Abstract
Blood samples were harvested from the antecubital vein of 20 fasting patients with acute cerebral infarction at 1, 7 and 15 days after onset to prepare blood platelet suspension. Fasting antecubital vein blood was collected from an additional 20 normal adults as controls. Under transmission tron microscope, platelet Golgi tubules and vesicles became significantly thickened, enlarged, and irregular after acute cerebral infarction. Alpha granules in platelets significantly reduced in number, especially 1 day after cerebral infarction. Under immunoelectron microscopy, a few alpha granules aggregated around Golgi tubules and vesicles after infarction. These results suggested that platelet Golgi apparatus displayed significant morphological changes, which were possibly associated with enhanced synthetic and secretory functions of activated platelets after acute cerebral infarction. This study used Golgi apparatus blocking agent Brefeldin A to block Golgi apparatus in an aim to study the effects of Golgi apparatus on CD40L expression on the surface of activated platelets. Flow cytometry revealed that CD40L expression on activated platelet surfaces decreased significantly when Golgi apparatus was blocked, which indicated that Golgi apparatus participated in the synthesis and transport of CD40L to the platelet surface.
Keywords: neural regeneration, brain injury, Golgi apparatus, CD40L, alpha granules, platelet, ultrastructure, cerebral infarction, transport, secretion, Brefeldin A, grants-supported paper, neuroregeneration
Research Highlights
(1) No reports concerning the association of platelet Golgi apparatus with acute cerebral infarction or Golgi apparatus participation in platelet CD40L expression have been published. The precise pathway of CD40L transferring to the platelet cell membrane remains unclear.
(2) After acute cerebral infarction, platelet Golgi tubules and vesicles became significantly thickened and enlarged. Alpha granule number in the platelets was significantly reduced. Only a few alpha granules aggregated around Golgi tubules and vesicles after infarction. Golgi apparatus blocking agent Brefeldin A was used, causing a decrease in CD40L expression.
INTRODUCTION
Stroke is a leading cause of death and disability globally, and presents huge burden to families and society[1]. To further complicate the issue, the pathophysiological mechanisms underlying stroke are considered to be heterogeneous[2]. Platelets are known to participate in the pathophysiological progress of stroke, and antithrombotic therapy targeting platelet activation is widely used for treatment of ischemic stroke. Although platelet activation after infarction is an issue that is generally gaining attention, assessment of platelet reactivity after ischemic injury remains under investigated. The specific role of platelet reactivity and activity is not fully understood, as only sparse and conflicting information exists in relation to acute ischemic stroke. Some scientists support the idea that activated platelets participate in the progress of acute cerebral infarctions[3,4,5,6], while others provide evidence corroborating the contrary[7]. Clinically, patients are considered at high risk for ischemic stroke when platelets aggregate in the blood, as platelet aggregation is associated with increased carotid artery intima-media thickness[8]. A previous study found that as platelet adhesiveness and aggregation increased, a variety of pro-in-flammatory cytokines were synthesized and simultaneously released during the pathogenesis or propagation of acute cerebral infarction[9]. Platelet activation is complicated and may play an important role in the mechanism of cerebral infarction. Considering platelet structure is strongly associated with its function, research has focused on two main aspects: platelet activation and ultrastructural changes. Littleton-Kearney et al[10] found that temporary cerebral ischemia would arouse platelet activation and intracerebral platelet aggregation. Some scholars found that ultrastructural changes in platelets were significant after observation in patients with acute ischemic infarction[11,12]. These changes included more pseudopodium, aggregation and fusion, and visibly fewer alpha granules. Compared with controls, the number of alpha granules and mitochondria was significantly lower in platelets from patients with acute ischemic stroke. The number of dense bodies also tended to decrease in platelets from stroke patients. These observations led to the notion that there is an essential connection between platelet structure change and cerebral infraction. In the pathological process of cerebral infarction, platelet secretions may be of much importance to mechanical occlusion of blood vessels due to ensuing platelet aggregation.
Golgi apparatus[13] is a pivotal organelle involved in cell synthesis, packaging, elaboration and axoplasmic flow of proteins. They are regarded as a focal point in the secretory pathway[14]. Platelets derived from bone marrow megakaryocytes retain several mRNA and organelles, such as Golgi apparatus. Previous studies from our group found that the morphology of Golgi apparatus in neurons obviously changed in a gerbil model of transient cerebral ischemia[15,16]. White[12] also confirmed that the presence of fully developed Golgi apparatus obviously changed in patients with white platelet syndrome. These changes were accompanied by centrioles, and deficient numbers or absent alpha granules in circulating platelets. However, no study has reported whether platelet Golgi apparatus is associated with cerebral infarction.
CD40L, a trimeric, transmembrane protein of the tumor necrosis factor family of ligands, plays a major role in immune responses via its receptor, CD40. This receptor is present on various types of human cells, binding and signaling as the surface receptor of soluble CD40L. Moreover, it was subsequently found that CD40L and CD40 are also present on several cells of the vasculature, including endothelial cells, smooth muscle cells, monocytes, and macrophages[18]. Emerging data suggest that CD40L may be at the heart of thrombogenesis. Expression of soluble CD40L has been shown to increase significantly in conditions such as stroke, myocardial infarction, unstable angina, high cholesterol, or other cardiovascular events[19,20]. Recently, CD40L has been detected on the surfaces of activated platelets and shown to activate endothelium[21].
Research has shown that absence of CD40L impairs the stability of arterial thrombi and delays arterial occlusion[22]. As a platelet-rich abundant protein, CD40L contributes to atherosclerotic lesion progression, thrombosis, and restenosis during inflammatory processes. Soluble CD40L, which is released from platelet-rich thrombi following platelet activation, also contributes to these processes[23]. Soluble CD40L induces the synthesis and release of proinflammatory cytokines from vascular cells and matrix metalloproteinases from resident cells in the atheroma[24]. It also stabilizes platelet-rich thrombi and inhibits the re-endothelialization of the injured vessel, which potentially leads to the activation and proliferation of smooth muscle cells[19]. Normally, CD40L lies dormant in unstimulated platelets, but is rapidly presented to the cell membrane and released when platelets are activated by agonists such as adenosine diphosphate, thrombin, or collagen[25]. The surface-expressed CD40L is subsequently cleaved over a period of minutes to hours, generating a soluble fragment termed soluble CD40L. 95% of the circulating CD40L exists in activated platelets[19]. The translocation of CD40L is assumed to coincide with the release of platelet alpha-granule contents, including platelet-derived growth factor, transforming growth factor beta, platelet factor 4, and thrombospondin[26]. However, the specific pathway of the transition of CD40L is not elucidated, and whether Golgi apparatus is involved in the expression of platelet CD40L still needs to be proven. Therefore, we hypothesize that Golgi apparatus may play an important role in the expression of CD40L derived from activated platelets.
