Abstract
Stem cell transplantation can promote functional restoration following acute spinal cord injury (injury time < 3 months), but the safety and long-term efficacy of this treatment need further exploration. In this study, 25 patients with traumatic spinal cord injury (injury time > 6 months) were treated with human umbilical cord blood stem cells via intravenous and intrathecal injection. The follow-up period was 12 months after transplantation. Results found that autonomic nerve functions were restored and the latent period of somatosensory evoked potentials was reduced. There were no severe adverse reactions in patients following stem cell transplantation. These experimental findings suggest that the transplantation of human umbilical cord blood stem cells is a safe and effective treatment for patients with traumatic spinal cord injury.
Keywords: neural regeneration, spinal cord injury, human umbilical cord blood stem cells, transplantation, paraplegia, American Spinal Cord Injury Association score, neurological function, secretion, somatosensory evoked potentials, spasm, safety, photographs-containing paper, neurogeneration
Research Highlights
(1) The safety of human umbilical cord blood stem cell transplantation in the treatment of traumatic spinal cord injury (injury time > 6 months) was observed.
(2) The restoration of neurological function was explored at 12 months after stem cell transplantation in patients with traumatic spinal cord injury. In addition, a neuroelectrophysiological monitoring system, the somatosensory evoked potential test, was performed to determine the nerve conduction functions of patients, thus reflecting the conduction of the spinal cord.
INTRODUCTION
The existing methods of treatment for patients with traumatic spinal cord injury have limited effectiveness, which leads to an increased number of patients with neurological complications in the late stages of injury[1,2,3,4,5]. An attractive source for cell transplantation is human umbilical cord blood[6], and there have been several studies in vitro showing that umbilical cord blood cells secrete a number of cytokines that could be beneficial to recovery following spinal cord injury[7,8,9]. There are also a number of animal experiments that demonstrate the capacity of umbilical cord blood cells to differentiate into neural and glial cells[10,11,12,13,14]. These properties are similar to multipotent mesenchymal cells found in bone marrow[11]. However, the conclusions of these studies are based on experimental animals, with rare studies of the safety and therapeutic effect of human umbilical cord blood stem cells in human being.
These studies concluded that intravenous injection is a safe approach and that infusion as close as possible to the injury site is the most effective[15,16,17,18]. Although these therapies were effective in the short-term, the long-term results of stem cell therapy have not been reported, and reports of the clinical application of human umbilical cord blood stem cells are rare. The aims of this study are to explore the long-term effect of human umbilical cord blood stem cell therapy, which combined intravenous injection and direct epidural injection. To evaluate the effect of stem cell therapy, we observed the patients’ American Spinal Cord Injury Association score, autonomic nerve function, Ashworth scale, and somatosensory evoked potential value in limbs at different time points before and after treatment.
RESULTS
Quantitative analysis and baseline information of patients
Twenty-five patients with late-stage spinal cord injury (9 females and 16 males; injury time > 6 months) receiving human umbilical cord blood stem cell transplantation and traditional rehabilitation were chosen as the treatment group, and another 25 patients with late-stage spinal cord injury (10 females and 15 males) receiving only traditional rehabilitation served as the control group. Both groups were followed-up for 12 months after treatment (Table 1).
Table 1.
Baseline analysis of involved patients in two groups
Complications of spinal cord injury patients after human umbilical cord blood stem cell transplantation
The stem cell treatment group comprised 9 females and 16 males, 5 (20%) of which were quadriplegic and 20 (80%) were paraplegic. Through regular and MRI examinations, there were no severe complications, neoplasm or aggravated neurological symptoms shown. Three (12%) patients had fever after infusion of stem cells; their body temperature was maintained at 37–38°C, there were no abnormities in the levels of white blood cells and the fever was retained for less than 24 hours. The fever could be controlled by physical hypothermia. There was no statistically significant difference in terms of complication rates between the paraplegic and the quadriplegic patients (P > 0.05).
Improvements of neurological function in patients with spinal cord injury after human umbilical cord blood stem cell transplantation (Table 2)
Table 2.
