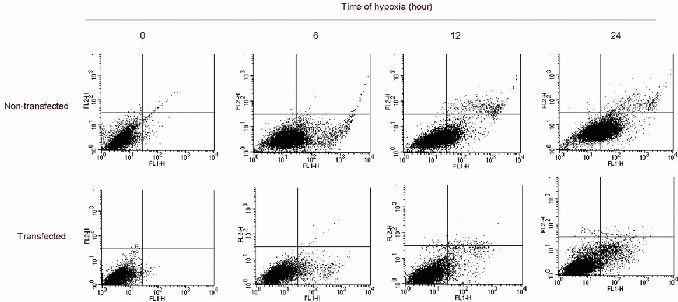
Detection of SH-SY5Y cell apoptosis induced by hypoxia (flow cytometry).
The proportion of non-transfected normal cells significantly decreased with increasing duration of hypoxia, and the proportion of early apoptotic cells significantly increased. The proportion of transfected normal cells also significantly decreased with increasing duration of hypoxia, and the proportion of early apoptotic cells significantly increased. The proportion of normal cells in the transfected group was substantially higher than in the non-transfected group. The proportion of apoptotic cells in the transfected group was noticeably lower than in the non-transfected group.
Table 3.
Effects of cytoglobin overexpression on the normal cell ratio (%) in SH-SY5Y cells under hypoxia
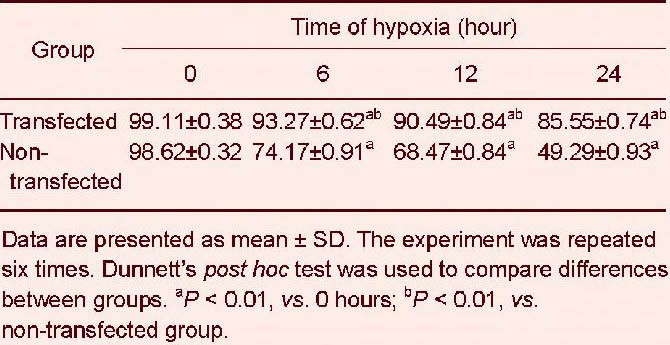
Table 4.
Effects of cytoglobin overexpression on apoptotic ratio (%) in SH-SY5Y cells under hypoxia
DISCUSSION
PAcGFP1-C1 is composed of a GFP reporter gene, the SV40 promoter, restriction enzyme sites and the kanamycin and neomycin resistance gene. Under the fluorescence microscope, using about 490 nm ultraviolet excitation, green fluorescence emitted by the GFP reporter can be observed. It is easy to construct fusion proteins with the GFP reporter while maintaining its ability to emit fluorescence. Cobalt chloride is a chemical that is widely-used to simulate hypoxia[12]. The Fe2+ in heme on the cell surface can be replaced by CO2+, thereby impairing oxygen signaling and transport and inducing hypoxia. In this study, we generated SH-SY5Y cells expressing GFP-cytoglobin, and green fluorescence was emitted by these cells under the fluorescence microscope, which shows that pAcGFP1-Cl-cytoglobin was successfully transfected into the cells and that the fusion protein was expressed. Many studies have shown that cytoglobin is upregulated by hypoxia[15,16,17,18]. The expression of cytoglobin in mouse brain, liver, heart and skeletal muscle is dependent on oxygen concentration, and varies according to tissue and developmental stage. In this study, cytoglobin expression was low in the non-transfected group, but high in the transfected group. With increasing length of hypoxia, cytoglobin expression steadily increased in both groups. This result is similar to a previously published study[5].
Wystub et al[7] demonstrated that the oxygen dependence of cytoglobin expression was mediated by hypoxia-inducible factor-1. In the present study, cytoglobin expression was detectable after hypoxia and gradually increased in a time-dependent manner. These results verified that acute hypoxia upregulates cytoglobin and hypoxia-inducible factor-1 expression, which indicates that cytoglobin expression increases in hypoxic cells through the hypoxia-inducible factor-1 pathway.