This study sought to observe the dynamic, morphological changes in platelet Golgi apparatus in patients with acute cerebral infarction. The role of Golgi apparatus in the expression of platelet CD40L was also investigated using flow cytometry.
RESULTS
Quantitative analysis of subjects
Twenty patients with acute cerebral infarction who were hospitalized within 24 hours (patient group) and 20 normal adults (control group) were recruited in this study. A total of 40 patients were included in the final analysis.
Patient characteristics
We performed analysis of covariance (adjusting for age and sex) for each measurement. No significant differences in age or gender were detected between patient and control groups (P > 0.05; Table 1).
Table 1.
Baseline data of acute cerebral infarction patients and normal adults
Morphological and alpha granule alteration in platelet Golgi apparatus
Ultrastructural observation of platelets from patients who had experienced an acute cerebral infarction showed characteristics that were both similar to and different from those of platelets from controls. Shared features included the presence of microtubules, masses of glycogen particles, Golgi apparatus, and the dense tubular system. All constituent parts of the organellar zone (dense bodies, alpha granules, and mitochondria) were also observed in platelets from the two groups. More specific morphological change analysis and quantification of organelles demonstrated that platelets from patients who had suffered an acute ischemic stroke were altered.
Platelets from the control group were clearly round or elliptic with smooth and complete clear-cut surfaces. These platelets contained clear and homogeneous dispersed intracellular granules, and more alpha granules. Golgi tubules and vesicles were arranged regularly. At 1, 7 and 15 days after infarction, platelets showed irregular morphology, more pseudopodium, aggregation and fusion, and some platelet structures disappeared. At each time point, alpha granules decreased (characteristics are presented in Figures 1 and 2), with the most significant decrease at 1 day after cerebral infarction (compared with each experimental group, P < 0. 05). Figure 3 illustrates irregularly arranged Golgi tubules and vesicles that were apparently increased both in width and size.
Figure 1.
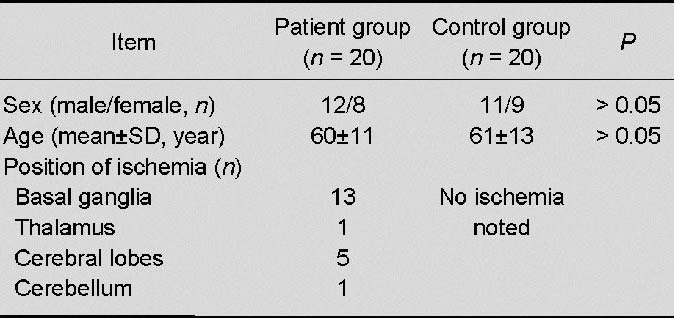
Effects of acute cerebral infarction on platelet Golgi apparatus ultrastructure (transmission electron microscope, × 15 000).
Platelets from controls (A) showed clear profiles with more distributed alpha granules. Golgi tubules and vesicles were regularly arranged. In samples from 1, 7 and 15 days after infarction, aggregated platelets showed irregular morphology, more pseudopodium (arrow), and some platelet structures even disappeared. At each time point, alpha granules decreased visibly. Image from 1 day post infarction is used as a representation (B). Scale bars: 2 μm.
Figure 2.
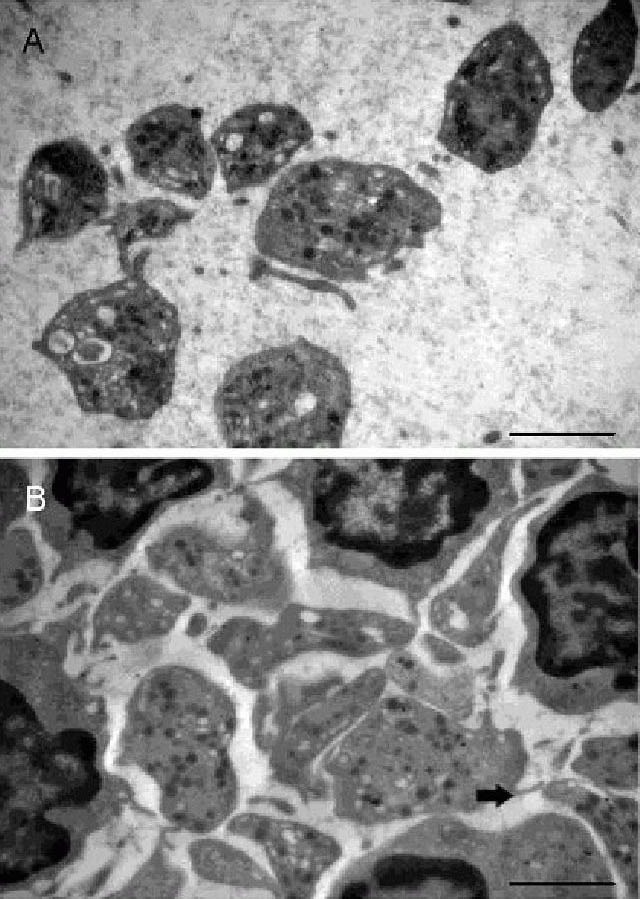
Alteration of alpha granule numbers in patients with acute cerebral infarction.
Data are expressed as mean ± SD. There are 20 patients and controls in each group at each time point. aP < 0.05, vs. normal (control group); bP < 0.05, vs. 7 and 15 days postocclusion group (Student's t-test followed one-way analysis of variance). At each time point, alpha granules were lower than those from control, and showed significant decrease at 1 day after infarction.
Figure 3.
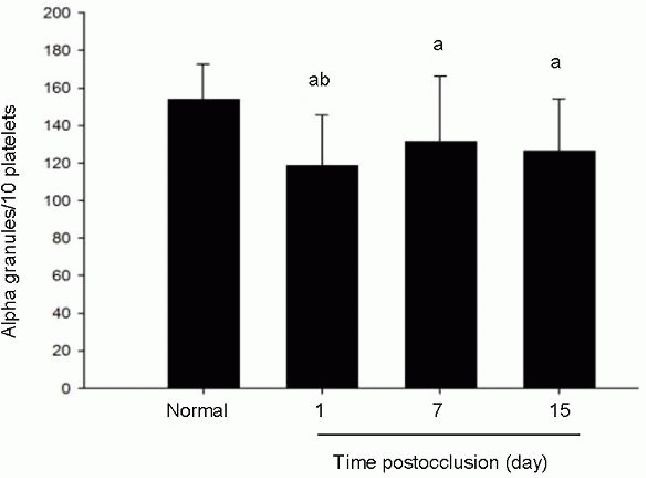
Effects of acute cerebral infarction on platelet ultrastructure (transmission electron microscope, × 20 000).