The amount and percentage of patients [n (%)] with improvements in different functions after human umbilical cord blood stem cell transplantation
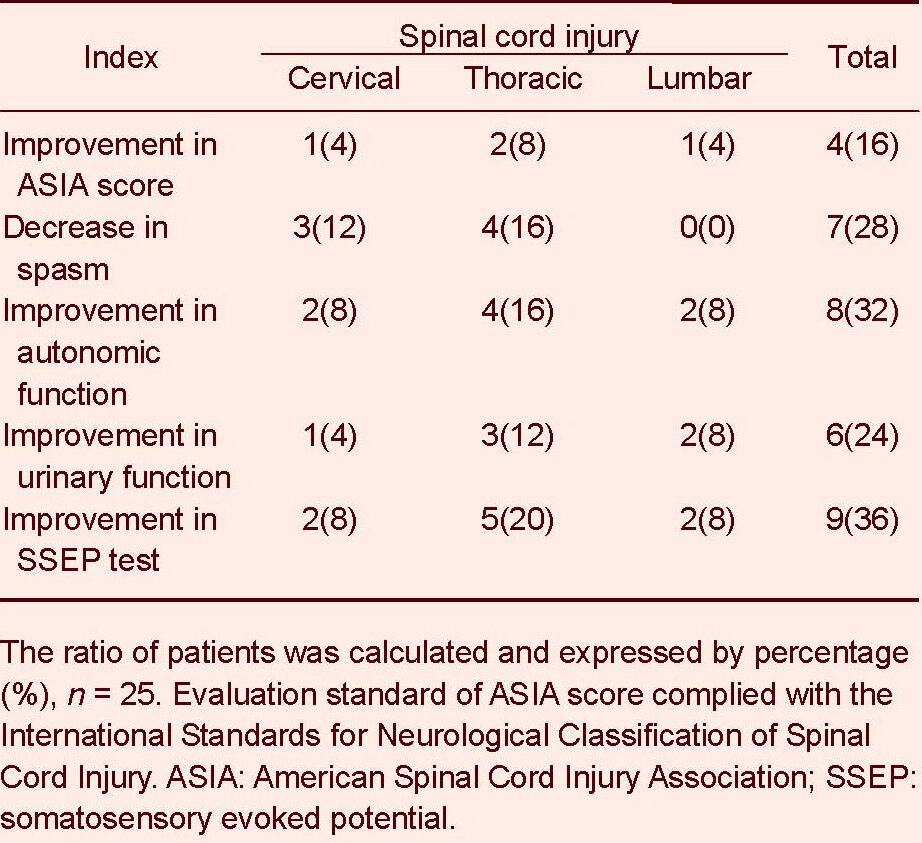
At 12 months after stem cell therapy, 4 patients (16 %) showed improvements in American Spinal Cord Injury Association score: one case was cervical spinal cord injury, two cases were thoracic spinal cord injury, and one case was lumbar spinal cord injury. Spasm decreased in seven patients (28%) after stem cell therapy, including three cases with cervical spinal cord injury and four cases with thoracic spinal cord injury. Eight patients (32%) had improved autonomic function after stem cell therapy, including two cases with cervical spinal cord injury, four cases with thoracic spinal cord injury, and two cases with lumbar spinal cord injury. Six patients (24%) had improved urinary function after stem cell therapy, including one case with cervical spinal cord injury, three cases with thoracic spinal cord injury, and two cases with lumbar spinal cord injury (Table 2, Figure 1). Nine patients (36%) had improved somatosensory evoked potential tests after stem cell therapy, including two cases with cervical spinal cord injury, five cases with thoracic spinal cord injury, and two cases with lumbar spinal cord injury (Table 2).
Figure 1.
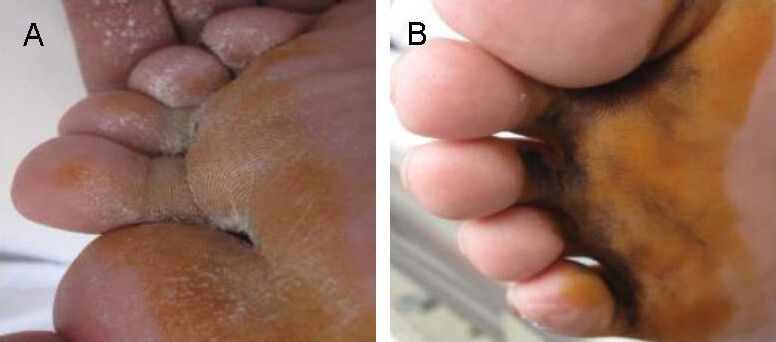
The improvement of sweating test results before and after stem cell therapy.
