Abstract
Transtentorial herniation is one of the causes of motor weakness in traumatic brain injury. In this study, we report on a patient who underwent decompressive craniectomy due to traumatic intracerebral hemorrhage. Brain CT images taken after surgery showed intracerebral hemorrhage in the left fronto-temporal lobe and left transtentorial herniation. The patient presented with severe paralysis of the right extremities at the time of intracerebral hemorrhage onset, but the limb motor function recovered partially at 6 months after onset and to nearly normal level at 27 months. Through diffusion tensor tractography, the left corticospinal tract was disrupted below the cerebral peduncle at 1 month after onset and the disrupted left corticospinal tract was reconstructed at 27 months. These findings suggest that recovery of limb motor function in a patient with traumatic transtentorial herniation can come to be true by recovery of corticospinal tract.
Keywords: neural regeneration, neuroimaging, diffusion tensor imaging, diffusion tensor tractography, transcranial magnetic stimulation, traumatic brain injury, intracerebral hemorrhage, transtentorial herniation, corticospinal tract, motor paralysis, neuroimaing, grants-supported paper, photographs-containing paper, neuroregeneration
Research Highlights
(1) A male patient revealed left transtentorial herniation following traumatic intracerebral hemorrhage in the left fronto-temporal lobe by diffusion tensor tractography.
(2) The patient presented with severe paralysis of the right extremities at the time of intracerebral hemorrhage onset, however, the paralysis had recovered to a nearly normal state at 27 months after onset.
(3) Through diffusion tensor tractography, the left corticospinal tract was disrupted below the cerebral peduncle at 1 month after onset and the disrupted left corticospinal tract was reconstructed at 27 months after onset.
(4) Recovery of limb motor function in a patient with traumatic transtentorial herniation can come to be true by recovery of corticospinal tract.
INTRODUCTION
Traumatic brain injury is one of the most common neurologic disorders causing disability[1,2]. Previous studies have reported the incidence of motor weakness following traumatic brain injury as 9–56%[1,3,4]. Transtentorial herniation is one of the causes of motor weakness in traumatic brain injury, along with diffuse axonal injury, deep cerebral hemorrhage, focal cortical contusion, and hypoxic-ischemic injury[1,5]. Transtentorial herniation can bring about corticospinal tract injury by compression of the cerebral peduncle, which was caused by downward displacement of medial brain structures out of the cranium through the tentorial notch following head trauma[1,6,7]. Conventional brain MRI cannot visualize or estimate a neural tract at the cerebral peduncle; therefore, it is difficult to demonstrate the recovery of the corticospinal tract injured by transtentorial herniation.
By contrast, diffusion tensor imaging allows for visualization and estimation of the corticospinal tract in three dimensions[8,9,10]. Some recent diffusion tensor imaging studies have reported on the usefulness of diffusion tensor imaging in diagnosis of transtentorial herniation in patients with traumatic brain injury; however, little is known about recovery of the corticospinal tract injured by transtentorial herniation[5,11,12].
In this study, we present with a patient who showed recovery of a corticospinal tract injured by transtentorial herniation following head trauma, using diffusion tensor imaging.
CASE REPORT
A 39-year-old, right-handed male patient underwent decompressive craniectomy due to traumatic intracerebral hemorrhage, which occurred after falling from a height of 3 meters. Brain CT images, which were taken after surgery, showed intracerebral hemorrhage in the left fronto-temporal lobe and left transtentorial herniation (Figure 1A). Brain MRI (1 and 27 months after intracerebral hemorrhage onset) revealed shrinkage in the left cerebral peduncle (Figure 1A).
Figure 1.