Golgi tubules and vesicles showed irregular arrangement in platelets from patients 1 day after infarction (B). These structures apparently increased in width and enlarged, compared with platelets from controls (A), in which Golgi internal structure is normal and regular. Arrows point to Golgi apparatus. Scale bars: 1 μm.
Immunoelectron microscopy of the distribution of alpha granules in platelets
The Golgi apparatus from patients after infarction was further observed by immunoelectron microscopy. We used TGN46 as a marker for Golgi apparatus, which is a putative cargo-binding protein that maintains a steady-state level in the dynamic trans-Golgi network structure by active retention and recycling[27]. Colloidal Gold was used for labeling.
Even though alpha granules of platelets from these patients were significantly decreased, particles could still be observed gathered near Golgi tubules and vesicles (Figures 3 and 4).
Figure 4.
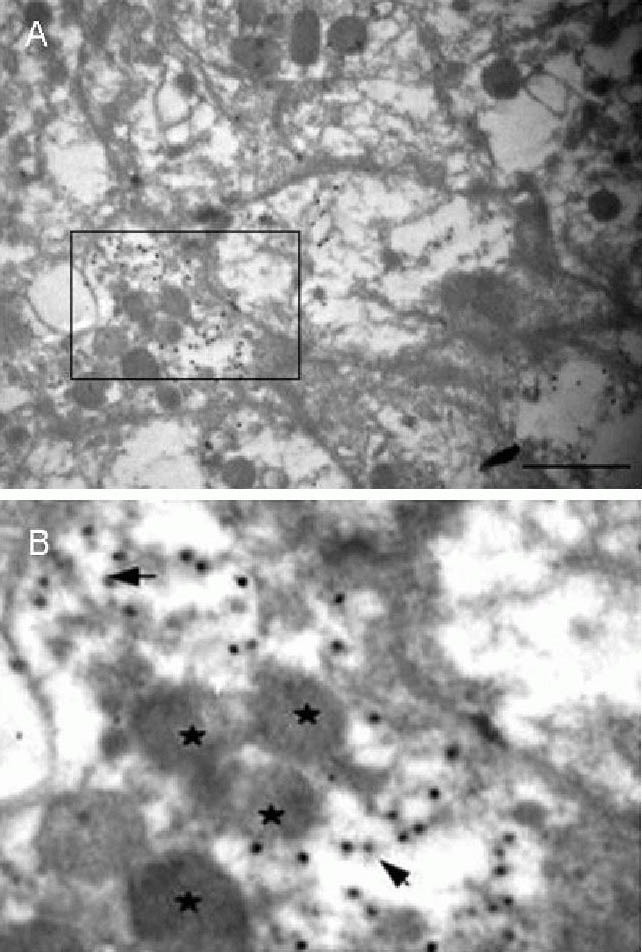
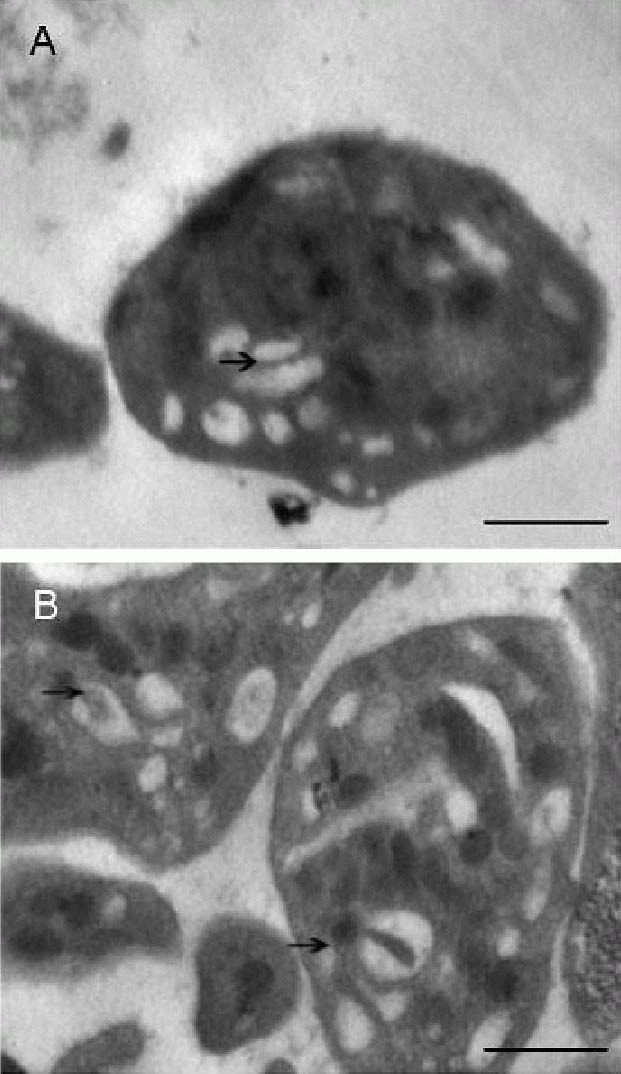
Effects of acute cerebral infarction on platelet Golgi apparatus and alpha granule distribution (immunoelectron microscopy, × 30 000).
(B) is the magnification of a box in (A), scale bar: 1 μm. Alpha granules aggregate around anti-TGN46 and colloidal gold immunostained Golgi tubules. ★ represents alpha granules;  represents colloidal gold particles conjugated with anti-TGN46.
represents colloidal gold particles conjugated with anti-TGN46.
Expression of CD40L on platelets
The surface expression of CD40L observed by flow cytometry of normal platelets at resting condition was only 2.70 ± 0.36%. Compared with nonactivated normal platelets, the surface expression of CD40L on platelets in response to adenosine diphosphate reached up to 14.38 ± 0.19% (P < 0.05). When Golgi was initially blocked by Brefeldin A, followed by platelet activation using adenosine diphosphate, the expression of CD40L decreased to 10.23 ± 0.71% (P < 0.05).