Images are of a male patient with traumatic spinal cord injury, 35 years old, injury time > 6 months and injury level was L1. The patient lost his sweating function before treatment, the negative result in sweating test indicated a loss in motor function, sensation and sweating function (A). At 6 months after treatment, a positive result in sweating test in the same patient indicated recovery of sweating function (B).
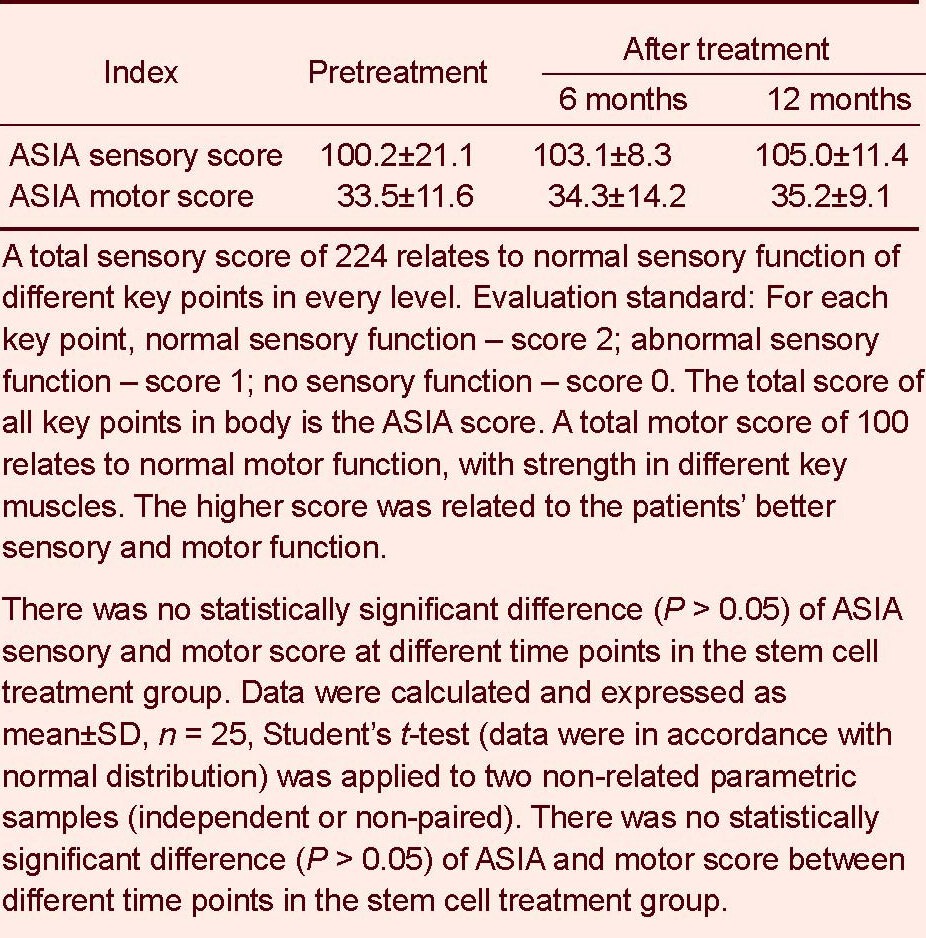
Compared with the stage before stem cell therapy, no statistically significant difference in American Spinal Cord Injury Association score was found after stem cell therapy (P > 0.05; Table 3).
Table 3.
The American Spinal Cord Injury Association (ASIA) score before and after stem cell treatment
Improvements in somatosensory evoked potential test after human umbilical cord blood stem cell transplantaion
Nine cases (36%) showed a positive response in somatosensory evoked potential (Table 2). After stem cell therapy, there were statistically significantly differences in latency time (milliseconds), which were measured by evoked potentials (p40Fcortex) of the right and left lower limbs compared with the stage before therapy (P < 0.05; Table 4, Figure 2). In those patients who showed improvement in somatosensory evoked potential, there was a mean time of 6 months between infusion and lower limb improvement.
Table 4.
Somatosensory evoked potentials measured by evoked potentials (p40Fcortex) of the right and left lower limbs after human umbilical cord blood stem cell transplantation
Figure 2.