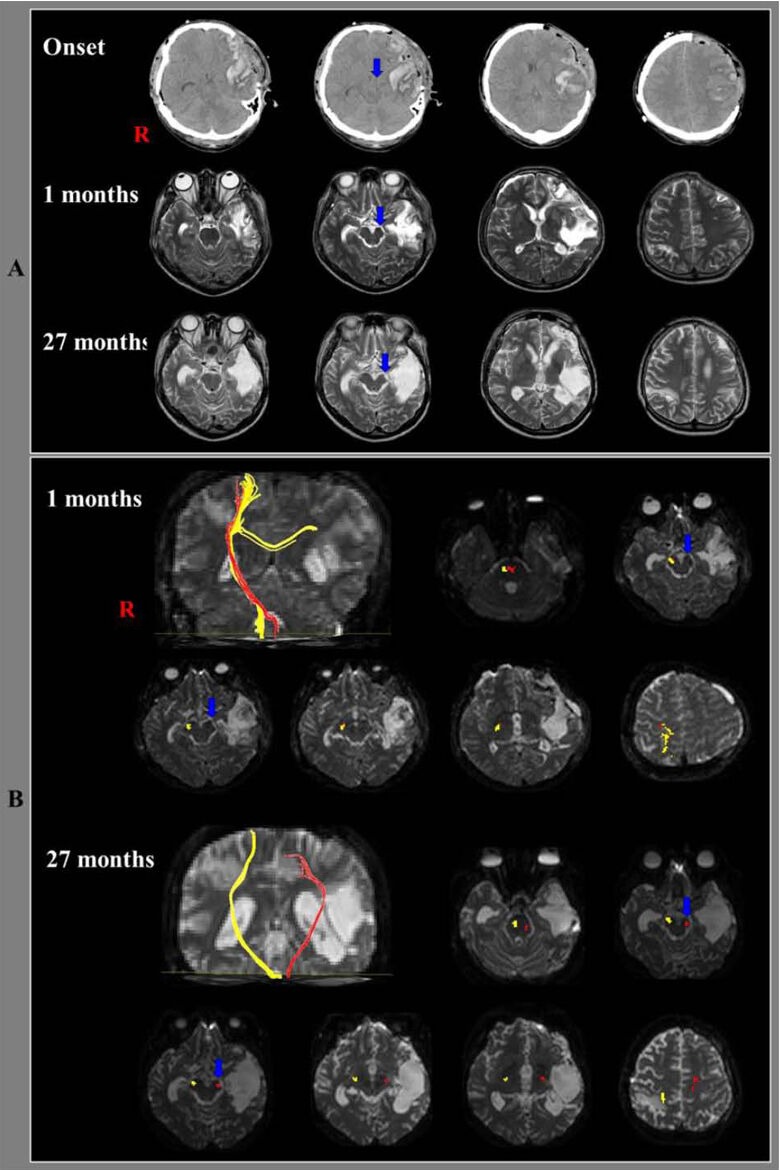
Brain CT, MRI images and diffusion tensor tractography results of the corticospinal tract in the included patient.
(A) Brain CT images after surgery show intracerebral hemorrhage in the left fronto-temporal lobes and left transtentorial herniation (arrow). Brain MRI images (1 and 27 months after onset) reveal shrinkage of the left cerebral peduncle (arrow). R: Right.
(B) Results of diffusion tensor tractography. The first (1 month after head trauma) and second (27 months after onset) diffusion tensor tractography for the corticospinal tracts (yellow) in the right hemispheres showed that fiber tracts passed along the known corticospinal tract pathway. On the first diffusion tensor tractography of the affected (left) hemisphere, the corticospinal tract (red) was disrupted below the cerebral peduncle (blue arrow) and connected to the right hemisphere via transpontine fibers. The transpontine connection fibers (red) in the right hemisphere may be related to compensatory mechanism after motor weakness or corticospinal tract injury. However, the left corticospinal tract (red) originated from the left primary motor cortex and descended through the left cerebral peduncle (blue arrow) on the second diffusion tensor tractography. R: Right.