DISCUSSION
The process of platelet activation comprises the release of adenosine diphosphate from dense granules, the synthesis of thromboxane A2 through a series of enzymatic reactions, and the formation of thrombin via a coagulation cascade[8]. Moreover, adenosine diphosphate can be released from endothelial cells at the site of vascular injury[9]. These mediators are able to bind to G protein-coupled receptors on the platelet membrane, which can change the shape of platelets from smooth discs to irregular spheroids with extrusion of filopodia. Platelet membrane GP IIb/IIIa receptors via complex signal transduction mechanisms are then activated. Activated GP IIb/IIIa binds to fibrinogen, finally forming platelet aggregates. A large body of evidence has demonstrated that platelet activation plays an important role in the complicated mechanism of cerebral infraction[3,4]. Activated platelets were able to secrete products containing large amounts of vasoactive substances, such as thromboxane, adenosine diphosphate, ATP, calcium and so on.
The release of these substances (particularly thromboxane and serotonin) may result in vascular and neuronal injury. In addition, the products released by platelets can accelerate further aggregation and adhesion until a thrombus forms, and even lead to the obstruction of the lumen. This study confirmed that compared with control, the irregular profile of aggregated and fused platelets and significant decrease in alpha granules can be observed at 24 hours after acute cerebral infarction. These results are nearly identical to previous studies[11,12]. Recently, the presence of morphological changes in platelets in ischemic stroke has also been demonstrated. These changes included membrane tears and swollen platelets, and may have been due to the pro-coagulant characteristic of the disease[28]. These results suggest that the process of platelet morphology change may be an important step in platelet activation in ischemic stroke. Platelet cytoplasmic granules contain a huge number of biologically active molecules which are grouped under their respective distinct ultrastructures, densities and content[10]. The alpha granule is a unique secretory organelle in that it exhibits further compartmentalization. The alpha granules stem from small precursor granules which can be observed budding from the trans-Golgi network within megakaryocytes, the platelet precursor cell[11]. Alpha granules increased remarkably with the maturation of megakaryocytes and were finally packaged into platelets during thrombopoiesis. During platelet activation, following vessel wall injury, the alpha granules release various activated materials and thus play an important role in hemostasis, inflammation, wound repair and the pathogenesis of atherosclerosis[10].
They participate in hemostasis by stimulating platelets, promoting vasoconstriction and inducing fibrinogen synthesis[12]. On further investigation, our team found more pseudopodium, malformed platelets, where Golgi tubules and vesicles appeared irregularly arranged, and apparently increased in width and size. Moreover, small quantities of alpha granules gathered near Golgi vesicles and networks could also be observed through immunoelectron microscopy. Degranulation is how activated platelets release molecules[29], during the process, small amounts of granules are gathered around the Golgi network. This observation indicates that Golgi apparatus may be involved in the formation and release of alpha granules after cerebral infraction.
Golgi apparatus is an elaborate factory and filtering station for modifying intracellular proteins and lipids[30,31]. This organelle also accepts newly synthesized proteins and lipids from the endoplasmic reticulum. In neurons, Golgi apparatus is mainly responsible for the packaging and axoplasmic flow of endogenous proteins and of exogenous macromolecules transported by the orthograde, retrograde and transsynaptic routes[13]. Golgi apparatus is central to the cellular secretory pathway for newly synthesized protein and lipids. After being transcribed and modified, these macromolecules are transferred to cytomembrane and secretory granules by the Golgi apparatus[15,32]. The morphology and quantity of Golgi apparatus changed markedly in patients with thrombasthenia[17]. Our previous study also showed morphological change of Golgi apparatus after transient ischemia[15]. This study found that platelet Golgi apparatus also changed after acute cerebral ischemia, and platelet structure is strongly associated with its function[33,34,35,36,37,38]. Based on existing research and our experimental results, we can conclude that the change in Golgi apparatus has some connection with the increasing synthesis and secretory function of platelets after cerebral infarction. This indicates that the Golgi apparatus in platelets has an essential relationship with the occurrence and development of cerebral infarction.
CD40L is a transmembrane glycoprotein with a molecular weight of 39 kDa. It is hidden in unstimulated platelets but is rapidly presented to the cell membrane after platelet stimulation[14], where it is subsequently hydrolyzed into a soluble fragment termed soluble CD40L. Soluble CD40L and membrane-bound CD40L have the same biological effect. Clinically, high levels of circulating soluble CD40L have been associated with many diseases including ischemic stroke, hypercholesterolemia, acute coronary syndromes, and diabetes, and predict increased restenosis after percutaneous coronary intervention[39]. CD40L is already known to be important in the pathologic process of atherosclerosis. In addition, CD40L appears to be particularly relevant because high levels of circulating soluble CD40L are released in response to platelet thrombosis, making elevated levels of soluble CD40L a reliable predictor of cardiovascular events. Moreover, soluble CD40L is also a ligand for GP IIb/IIIa and involved in the mechanism of thrombus stabilization and platelet activation. A growing body of clinical evidence supports the potential importance of elevated plasma soluble CD40L in the mechanism of ischemic strokes. A previous study proved soluble CD40L increased greatly in patients with acute cerebral infarction[7]. Moreover, CD40L plays a significant role in the formation of cerebral infarction[40,41,42]. 95% of soluble CD40L in circulation was from activated platelets[12]. Some thoughts that the expression of CD40L might associate with alpha granules have been expressed[43], but how CD40L is transported intracelluarly to the membrane still requires further elucidation[40,44,45,46]. In the inactivated form, platelets do not express the CD40L glycoprotein. CD40L is transported from the intracellular region to the membrane after platelet activation, and is then split into soluble CD40L fragments by hydratase and released[40,44,47]. A GPIIb/IIIa inhibitor, such as abciximab, eptifibatide or tirofiban, can inhibit the release of soluble CD40L from platelet membranes[48,49,50,51], but cannot inhibit the transporting process[47]. Thienopyridines suppress the platelet aggregation adenosine diphosphate pathway by inhibiting the platelet P2Y12 subtype of adenosine diphosphate receptor, while clopidogrel lowers the expression of CD40L by inhibiting platelet stimulation by adenosine diphosphate[52]. Therefore, in summary inhibiting CD40L requires targeting multiple pathways. Recent studies, however, have revealed that approximately 10% to 40% patients showed hyporesponsiveness or even non-responsiveness to clopidogrel[15]. Thus, it would be worthwhile to probe an effective way to inhibit the expression of CD40L. However, the role of Golgi apparatus in the expression of platelet CD40L has not yet been evaluated in cerebral infarction. An important finding from this study was that blocking Golgi apparatus function with Brefeldin A before activation demonstrated that the expression of CD40L was greatly decreased if the function of Golgi apparatus was blocked. These results suggest that Golgi apparatus may be involved in the expression of platelet CD40L.