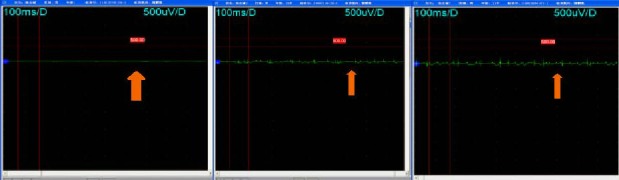
Results of somatosensory evoked potentials (SSEP) test in lower limbs at different time points before and after stem cell therapy.
The orange arrows revealed the change in shape of the SSEP wave at different time points. Distinct improvements in SSEP results was found at different time points after stem cell trerapy. Before stem cell therapy, the latency time was at a high level (left); 6 months after stem cell therapy, the latency time (s) was decreased (middle); 12 months after therapy, there was a statistically significant difference compared with both 6 and 12 months (right).
DISCUSSION
Nowadays, there are no effective therapies for spinal cord injury because of the limited spontaneous endogenous regeneration of damaged/lost oligodendrocytes in the spinal cord[19]. Potential strategies to treat spinal cord injury could be aimed at promoting remyelination via oligodendrocyte transplantation[20], controlling apoptosis, and promoting endogenous regeneration of dead cells[21]. In previous studies, human umbilical cord blood stem cells have been shown to have the following properties: they have the potential to differentiate in vitro into cells that are morphologically similar to oligodendrocytes and express oligodendrocyte markers; they secrete factors that prevent further injury; have tropism for the injured area in the spinal cord; can be effective even through remote infusion either by intravascular or intrathecal administration; and improve neurological function in animal studies[22,23,24]. Therefore, the clinical application of human umbilical cord blood stem cells to treat spinal cord injury is very appealing. They concluded that intravenous injection is a safe approach and infusion applied as close as possible to the injury site provides the best results[25,26]. However, reports on the clinical application of human umbilical cord blood stem cells, which are easier to acquire and cultivate are rare. Our study supplies some important information relating to the safety and effects of human umbilical cord blood stem cells in patients with spinal cord injury.
The study samples were homogeneous as we chose patients in the late stages of traumatic spinal cord injury, which is the most widely studied and well-established condition[27,28]. Improvements in neurological function and somatosensory evoked potential tests were observed in some patients. There was no neoplasm or aggravated neurological symptoms after human umbilical cord blood stem cell therapy. Three patients had low-grade fever shortly (< 24 hours) after infusion of stem cells, but they had no abnormities in the blood or cerebral spinal fluid. One of the patients with low-grade fever showed improvement in autonomic function, so we considered the fever was a slight immune reaction, which had no influence on the safety and therapeutic effect of the treatment. The causes and effects of fever reaction need further exploration. Eleven cases (44%) showed improved neurological function in different aspects; the majority (40%) improved in autonomic neurological function (sweating function or bladder function), nine cases (36%) improved in electrophysiology test, and four cases (16%) improved in the American Spinal Cord Injury Association score. The improvement in neurological function and cortical response to peripheral stimuli may be explained by the formation of new synapses between host neurons and neurons formed after stem cell transplantation[29], or by newly myelinated glial cells derived from the transplanted stem cells[30,31].
The results of this study are very appealing as human umbilical cord blood stem cells are easy to acquire and its safety and effects were confirmed. The subjects of the study were patients with spinal cord injury for at least 6 months, as it is well-known that neurological recovery in patients with spinal cord injury largely occurs in the first 6 months post-injury[32]. Hence, any change in the neurological status of these patients could be attributed to the therapeutic approach under investigation. This study confirmed the safety and effects of stem cell therapy in the primary trial. Human umbilical blood stem cell treatment in patients with traumatic spinal cord injury in late stages resulted in improvement of neurological status, and confirmed the safety and therapeutic effects of human umbilical blood stem cells.
MATERIALS AND METHODS
Design
A clinical retrospective study.
Time and setting
The study was accomplished from July 2010 to March 2011 in the Second Affiliated Hospital of Kunming Medical University, China.
Subjects
Twenty-five patients with spinal cord injury in the late stage (injury time > 6 months) were included as the treatment group, aged 18–48 years.
All cases had complete or incomplete traumatic cervical, thoracic and lumbar injury. Cases with primary spinal cord disease, such as myelitis, infection and tumor, were excluded.
Another 25 patients with spinal cord injury in the late stage (injury time > 6 months), aged over 18 years, who received only traditional rehabilitation therapy and no stem cell therapy were included as the control group. The inclusion and exclusion criteria were the same as treatment group.