Flow cytometry results showed that the proportion of cells in the early stages of apoptosis was remarkably reduced among cells transfected with cytoglobin, and cell viability was significantly higher, which suggests that cytoglobin can protect cells by suppressing hypoxia-induced cell death.
In summary, our findings indicate that cytoglobin overexpression reduces cobalt chloride-induced nerve cell death.
The recombinant cytoglobin gene was effectively transfected into neuronal cells and had a neuroprotective effect. Moreover, hypoxia-inducible factor-1α protein expression increased. These results indicate that the oxygen dependence of cytoglobin expression is associated with hypoxia-inducible factor-1.
MATERIALS AND METHODS
Design
A genetic engineering, in vitro controlled experiment.
Time and setting
This study was performed at the Laboratory of Shengjing Hospital of China Medical University and the Laboratory of Department of Ophthalmology, Shenyang No.4 People's Hospital, China from February 2010 to January 2012.
Materials
SH-SY5Y cells were obtained from the Cancer Institute of the Chinese Academy of Medical Sciences. pAcGFP1-C1-cytoglobin was obtained from Takara Biotechnology (Dalian) Co., Ltd., Liaoning Province, China.
Methods
SH-SY5Y cell transfection
SH-SY5Y cells were cultured in Dulbecco's modified Eagle's medium (Gibco, Grand Island, NY, USA) supplemented with 10% fetal bovine serum (Gibco) and incubated at 37°C in 5% CO2. The medium was replaced every 3 days, and cells were subcultured twice a week. The day before transfection, cells were seeded into every well of a 6-well plate at a density of 2 × 105 cells per well. Approximately 60–80% confluency was reached on the day of transfection. Cells in the 6-well plate were washed twice in serum-free Dulbecco's modified Eagle's medium, and then 1.5 mL serum-free medium was added. Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) was mixed with pAcGFP1-C1-cytoglobin for 5 hours at 37°C in 5% CO2. Supernatant was removed, and the medium was replaced with Dulbecco's modified Eagle's medium containing 20% serum. Cells were incubated in a 5% CO2 incubator for 48 hours at 37°C. Cell transfection was evaluated using a fluorescence microscope (Motic [Xiamen] Electric Group Co., Ltd., Xiamen, Fujian Province, China).
SH-SY5Y cell hypoxia induction
After 48 hours, hypoxia was induced. Non-transfected SH-SY5Y cells were cultured with cobalt chloride (No. C8661; Sigma, St. Louis, MO, USA) in high-glucose Dulbecco's modified Eagle's medium containing 10% fetal bovine serum. Transfected cells with Lipofectamine 2000 and pAcGFP1-C1-cytoglobin were incubated with high-glucose Dulbecco's modified Eagle's medium, supplemented with 250 μmol/L cobalt chloride, 400 μg/mL G418 (stable transfection; Merck KGaA, Darmstadt, Germany) and 10% fetal bovine serum at 37°C in a 5% CO2 incubator.
Western blot analysis of cytoglobin and hypoxia-inducible factor-1α protein expression in SH-SY5Y cells
Cells were examined at 0, 2, 6, 12 and 24 hours of hypoxia. Total protein was isolated. Protein concentrations were determined using the bicinchoninic acid method according to the manufacturer's protocol (Beyotime Institute of Biotechnology, Shanghai, China). After electrophoresis, proteins were transferred onto a membrane. The membrane was then immersed in Tris-buffered saline for 10 minutes, and blocked with 5% nonfat milk for 1 hour, washed twice in Tris-buffered saline (each for 5 minutes), and then probed overnight at 4°C with rabbit anti-rat cytoglobin and hypoxia-inducible factor-1α polyclonal antibodies (1:1 000; Santa Cruz Biotechnology, Santa Cruz, CA, USA). After two washes in Tris-buffered saline (each for 5 minutes), membranes were incubated with horseradish peroxidase-labeled goat anti-rabbit IgG (1:10 000; Beyo-time, Haimen, China) for 2 hours at room temperature. After two washes in Tween-Tris- buffered saline and two washes in Tris-buffered saline (each for 5 minutes), the immunoreactive bands were then visualized. β-actin was used as the internal control. The immunoblots were scanned using Chemi Imager 5500 V2.03 software (AlPha InnCh, Miami, FL, USA), and absorbance values were calculated using FluorChem 2.0 software (Olympus, Tokyo, Japan) and normalized against β-actin.