The present study of morphological alteration of platelet Golgi apparatus within patients after ischemic stroke and the relationship between Golgi apparatus and CD40L has provided considerable information concerning the important role played by Golgi apparatus after infarction. These changes appear to be in connection with platelet activation, synthesis and secretion, but further larger studies are required to confirm this hypothesis. The distinct relationship between Golgi apparatus and the expression of platelet CD40L is considered to be of potential value because a novel therapeutic target for ischemic insult could be elucidated with further evaluation.
SUBJECTS AND METHODS
Design
A controlled study based on clinical cases.
Time and setting
Experiments were performed at the Second Xiangya Hospital, Central South University, China from 2009 to 2011.
Subjects
We investigated 20 patients ranging in age from 46 to 76 years from 2009 to 2011 who had been hospitalized at the Second Xiangya Hospital, Central South University, China within 24 hours after the onset of acute ischemic stroke. All patients fit into the revised diagnostic standard from the Forth National Cerebrovascular Disease Conference of China[53], as was proven by head CT and MRI. Ischemic regions were found bilaterally or unilaterally in the basal ganglia, thalamus, and various other regions of the brain lobes. Patients showed hemiplegia, aphasia, sensory impairments or other clinical manifestations, depending on the damaged area. All stroke patients were nonsmokers and underwent a cranial CT to exclude cerebral hemorrhage. The clinical deficit after stroke lasted for at least 24 hours. None of the patients suffered from a new cerebral or cardiac ischemic event during the 15-day follow-up. They did not take antiplatelet medication for at least 2 weeks, and did not have diabetes mellitus, coronary heart disease or deep vein thrombosis before their ischemic event. They did however have hypertension.
A total of 20 controls at the same period were chosen from Medical Examination Center, Second Xiangya Hospital in China. Inclusion criteria were: healthy nonsmokers, aged from 28 to 69 years, and not taking antiplatelet medication for at least 2 weeks.
Exclusion criteria for all subjects were surgery; acute organ ischemia within the preceding 3 months; cancer; chronic inflammatory diseases; blood system diseases; fever; acute inflammatory or infectious conditions at study entry. For healthy control subjects, any vascular diseases (diabetes mellitus, coronary heart disease, deep vein thrombosis) were additional exclusion criteria.
The participating subjects gave informed consent. The study was consistent with the Declaration of Helsinki.
Methods
Sample collection
In stroke patients, blood samples were taken within 24 hours after ischemia and at 7 and 15 days after consistent therapy, including aspirin (p.o., 100 mg, once per day; Baeyer, Leverkusen, Germany), edaravone (i.v., 30 mg, once per day; Guorui, Heilongjiang Province, China), and monosialotetrahexosylganglioside sodium injection (i.v., 40 mg, once per day; Qilu, Shandong Province, China) in connection with acute ischemia. In 20 healthy control subjects, blood samples were taken once. All the samples were taken before breakfast. Venepuncture of forearm veins was performed with minimal stasis. We used a 2-syringe technique to reject the first few milliliters of the sample.
Preparation of platelets for electron microscopy
Blood was collected on an empty stomach from the antecubital vein (5 mL) and anticoagulated with 3.8% (w/v) trisodium citrate. Whole blood was collected and processed using solutions that optimally retain the organelles of platelets. Platelet-rich plasma (2 mL) was obtained by centrifuging citrated whole blood at 3.5 × g at 22°C for 15 minutes, then equally divided into two Eppendorf tubes, followed by centrifugation at 11 000 × g at 22°C for 5 minutes. The upper layer of platelet-rich plasma was removed, leaving platelets at the bottom of Eppendorf tubes, which was mixed with 1 mL of hydroxyethyl piperazine ethanesulfonic acid (HEPES) (pH 7.4; Sigma, St. Louis, MO, USA) containing 0.6% glutaraldehyde. This mixture was kept at room temperature for 15 minutes. The partially fixed platelets were then centrifuged at 11 000 × g for 5 minutes at 22°C to form a pellet. HEPES was then removed and replaced.
Electron microscopy
The prepared pellet was immersed in paraformaldehyde for 15–30 minutes at 4°C, and dehydrated in a series of graded ethanol solutions. The pellet was next broken into smaller pieces that were embedded in Maraglas. Ultrathin sections (50–70 nm) were cut from three blocks using an ultratome (Model 8801A, LKBProduckter AB, Stockholm, Sweden) and a diamond knife. Each section was mounted on a 200-mesh nickel grid and doubly stained with uranyl acetate and lead citrate. Using a transmission electron microscope (H-7500; Hitachi, Tokyo, Japan), we photographed platelets at five random fields. 10–20 photographs were usually produced per pellet. Alpha granules of ten platelets randomly selected from each sample were counted at 15 000-fold magnification.
Immunoelectron microscopy
For immunoelectron microscopy, sections were incubated in 1% hydrogen peroxide for 30 minutes to block endogenous peroxidase activity. They were then rinsed in double distilled water, incubated in goat serum, and rinsed in PBS again. Normal goat serum was used as a pre-block, after which the sections were incubated with rabbit anti-TGN46 polyclonal antibody (10 μg/mL; Sigma) at room temperature for 1 hour, and then at 4°C for 36 hours. After incubation with primary antibodies, sections were rinsed in PBS (3 × 10 minutes) and incubated in Colloidal Gold (10 nm in diameter; 1:100; Boster, Wuhan, China) at room temperature for 1 hour, followed by a wash in double distilled water. Subsequently, sections were incubated in 5% uranyl acetate for 5 minutes, washed in double distilled water, and finally stained with uranium citrate for 5 minutes. Photographs were taken under an immunoelectron microscope (H-7500; Hitachi).