Before receiving rehabilitation, all patients underwent spinal surgery in the orthopedics department of different hospitals. All patients had normal blood cell counts and had no tumor or coagulation disorders. There were 5 cases of cervical spinal injury, 11 cases of thoracic spinal cord injury and 9 cases of lumbar spinal cord injury. No patient had shown any neurological improvement before stem cell therapy. All patients received somatosensory evoked potentials tests[18] and other tests of neurological function before and after the stem cell therapy. We examined the sensation and muscle strength in key points of bilateral limbs, and used the American Spinal Cord Injury Association score to evaluate the sensory and motor function of patients. All patients were followed-up for 12 months after stem cell therapy. The moral principles of this study were in accordance with the Administrative Regulations of Medical Institutions formulated by the State Council of the People's Republic of China[33].
Methods
Preparation of human umbilical cord blood stem cells
Umbilical cord blood (100–150 mL) was collected from healthy unrelated donors, after obtaining signed informed consent forms in accordance with the sterile procurement guidelines for cord blood in each hospital[34]. Mononuclear cells were collected and washed twice in saline. Contaminating erythrocytes were lysed with lysis buffer (Beyotime, Shanghai, China) comprising of injection-grade water. Cell density was adjusted to 2–6 × 106/mL and seeded in DMEM/F12 culture medium with basic fibroblast growth factor and epidermal growth factor (Peprotech, Rocky Hill, NJ, USA) at a concentration of 20 ng/mL. Culture media (DMEM/F12; Gibco, New York, USA) was mixed with 2% v/v B-27 Stem Cell Culture Supplement (Gibco). Cells were cultured at 37°C with saturated humidity and 5% CO2 by volume. At this stage, all relevant information about the initial culture was entered in the batch information record, including test results for sterility, mycoplasma and endotoxins. Cell growth was regularly monitored and the inspection records were updated accordingly. Cells were harvested for clinical application after 1 week of cultivation with cell quantity ≥ 1 × 107 and viability ≥ 95%.
To ensure the quality of the umbilical cord blood-derived mononuclear cells, a number of parameters were confirmed before use. Raw material control: Tests for communicable diseases (hepatitis B virus, hepatitis C virus, human immunodeficiency virus, alanine transaminase and syphilis) for umbilical cord blood units were performed before any processing began. Testing was performed by a third party laboratory under local government-monitored conditions.
In-process control: Non-qualifying cells were eliminated in accordance with Beike's cell counting and morphology standards, which include a cell quantity of ≥ 1 × 107 and highly homogeneous cells that have a rounded shape and have detached from the culture flask.
Culture control: Any contaminated cell suspensions or unhealthy cells were eliminated upon discovery. Contamination was determined by the presence of mycoplasma or visible microorganisms by microscopy. Furthermore samples were required to have an endotoxin level ≤ 0.5 EU/mL and be negative of free DNA.
Finished product control: This incorporates a final cell count (≥ 1 × 107), containing 1.0–2.0% CD34+ cells as determined by flow cytometry (BD Bioscience Pharmingen Inc., San Diego, CA, USA), cell viability (≥ 95%) and sterility test.
Transplantation of human umbilical cord blood stem cells
Depending on the patient's condition, they were admitted to receive stem cell infusion by lumbar puncture and intravenous infusion, which was repeated four or five times. Treatments were separated by 1 week intervals. At the first time of therapy, a 30-mL intravenous infusion of cell suspension was administered through an intravenous catheter over a period of 20–30 minutes. Following this, the next three treatments were administered by lumbar puncture, which was performed in the lateral decubitus position, with the patient prepped and draped in sterile fashion, and the needle placed in the lumbar subarachnoid space. Flow of the cerebrospinal fluid into the syringe needle was evidence of the needle being in the correct place in subarachnoid space. Thus, the stem cells could be injected into the correct place successfully and accordingly exert their effects, which was the criterion of successful stem cell transplantation. 4 mL of cerebrospinal fluid was removed and replaced with 4 mL of cell suspension containing 1–3 × 107 cells. The color and pressure of the cerebrospinal fluid were observed and recorded to determine whether they were normal. During the progress, any abnormal reactions of the patients were observed. Stem cell therapy was implemented by Professor Ao, who was the item director of stem cell treatment for spinal cord injury.