Detection of SH-SY5Y cell apoptosis by flow cytometry
Cells were obtained at 0, 6, 12 and 24 hours of hypoxia. After the supernatant was removed, cells were washed twice in PBS and digested in 0.25% trypsin. The digestion was terminated with medium. A single cell suspension (1 × 106/mL) was prepared and centrifuged at 800 r/min for 10 minutes. The supernatant was discarded and the cell pellet was recovered (approximately 50 μL medium was left to prevent loss of cells). 1 mL pre-cooled PBS was added, and cells were resuspended and centrifuged at 800 r/min for 10 minutes. The supernatant was discarded (approximately 50 μL PBS was left) and cells were resuspended after adding 195 μL Annexin V-FITC (Bo Ling Ke Biological Science and Technology Co., Ltd., Beijing, China). Following addition of 5 μL Annexin V-FITC, the cells were incubated at room temperature in the dark for 10 minutes and centrifuged at 800 r/min for 10 minutes. Supernatant was carefully removed, and about 50 μL liquid was left. Cells were resuspended after addition of 190 μL Annexin V-FITC conjugate. Cells were stained with 10 μL Annexin propidium iodinate and stored in an ice bath in the dark. A fluorescence-activated cell sorting machine (Becton Dickinson, San Jose, CA, USA) was used to examine the protective role of cytoglobin and hypoxia-inducible factor-1α in SH-SY5Y cells following cobalt chloride-induced hypoxia.
Statistical analysis
All data were presented as mean ± SD. One-way analysis of variance was used to compare differences among groups using SPSS 13.0 software (SPSS, Chicago, IL, USA). Dunnett's post hoc test was applied to compare intergroup differences if one-way analysis of variance revealed a significant difference. A level of P < 0.05 was considered statistically significant.
Acknowledgments:
We thank Lv JY from the First Clinical Hospital of China Medical University and Qu Y from Shenyang No.4 People’s Hospital for technical assistance.
Footnotes
Conflicts of interest: None declared.
(Reviewed by Patel B, Rave W, Song JN, Duan RS)
(Edited by Yu J, Qiu Y, Li CH, Song LP, Liu WJ, Zhao M)
REFERENCES
- [1].Burmester T, Ebner B, Weich B, et al. Cytoglobin: a novel globin type ubiquitously expressed in vertebrate tissues. Mol Biol Evol. 2002;19(4):416–421. doi: 10.1093/oxfordjournals.molbev.a004096. [DOI] [PubMed] [Google Scholar]
- [2].Fordel E, Thijs L, Martinet W, et al. Neuroglobin and cytoglobin overexpression protects human SH-SY5Y neuroblastoma cells against oxidative stress-induced cell death. Neurosci Lett. 2006;410(2):146–151. doi: 10.1016/j.neulet.2006.09.027. [DOI] [PubMed] [Google Scholar]
- [3].Kuo NT, Benhayon D, Przybylski RJ, et al. Prolonged hypoxia increases vascular endothelial growth factor mRNA and protein in adult mouse brain. J Appl Physiol. 1999;86(1):260–264. doi: 10.1152/jappl.1999.86.1.260. [DOI] [PubMed] [Google Scholar]
- [4].Semenza GL. Regulation of mammalian O2 homeostasis by hypoxia-inducible factor 1. Annu Rev Cell Dev Biol. 1999;15:551–578. doi: 10.1146/annurev.cellbio.15.1.551. [DOI] [PubMed] [Google Scholar]
- [5].Fordel E, Thijs L, Martinet W, et al. Anoxia or oxygen and glucose deprivation in SH-SY5Y cells: a step closer to the unraveling of neuroglobin and cytoglobin functions. Gene. 2007;398(1-2):114–122. doi: 10.1016/j.gene.2007.03.022. [DOI] [PubMed] [Google Scholar]