Preparation of platelets for flow cytometry
Venous blood was obtained from 20 healthy individuals before breakfast. Platelets were isolated using the standard methods of Baenziger and Majerus[54]. Briefly, human blood from healthy volunteers was collected into 3.8% (v/v) sodium citrate (nine parts of blood to one part of sodium citrate). The blood was centrifuged at 150 × g for 15 minutes at 22°C. The upper phase was used as platelet-rich plasma and was divided into three parts (500 μL each), which were recentrifuged at 10 000 × g for 5 minutes at 22°C to form a pellet. The pellet was fixed with HEPES-Tyrode's buffer (10 mmol/L HEPES/137 mmol/L NaCl/2.68 mmol/L KCl/0.42 mmol/L NaH2PO4/1.7 mmol/L MgCl2/11.9 mmol/L NaHCO3/5 mmol/L glucose), to make the bulk volume to be 1 000 μL. The suspension in the first tube served as control, while 999 μL of platelets of the second suspension were removed to an Eppendorf tube containing 1 μL Brefeldin A (1:1 000; Biolegend, San Diego, CA, USA) using a transfer pipette, and this mixture was kept for 20 minutes. The platelets in the second and third tubes were both stimulated by adenosine diphosphate (adenosine-5’-diphosphate disodium salt; Sigma) for 3 minutes. Brefeldin A and adenosine diphosphate were stored in aliquots at –20°C. Brefeldin A was diluted by the suspension to achieve a final concentration of 1 μmol/L (incubated at 22°C for 5 minutes). Adenosine diphosphate was added to platelets at a concentration of 30 μmol/L (incubated at 22°C for 3 minutes). For flow cytometry analysis, 50 μL aliquots were taken from each of the three tubes. A phycoerythrin (PE)-labeled anti-human CD40L (BioLegend) monoclonal antibody (10 μL each) was added, with PE-mouse IgG 1, κ (BioLegend) served as non-specific controls. The mixtures were incubated in the dark for 30 minutes at 22°C. The platelets were fixed by addition of 1.5 mL of 3 g/L paraformaldehyde. Subsequently, 500 μL HEPES-Tyrode's buffer was added to resuspend the platelets. This platelet suspension was centrifuged at 10 000 × g for 5 minutes. The upper phase was removed, and platelets were resuspended in 500 μL HEPES-Tyrode's buffer.
The platelet suspensions were analyzed on a FACSCalibur flow cytometer with CellQuest Pro 4.0.2 software (BD Diagnostics, San Diego, CA, USA). Forward scatter, side scatter, and fluorescence data were obtained with gain settings in the logarithmic mode. For each sample, 10 000 platelets were acquired. To identify CD40L marker-positive events, thresholds were set based on samples incubated with similar concentrations of isotype-matched control antibodies.
Statistical analysis
All data including the number of alpha granules, expression of CD40L were expressed as mean ± SD. Statistical analysis was performed with one-way analysis of variance followed by Student's t-test. A value of P < 0.05 was considered statistically significant. All analyses were performed using SPSS 13.0 software (SPSS, Chicago, IL, USA).
Footnotes
Funding: This work was supported by grants from the National Natural Science Foundation of China, No. 81171239/H0914; Frontier Research Key Project, Central South University in China (2010-2011), No. 2177-721500065; and the Education Expenditure of Hunan Provincial Finance Department in China, No. 2010163.
Conflicts of interest: None declared.
Ethical approval: This research has been approved by the Ethics Committee of Second Xiangya Hospital, Central South University, China.
(Reviewed by Apricò K, Stow A, Wang YL, Zhang RX)
(Edited by Wang LM, Qiu Y, Li CH, Song LP, Liu WJ, Zhao M)
REFERENCES
- [1].Kurabayashi H, Tamura J, Naruse T, et al. Possible existence of platelet activation before the onset of cerebral infarction. Atherosclerosis. 2000;153(1):203–207. doi: 10.1016/s0021-9150(00)00399-3. [DOI] [PubMed] [Google Scholar]
- [2].Jaremo P, Eriksson M, Lindahl TL, et al. Platelets and acute cerebral infarction. Platelets. doi: 10.3109/09537104.2012.712168. in press. [DOI] [PubMed] [Google Scholar]
- [3].Sandercock PA, Counsell C, Gubitz GJ, et al. Antiplatelet therapy for acute ischaemic stroke. Cochrane Database Syst Rev. 2008;3:CD000029. doi: 10.1002/14651858.CD000029.pub2. [DOI] [PubMed] [Google Scholar]
- [4].McCabe DJ, Harrison P, Mackie IJ, et al. Platelet degranulation and monocyte-platelet complex formation are increased in the acute and convalescent phases after ischaemic stroke or transient ischaemic attack. Br J Haematol. 2004;125(6):777–787. doi: 10.1111/j.1365-2141.2004.04983.x. [DOI] [PubMed] [Google Scholar]
- [5].Sandercock P, Gubitz G, Foley P, et al. Antiplatelet therapy for acute ischaemic stroke. Cochrane Database Syst Rev. 2003;2:CD000029. doi: 10.1002/14651858.CD000029. [DOI] [PubMed] [Google Scholar]
- [6].Wang J, Cao L, He M. Flow cytometric 3-color analysis of circulating activated platelets and its clinical significance in ischemic cerebrovascular diseases. Zhonghua Yi Xue Za Zhi. 2000;80(7):499–502. [PubMed] [Google Scholar]
- [7].Järemo P, Eriksson M, Lindahl TL, et al. Platelets and acute cerebral infarction. Platelets. 2013;24(5):407–411. doi: 10.3109/09537104.2012.712168. [DOI] [PubMed] [Google Scholar]
- [8].Shimizu M, Yamamoto M, Takizawa S, et al. Platelet aggregates detected by a conventional hematology analyzer method is a risk factor for stroke or a predictive factor in patients with chronic-stage cerebral infarction. J Stroke Cerebrovasc Dis. 2011;20(4):275–281. doi: 10.1016/j.jstrokecerebrovasdis.2010.01.009. [DOI] [PubMed] [Google Scholar]
- [9].Schafer A, Bauersachs J. Endothelial dysfunction, impaired endogenous platelet inhibition and platelet activation in diabetes and atherosclerosis. Curr Vasc Pharmacol. 2008;6(1):52–60. doi: 10.2174/157016108783331295. [DOI] [PubMed] [Google Scholar]