Before receiving traditional rehabilitation, such as strength exercise and electrical stimulation, all patients received spinal fixation surgery by hospital orthopedic departments. Then, the patients in the treatment and control groups received their corresponding treatments between July 2010 and March 2011 in the Second Affiliated Hospital of Kunming Medical University, China. To evaluate the effect of stem cell therapy, the patients were informed to return to the hospital for functional evaluation at different time points during follow-up assessments.
Observation during 12-month follow-up after stem cell therapy
From the first day after infusion, abnormal reactions, such as fever, headache or lumbago, were recorded and the patients received rehabilitation training at an early stage if they had no abnormal reactions. The safety was evaluated by patient complication rates. The neurological functions were evaluated by American Spinal Cord Injury Association score[34], which evaluates the strength of 10 symmetrical muscle groups and different sensory levels in the body, and scores them as follows: A: complete injury; B: sensory function remains, but the motor function is lost; C: sensory and motor function remains, but more than half of the muscle groups have a strength < 3 below the injury level; D: sensory and motor function remains, but more than half of muscle groups have a strength > 3 below the injury level; E: normal function.
Other standards that were evaluated included autonomic nerve function (sweating), Ashworth scales, and somatosensory evoked potential values in limbs at different time points before and after treatment[18]. Sweating tests were performed using dry iodine and amylum. Dry iodine and dry amylum were placed on the skin of the patients’ toe; if the patient had normal sweating function, the iodine and amylum would become wet and the amylum would change color from white to blue. The somatosensory evoked potential tests were detected using an electromyogram instrument (NTS-2000; Nuo Cheng, Shanghai, China). The results of this study were conducted and evaluated by the same doctor. The positive effects of stem cell therapy were defined by improvement of American Spinal Cord Injury Association score (from A to E), improvement of autonomic nerve function (revival of sweating function), decreased spasm (Ashworth score from 5 to 0) and revival of neurological transmit function, which was indicated by a reduced response time(s) of lower limb(s) under stimulation in both paraplegic and quadriplegic patients after treatment.
Statistical analysis
Statistical analysis was performed using SPSS 17.0 statistical software (SPSS, Chicago, IL, USA). Rates of complication and effectiveness were calculated and expressed as percentage. Statistical data were expressed as mean ± SD, and intergroup comparisons were applied for the mean estimates, Student's t-test (the data was in accordance with normal distribution) was applied to two non-related parametric samples (independent or non-paired). A level of 5% was set as significant.[35]
Footnotes
Liqing Yao, M.D., Associate professor.
Conflicts of interest: None declared.
Ethical approval: This study was approved by Research Ethics Committee of the Second Affiliated Hospital of Kunming Medical University in China.
(Edited by Nan F, Nan XF/Yang Y/Song LP)
REFERENCES
- [1].Wyndaele M, Wyndaele JJ. Incidence, prevalence and epidemiology of spinal cord injury: what learns a worldwide literature survey? Spinal Cord. 2006;44(9):523–529. doi: 10.1038/sj.sc.3101893. [DOI] [PubMed] [Google Scholar]
- [2].Kwon BK, Stammers AM, Belanger LM, et al. Cerebrospinal fluid inflammatory cytokines and biomarkers of injury severity in acute human spinal cord injury. J Neurotrauma. 2010;27(4):669–682. doi: 10.1089/neu.2009.1080. [DOI] [PubMed] [Google Scholar]
- [3].Tator CH, Fehlings MG. Review of the secondary injury theory of acute spinal cord trauma with emphasis on vascular mechanisms. J Neurosurg. 1991;75(1):15–26. doi: 10.3171/jns.1991.75.1.0015. [DOI] [PubMed] [Google Scholar]