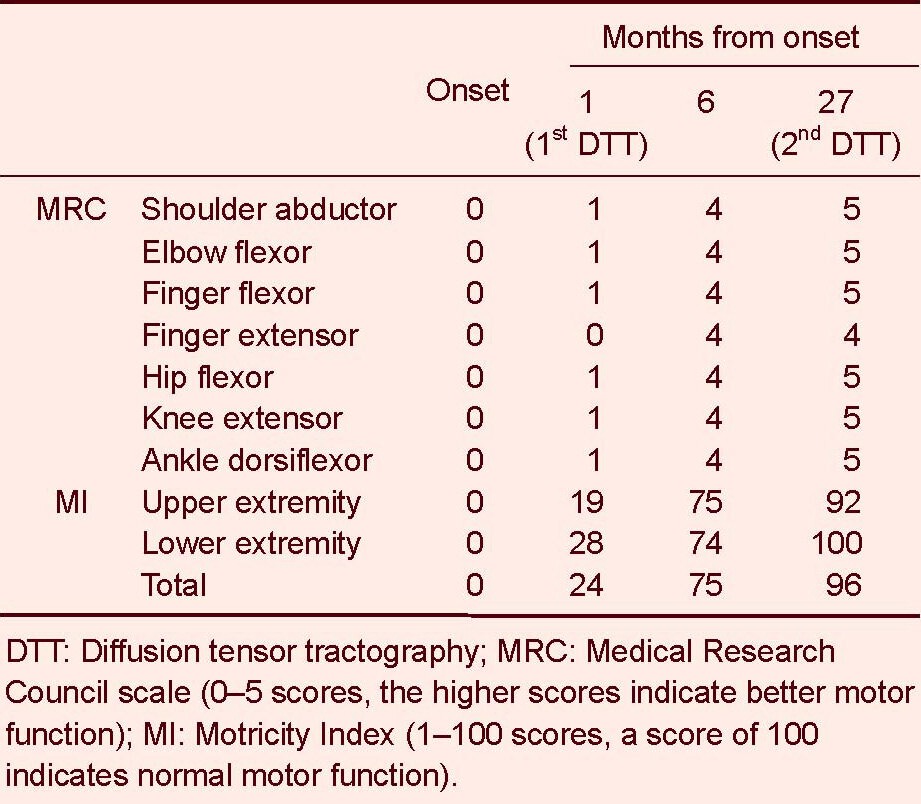
The standardized Motricity Index and Medical Research Council scale were used for determination of motor function at the time of intracerebral hemorrhage onset and 1, 6 and 27 months after onset. The Motricity Index is a measure of the integrity of extremity motor function, with a maximum score of 100. Morticity Index has two different evaluation scores between hand and other joints (shoulder, elbow, hip, knee and ankle); hand prehension: 0 (non-movement), 33 (beginning of prehension), 56 (grips cube without gravity), 65 (holds cube against gravity), 77 (grips against pull, but weaker than other side), and 100 (normal); other joints: 0 (non-movement), 28 (palpable contraction), 42 (movement without gravity), 56 (movement against gravity), 74 (movement against resistance, but weaker than normal), and 100 (normal). In addition, six categories are included in the Medical Research Council scale: 0, no contraction; 1, palpable contraction, but no visible movement; 2, movement without gravity; 3, movement against gravity; 4, movement against a resistance lower than the resistance overcome by the healthy side; 5, movement against a resistance equal to the maximum resistance overcome by the healthy side. Reliability and validity of the Motricity Index are well-established[13]. The patient presented with severe paralysis of the right extremities at the time of intracerebral hemorrhage onset (Motricity Index, onset: 0 point, 1 month after onset (first diffusion tensor imaging); 24 points), but slowly recovered some functions, to the point of being able to move the affected extremities against some resistance at about 6 months after onset (Motricity Index: 75 points) (Table 1). At 27 months after onset (second diffusion tensor imaging), the motor function of the right extremities in the patient had recovered to a nearly normal state (Motricity Index: 96 points).
Table 1.