- [10].Littleton-Kearney MT, Hurn PD, Kickler TS, et al. Incomplete global cerebral ischemia alters platelet biology in neonatal and adult sheep. Am J Physiol. 1998;274(4 Pt 2):H1293–1300. doi: 10.1152/ajpheart.1998.274.4.H1293. [DOI] [PubMed] [Google Scholar]
- [11].Joseph R, Riddle JM, Welch KM, et al. Platelet ultrastructure and secretion in acute ischemic stroke. Stroke. 1989;20(10):1316–1319. doi: 10.1161/01.str.20.10.1316. [DOI] [PubMed] [Google Scholar]
- [12].Xue SW, Guo SH, Zhang FZ. An ultrastructural study of platelets in patients with acute cerebrovascular disease. Zhonghua Shenjingke Zazhi. 1996;29(1):45. [Google Scholar]
- [13].Albarran MT, Lopez-Burillo S, Pablos MI, et al. Endogenous rhythms of melatonin, total antioxidant status and superoxide dismutase activity in several tissues of chick and their inhibition by light. J Pineal Res. 2001;30(4):227–233. doi: 10.1034/j.1600-079x.2001.300406.x. [DOI] [PubMed] [Google Scholar]
- [14].Lippincott-Schwartz J, Roberts TH, Hirschberg K. Secretory protein trafficking and organelle dynamics in living cells. Annu Rev Cell Dev Biol. 2000;16:557–589. doi: 10.1146/annurev.cellbio.16.1.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Hu Z, Zeng L, Xie L, et al. Morphological alteration of Golgi apparatus and subcellular compartmentalization of TGF-beta1 in Golgi apparatus in gerbils following transient forebrain ischemia. Neurochem Res. 2007;32(11):1927–1931. doi: 10.1007/s11064-007-9382-1. [DOI] [PubMed] [Google Scholar]
- [16].Jiang Z, Hu Z, Zeng L, et al. The role of the Golgi apparatus in oxidative stress: is this organelle less significant than mitochondria? Free Radic Biol Med. 2011;50(8):907–917. doi: 10.1016/j.freeradbiomed.2011.01.011. [DOI] [PubMed] [Google Scholar]
- [17].White JG. Golgi complexes in hypogranular platelet syndromes. Platelets. 2005;16(1):51–60. doi: 10.1080/0953710042000260173. [DOI] [PubMed] [Google Scholar]
- [18].Croce K, Libby P. Intertwining of thrombosis and inflammation in atherosclerosis. Curr Opin Hematol. 2007;14(1):55–61. doi: 10.1097/00062752-200701000-00011. [DOI] [PubMed] [Google Scholar]
- [19].Andre P, Nannizzi-Alaimo L, Prasad SK, et al. Platelet-derived CD40L: the switch-hitting player of cardiovascular disease. Circulation. 2002;106(8):896–899. doi: 10.1161/01.cir.0000028962.04520.01. [DOI] [PubMed] [Google Scholar]
- [20].Oberheiden T, Nguyen XD, Fatar M, et al. Platelet and monocyte activation in acute ischemic stroke--is there a correlation with stroke etiology? Clin Appl Thromb Hemost. 2012;18(1):87–91. doi: 10.1177/1076029611412359. [DOI] [PubMed] [Google Scholar]
- [21].Andre P, Prasad KS, Denis CV, et al. CD40L stabilizes arterial thrombi by a beta3 integrin--dependent mechanism. Nature medicine. 2002;8(3):247–252. doi: 10.1038/nm0302-247. [DOI] [PubMed] [Google Scholar]
- [22].Wenzel F, Gunther W, Baertl A, et al. Comparison of soluble CD40L concentrations and release capacities in apheresis and prestorage pooled platelet concentrates. Clin Hemorheol Microcirc. 2011;47(4):269–278. doi: 10.3233/CH-2011-1407. [DOI] [PubMed] [Google Scholar]
- [23].Ferro D, Loffredo L, Polimeni L, et al. Soluble CD40 ligand predicts ischemic stroke and myocardial infarction in patients with nonvalvular atrial fibrillation. Arterioscler Thromb Vasc Biol. 2007;27(12):2763–2768. doi: 10.1161/ATVBAHA.107.152777. [DOI] [PubMed] [Google Scholar]
- [24].Tayebjee MH, Lip GY, Tan KT, et al. Plasma matrix metalloproteinase-9, tissue inhibitor of metalloproteinase-2, and CD40 ligand levels in patients with stable coronary artery disease. Am J Cardiol. 2005;96(3):339–345. doi: 10.1016/j.amjcard.2005.03.072. [DOI] [PubMed] [Google Scholar]
- [25].Delmas Y, Viallard JF, Villeneuve J, et al. Platelet-associated CD154: a new interface in haemostasis and in the inflammatory reaction. Med Sci (Paris) 2005;21(10):825–831. doi: 10.1051/medsci/20052110825. [DOI] [PubMed] [Google Scholar]
- [26].Inwald DP, McDowall A, Peters MJ, et al. CD40 is constitutively expressed on platelets and provides a novel mechanism for platelet activation. Circ Res. 2003;92(9):1041–1048. doi: 10.1161/01.RES.0000070111.98158.6C. [DOI] [PubMed] [Google Scholar]
- [27].Cobbold C, Coventry J, Ponnambalam S, et al. Actin and microtubule regulation of trans-Golgi network architecture, and copper-dependent protein transport to the cell surface. Mol Membr Biol. 2004;21(1):59–66. doi: 10.1080/096870310001607350. [DOI] [PubMed] [Google Scholar]
- [28].Pretorius E, Engelbrecht MJ, Duim W. Thromboembolic ischemic stroke and the presence of necrotic platelets: a scanning electron microscopy investigation. Ultrastruct Pathol. 2012;36(1):19–22. doi: 10.3109/01913123.2011.620219. [DOI] [PubMed] [Google Scholar]
- [29].Harrison P, Cramer EM. Platelet alpha-granules. Blood Rev. 1993;7(1):52–62. doi: 10.1016/0268-960x(93)90024-x. [DOI] [PubMed] [Google Scholar]
- [30].Xu H, Su W, Cai M, et al. The asymmetrical structure of Golgi apparatus membranes revealed by in situ atomic force microscope. PLoS One. 2013;8(4):e61596. doi: 10.1371/journal.pone.0061596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Koreishi M, Gniadek TJ, Yu S, et al. The golgin tether giantin regulates the secretory pathway by controlling stack organization within Golgi apparatus. PLoS One. 2013;8(3):e59821. doi: 10.1371/journal.pone.0059821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Pelham HR. Traffic through the Golgi apparatus. J Cell Biol. 2001;155(7):1099–1101. doi: 10.1083/jcb.200110160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Corash L. Inactivation of viruses, bacteria, protozoa, and leukocytes in platelet concentrates. Vox Sang. 1998;74(Suppl 2):173–176. doi: 10.1111/j.1423-0410.1998.tb05418.x. [DOI] [PubMed] [Google Scholar]