- [4].Dusart I, Schwab ME. Secondary cell death and the inflammatory reaction after dorsal hemisection of the rat spinal cord. Eur J Neurosci. 1994;6(5):712–724. doi: 10.1111/j.1460-9568.1994.tb00983.x. [DOI] [PubMed] [Google Scholar]
- [5].Bracken MB, Holford TR. Neurological and functional status 1 year after acute spinal cord injury: estimates of functional recovery in National Acute Spinal Cord Injury Study II from results modeled in National Acute Spinal Cord Injury Study III. J Neurosurg. 2002;96(3 Suppl):259–266. doi: 10.3171/spi.2002.96.3.0259. [DOI] [PubMed] [Google Scholar]
- [6].Kobylka P, Ivanyi P, Breur-Vriesendorp BS. Preservation of immunological and colony- forming capacities of long-term (15 years) cryopreserved cordblood cells. Transplantation. 1998;65(9):1275–1278. doi: 10.1097/00007890-199805150-00024. [DOI] [PubMed] [Google Scholar]
- [7].Liu CH, Hwang SM. Cytokine interactions in mesenchymal stem cells from cord blood. Cytokine. 2005;32(6):270–279. doi: 10.1016/j.cyto.2005.11.003. [DOI] [PubMed] [Google Scholar]
- [8].Newman MB, Willing AE, Manresa JJ, et al. Cytokines produced by cultured human umbilical cord blood (HUCB) cells: implications for brain repair. Exp Neurol. 2006;199(1):201–208. doi: 10.1016/j.expneurol.2006.04.001. [DOI] [PubMed] [Google Scholar]
- [9].Neuhoff S, Moers J, Rieks M, et al. Proliferation, differentiation, and cytokine secretion of human umbilical cord blood-derived mononuclear cells in vitro. Exp Hematol. 2007;35(7):1119–1131. doi: 10.1016/j.exphem.2007.03.019. [DOI] [PubMed] [Google Scholar]
- [10].Chua SJ, Bielecki R, Wong CJ, et al. Neural progenitors, neurons and oligodendrocytes from human umbilical cord blood cells in a serum-free, feeder free cell culture. Biochem Biophys Res Commun. 2009;379(2):217–221. doi: 10.1016/j.bbrc.2008.12.045. [DOI] [PubMed] [Google Scholar]
- [11].Chen N, Hudson JE, Walczak P, et al. Human umbilical cord blood progenitors: the potential of these hematopoietic cells to become neural. Stem Cells. 2005;23(10):1560–1570. doi: 10.1634/stemcells.2004-0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Lee MW, Moon YJ, Yang MS, et al. Neural differentiation of novel multipotent progenitor cells from cryopreserved human umbilical cord blood. Biochem Biophys Res Commun. 2007;358(2):637–643. doi: 10.1016/j.bbrc.2007.04.181. [DOI] [PubMed] [Google Scholar]
- [13].Ortiz-Gonzalez XR, Keene CD, Verfaillie CM, et al. Neural induction of adult bone marrow and umbilical cord stem cells. Curr Neurovasc Res. 2004;1(3):207–213. doi: 10.2174/1567202043362342. [DOI] [PubMed] [Google Scholar]
- [14].Li JY, Christophersen NS, Hall V, et al. Critical issues of clinical human embryonic stem cell therapy for brain repair. Trends Neurosci. 2008;31(3):146–153. doi: 10.1016/j.tins.2007.12.001. [DOI] [PubMed] [Google Scholar]
- [15].Sykova’ E, Jendelova’ P, Urdzı’kova’ L, et al. Bone marrow stem cells and polymer hydrogels–two strategies for spinal cord injury repair. Cell Mol Neurobiol. 2006;7-8:1113–1129. doi: 10.1007/s10571-006-9007-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Park HC, Shim YS, Ha Y, et al. Treatment of complete spinal cord injury patients by autologous bone marrow cell transplantation and administration of granulocyte-macrophage colony stimulating factor. Tissue Eng. 2005;11(5-6):913–922. doi: 10.1089/ten.2005.11.913. [DOI] [PubMed] [Google Scholar]
- [17].Tator CH. Review of treatment trials in human spinal cord injury: issues, difficulties, and recommendations. Neurosurgery. 2006;59(5):957–982. doi: 10.1227/01.NEU.0000245591.16087.89. [DOI] [PubMed] [Google Scholar]
- [18].Stokic DS, Curt A. Stem cells in the treatment of chronic spinal cord injury: evaluation of somatosensitive evoked potentials in 39 patients. Spinal Cord. 2010;48(8):649. doi: 10.1038/sc.2009.187. [DOI] [PubMed] [Google Scholar]
- [19].McDonald JW, Belegu V. Demyelination and remyelination after spinal cord injury. Neurotrauma. 2006;23(3-4):345–359. doi: 10.1089/neu.2006.23.345. [DOI] [PubMed] [Google Scholar]