- [6].Sundaram J, Mellein BR, Mitragotri S. An experimental and theoretical analysis of ultrasound-induced permeabilization of cell membranes. Biophys J. 2003;84(5):3087–3101. doi: 10.1016/S0006-3495(03)70034-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Wystub S, Ebner B, Fuchs C, et al. Interspecies comparison of neuroglobin, cytoglobin and myoglobin: sequence evolution and candidate regulatory elements. Cytogenet Genome Res. 2004;105(1):65–78. doi: 10.1159/000078011. [DOI] [PubMed] [Google Scholar]
- [8].Fordel E, Geuens E, Dewilde S, et al. Cytoglobin expression is upregulated in all tissues upon hypoxia: an in vitro and in vivo study by quantitative real-time PCR. Biochem Biophys Res Commun. 2004;319(2):342–348. doi: 10.1016/j.bbrc.2004.05.010. [DOI] [PubMed] [Google Scholar]
- [9].Fordel E, Geuens E, Dewilde S, et al. Hypoxia/ischemia and the regulation of neuroglobin and cytoglobin expression. IUBMB Life. 2004;56(11-12):681–687. doi: 10.1080/15216540500037406. [DOI] [PubMed] [Google Scholar]
- [10].Fago A, Hundahl C, Malte H, et al. Functional properties of neuroglobin and cytoglobin. Insights into the ancestral physiological roles of globins. IUBMB Life. 2004;56(11-12):689–696. doi: 10.1080/15216540500037299. [DOI] [PubMed] [Google Scholar]
- [11].Huang J, Gao WX, Gao YQ, et al. Hypoxia upregulates the expression of cytoglobin in lung cancer cells. Zhonghua Yi Xue Za Zhi. 2006;86(5):321–324. [PubMed] [Google Scholar]
- [12].Chávez JC, Agani F, Pichiule P, et al. Expression of hypoxia-inducible factor-1alpha in the brain of rats during chronic hypoxia. J Appl Physiol. 2000;89(5):1937–1942. doi: 10.1152/jappl.2000.89.5.1937. [DOI] [PubMed] [Google Scholar]
- [13].Boero JA, Ascher J, Arregui A, et al. Increased brain capillaries in chronic hypoxia. J Appl Physiol. 1999;86(4):1211–1219. doi: 10.1152/jappl.1999.86.4.1211. [DOI] [PubMed] [Google Scholar]
- [14].Kunz M, Hartmann A, Flory E, et al. Anoxia-induced up-regulation of interleukin-8 in human malignant melanoma. A potential mechanism for high tumor aggressiveness. Am J Pathol. 1999;155(3):753–763. doi: 10.1016/S0002-9440(10)65174-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Semenza GL. Expression of hypoxia-inducible factor 1: mechanisms and consequences. Biochem Pharmacol. 2000;59(1):47–53. doi: 10.1016/s0006-2952(99)00292-0. [DOI] [PubMed] [Google Scholar]
- [16].McRonald FE, Liloglou T, Xinarianos G, et al. Down- regulation of the cytoglobin gene, located on 17q25, in tylosis with oesophageal cancer (TOC): evidence for trans-allele repression. Hum Mol Genet. 2006;15(8):1271–1277. doi: 10.1093/hmg/ddl042. [DOI] [PubMed] [Google Scholar]
- [17].Guo X, Philipsen S, Tan-Un KC. Characterization of human cytoglobin gene promoter region. Biochim Biophys Acta. 2006;1759(5):208–215. doi: 10.1016/j.bbaexp.2006.04.002. [DOI] [PubMed] [Google Scholar]
- [18].Ruffels J, Griffin M, Dickenson JM. Activation of ERK1/2, JNK and PKB by hydrogen peroxide in human SH-SY5Y neuroblastoma cells: role of ERK1/2 in H2O2-induced cell death. Eur J Pharmacol. 2004;483(2-3):163–173. doi: 10.1016/j.ejphar.2003.10.032. [DOI] [PubMed] [Google Scholar]