- [34].Weyrich AS, Dixon DA, Pabla R, et al. Signal-dependent translation of a regulatory protein, Bcl-3, in activated human platelets. Proc Natl Acad Sci U S A. 1998;95(10):5556–5561. doi: 10.1073/pnas.95.10.5556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Pabla R, Weyrich AS, Dixon DA, et al. Integrin-dependent control of translation: engagement of integrin alphaIIbbeta3 regulates synthesis of proteins in activated human platelets. J Cell Biol. 1999;144(1):175–184. doi: 10.1083/jcb.144.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Rivera J, Lozano ML, Corral J, et al. Quality assessment of platelet concentrates supplemented with second-messenger effectors. Transfusion. 1999;39(2):135–143. doi: 10.1046/j.1537-2995.1999.39299154726.x. [DOI] [PubMed] [Google Scholar]
- [37].Lindemann S, Tolley ND, Dixon DA, et al. Activated platelets mediate inflammatory signaling by regulated interleukin 1beta synthesis. J Cell Biol. 2001;154(3):485–490. doi: 10.1083/jcb.200105058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Lindemann S, Tolley ND, Eyre JR, et al. Integrins regulate the intracellular distribution of eukaryotic initiation factor 4E in platelets. A checkpoint for translational control. J Biol Chem. 2001;276(36):33947–33951. doi: 10.1074/jbc.M104281200. [DOI] [PubMed] [Google Scholar]
- [39].Jefferis BJ, Whincup PH, Welsh P, et al. Prospective study of circulating soluble CD40 ligand concentrations and the incidence of cardiovascular disease in a nested prospective case-control study of older men and women. J Thromb Haemost. 2011;9(8):1452–1459. doi: 10.1111/j.1538-7836.2011.04415.x. [DOI] [PubMed] [Google Scholar]
- [40].Henn V, Steinbach S, Buchner K, et al. The inflammatory action of CD40 ligand (CD154) expressed on activated human platelets is temporally limited by coexpressed CD40. Blood. 2001;98(4):1047–1054. doi: 10.1182/blood.v98.4.1047. [DOI] [PubMed] [Google Scholar]
- [41].Andre P, Prasad KS, Denis CV, et al. CD40L stabilizes arterial thrombi by a beta3 integrin--dependent mechanism. Nat Med. 2002;8(3):247–252. doi: 10.1038/nm0302-247. [DOI] [PubMed] [Google Scholar]
- [42].Urbich C, Dernbach E, Aicher A, et al. CD40 ligand inhibits endothelial cell migration by increasing production of endothelial reactive oxygen species. Circulation. 2002;106(8):981–986. doi: 10.1161/01.cir.0000027107.54614.1a. [DOI] [PubMed] [Google Scholar]
- [43].Michelson AD, Ellis PA, Barnard MR, et al. Downregulation of the platelet surface glycoprotein Ib-IX complex in whole blood stimulated by thrombin, adenosine diphosphate, or an in vivo wound. Blood. 1991;77(4):770–779. [PubMed] [Google Scholar]
- [44].Aukrust P, Muller F, Ueland T, et al. Enhanced levels of soluble and membrane-bound CD40 ligand in patients with unstable angina. Possible reflection of T lymphocyte and platelet involvement in the pathogenesis of acute coronary syndromes. Circulation. 1999;100(6):614–620. doi: 10.1161/01.cir.100.6.614. [DOI] [PubMed] [Google Scholar]
- [45].Jin Y, Nonoyama S, Morio T, et al. Characterization of soluble CD40 ligand released from human activated platelets. J Med Dent Sci. 2001;48(1):23–27. [PubMed] [Google Scholar]
- [46].Nannizzi-Alaimo L, Alves VL, Phillips DR. Inhibitory effects of glycoprotein IIb/IIIa antagonists and aspirin on the release of soluble CD40 ligand during platelet stimulation. Circulation. 2003;107(8):1123–1128. doi: 10.1161/01.cir.0000053559.46158.ad. [DOI] [PubMed] [Google Scholar]
- [47].Henn V, Slupsky JR, Grafe M, et al. CD40 ligand on activated platelets triggers an inflammatory reaction of endothelial cells. Nature. 1998;391(6667):591–594. doi: 10.1038/35393. [DOI] [PubMed] [Google Scholar]
- [48].Judge HM, Buckland RJ, Holgate CE, et al. Glycoprotein IIb/IIIa and P2Y12 receptor antagonists yield additive inhibition of platelet aggregation, granule secretion, soluble CD40L release and procoagulant responses. Platelets. 2005;16(7):398–407. doi: 10.1080/09537100500163226. [DOI] [PubMed] [Google Scholar]
- [49].Furman MI, Krueger LA, Linden MD, et al. GPIIb-IIIa antagonists reduce thromboinflammatory processes in patients with acute coronary syndromes undergoing percutaneous coronary intervention. J Thromb Haemost. 2005;3(2):312–320. doi: 10.1111/j.1538-7836.2005.01124.x. [DOI] [PubMed] [Google Scholar]
- [50].Welt FG, Rogers SD, Zhang X, et al. GP IIb/IIIa inhibition with eptifibatide lowers levels of soluble CD40L and RANTES after percutaneous coronary intervention. Catheter Cardiovasc Interv. 2004;61(2):185–189. doi: 10.1002/ccd.10763. [DOI] [PubMed] [Google Scholar]
- [51].Bertram U, Moser M, Peter K, et al. Effects of different thrombolytic treatment regimen with abciximab and tirofiban on platelet aggregation and platelet-leukocyte interactions: a subgroup analysis from the GUSTO V and FASTER trials. J Thromb Thrombolysis. 2002;14(3):197–203. doi: 10.1023/a:1025044625487. [DOI] [PubMed] [Google Scholar]
- [52].Furman MI, Krueger LA, Linden MD, et al. Release of soluble CD40L from platelets is regulated by glycoprotein IIb/IIIa and actin polymerization. J Am Coll Cardiol. 2004;43(12):2319–2325. doi: 10.1016/j.jacc.2003.12.055. [DOI] [PubMed] [Google Scholar]
- [53].The Editorial Board of the Fourth National Cerebrovascular Disease Conference of Chinese Medical Association. Elements of various types of cerebral vascular disease diagnosis. Zhonghua Shenjingke Zazhi. 1996;29(6):379. [Google Scholar]
- [54].Baenziger NL, Majerus PW. Isolation of human platelets and platelet surface membranes. Methods Enzymol. 1974;31:149–155. doi: 10.1016/0076-6879(74)31015-4. [DOI] [PubMed] [Google Scholar]