- [20].McDonald JW. Repairing the damaged spinal cord: from stem cells to activity-based restoration therapies. Clin Neurosurg. 2004;51:207–227. [PubMed] [Google Scholar]
- [21].Becker D, Sadowsky CL, McDonald JW. Restoring function after spinal cord injury. Neurologist. 2003;9(1):1–15. doi: 10.1097/01.nrl.0000038587.58012.05. [DOI] [PubMed] [Google Scholar]
- [22].Chua SJ, Bielecki R, Wong CJ, et al. Neural progenitors, neurons and oligodendrocytes from human umbilical cord blood cells in a serum-free, feederfree cell culture. Biochem Biophys Res Commun. 2009;379(2):217–221. doi: 10.1016/j.bbrc.2008.12.045. [DOI] [PubMed] [Google Scholar]
- [23].Chua SJ, Bielecki R, Yamanaka N, et al. The effect of umbilical cord blood cells on outcomes after experimental traumatic spinal cord injury. Spine. 2010;35(16):1520–1526. doi: 10.1097/BRS.0b013e3181c3e963. [DOI] [PubMed] [Google Scholar]
- [24].Veeravalli KK, Dasari VR, Tsung AJ, et al. Human umbilical cord blood stem cells upregulate matrix metalloproteinase-2 in rats after spinal cord injury. Neurobiol Dis. 2009;36(1):200–212. doi: 10.1016/j.nbd.2009.07.012. [DOI] [PubMed] [Google Scholar]
- [25].Sykova’ E, Jendelova’ P, Urdzı’kova’ L, et al. Bone marrow stem cells and polymer hydrogels–two strategies for spinal cord injury repair. Cell Mol Neurobiol. 2006;26(7-8):1113–1129. doi: 10.1007/s10571-006-9007-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Park HC, Shim YS, Ha Y, et al. Treatment of complete spinal cord injury patients by autologous bone marrow cell transplantation and administration of granulocyte-macrophage colony stimulating factor. Tissue Eng. 2005;11(5-6):913–922. doi: 10.1089/ten.2005.11.913. [DOI] [PubMed] [Google Scholar]
- [27].Burns AS, Lee BS, Ditunno JF, Jr, et al. Patient selection for clinical trials: the reliability of the early spinal cord injury examination. J Neurotrauma. 2003;20(5):477–482. doi: 10.1089/089771503765355540. [DOI] [PubMed] [Google Scholar]
- [28].Marino RJ, Barros T, Biering-Sorensen F, et al. International standards for neurological classification of spinal cord injury. J Spinal Cord Med. 2003;26(Suppl 1):S50–56. doi: 10.1080/10790268.2003.11754575. [DOI] [PubMed] [Google Scholar]
- [29].Ogawa Y, Sawamoto K, Miyata T, et al. Transplantation of in vitro-expanded fetal neural progenitor cells results in neurogenesis and functional recovery after spinal cord contusion injury in adult rats. J Neurosci Res. 2002;69(6):925–933. doi: 10.1002/jnr.10341. [DOI] [PubMed] [Google Scholar]
- [30].Sasaki M, Honmou O, Akiyama Y, et al. Transplantation of an acutely isolated bone marrow fraction repairs demyelinated adult rat spinal cord axons. Glia. 2001;35(1):26–34. doi: 10.1002/glia.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Liu S, Qu Y, Stewart TJ, et al. Embryonic stem cells differentiate into oligodendrocytes and myelinate in culture and after spinal cord transplantation. Proc Natl Acad Sci U S A. 2000;97(11):6126–6131. doi: 10.1073/pnas.97.11.6126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Burns AS, Ditunno JF. Establishing prognosis and maximizing functional outcomes after spinal cord injury: a review of current and future directions in rehabilitation management. Spine. 2001;26(24 Suppl):S137–145. doi: 10.1097/00007632-200112151-00023. [DOI] [PubMed] [Google Scholar]
- [33].State Council of the People's Republic of China. Administrative Regulations on Medical Institution. 1994-09-01 [Google Scholar]
- [34].Yang WZ, Zhang Y, Wu F, et al. Safety evaluation of allogeneic umbilical cord blood mononuclear cell therapy for degenerative conditions. J Transl Med. 2010;8:75–80. doi: 10.1186/1479-5876-8-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Kirshblum SC, Memmo P, Kim N, et al. Comparision of the revised 2000 American Spinal Injury Association classification standards with the 1966 guidelines. Am J Phys Med Rehabili. 2002;81(7):502–505. doi: 10.1097/00002060-200207000-00006. [DOI] [PubMed] [Google Scholar]