Changes of motor function in the included patient
Diffusion tensor tractography
Diffusion tensor imagings were obtained twice (1 month and 27 months after onset) using a multi-channel head coil on a 1.5-T Philips Gyroscan Intera (Philips, Best, the Netherlands) with single-shot echo-planar imaging. Imaging was performed using a 6-channel head coil. For each of the 32 non-collinear diffusion-sensitizing gradients, we acquired 67 contiguous slices parallel to the anterior commissure-posterior commissure line. Imaging parameters were as follows: acquisition matrix = 96 × 96, reconstruction matrix = 128 × 128 matrix, field of view = 221 × 221 mm2, repetition time/echo time = 10 726/76 ms, parallel imaging reduction factor (SENSE factor) = 2, echo planar imaging factor = 49 and b = 1 000 s/mm2, number of excitations = 1, and a slice thickness of 2.3 mm (acquired isotropic voxel size 2.3 × 2.3 × 2.3 mm3). Eddy current-induced image distortions and motion artifacts were removed using affine multi-scale two-dimensional registration[14]. Preprocessing of diffusion tensor imaging datasets was performed using the Oxford Centre for Functional Magnetic Resonance Imaging of Brain Software Library. We evaluated the corticospinal tract using diffusion tensor imaging -Studio software (CMRM, Johns Hopkins Medical Institute, USA). For analysis of the corticospinal tract, the region of interest was placed on the corticospinal tract portion of the pons (anterior blue portion on the color map). Fiber tracking was performed with an FA threshold of > 0.2 and direction threshold < 60°[10].
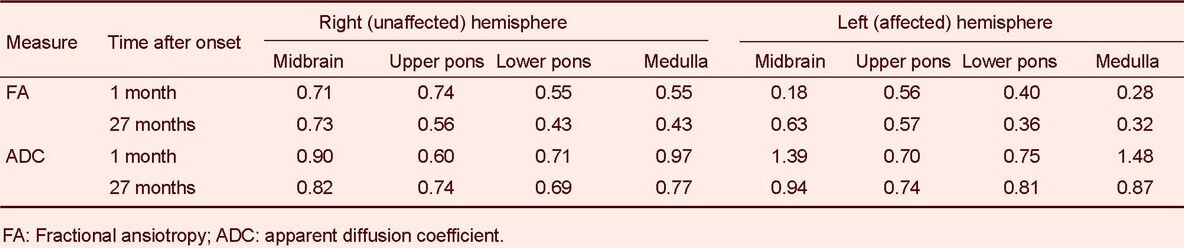
Diffusion tensor tractography for the corticospinal tracts in the right hemispheres showed that fiber tracts originated from the primary motor cortex, and passed along the known corticospinal tract pathway (Figure 1B). Through the diffusion tensor tractography, the corticospinal tracts on the affected (left) hemisphere were disrupted below the cerebral peduncle and crossed to the right hemisphere via the transpontine fibers at 1 month after onset, and they originated from the left primary motor cortex and descended through the left cerebral peduncle at 27 months after onset. Diffusion tensor imaging parameters of regions of interest on the corticospinal tract pathway are summarized in Table 2. Through the diffusion tensor imaging, the fractional anisotropy value (0.18) of the corticospinal tract area in the left cerebral peduncle was lower than that (0.71) of the right hemisphere at 1 month after onset and it increased to 0.63 at 27 months after onset. By contrast, the apparent diffusion coefficient value of that area increased to 1.39 at 1 month after onset and it decreased to 0.94 at 27 months after onset.
Table 2.
Diffusion tensor image parameters in regions of interest on the corticospinal tract pathway
DISCUSSION
In this study, we demonstrated recovery of a corticospinal tract injured by transtentorial herniation in a patient with traumatic brain injury by diffusion tensor tractography and diffusion tensor imaging findings as well as clinical symptoms. Through the diffusion tensor tractography, the left corticospinal tract was disrupted below the cerebral peduncle and connected to the right hemisphere via transpontine fibers at 1 month after onset[15] and the disrupted left corticospinal tract was restored at 27 months after onset. These diffusion tensor tractography findings are consistent with diffusion tensor imaging findings: fractional anisotropy and apparent diffusion coefficient values of the corticospinal tract area in the cerebral peduncle were decreased and increased, respectively, compared with those of the unaffected side. However, fractional anisotropy value increased and apparent diffusion coefficient value decreased at 27 months after onset.
Fractional anisotropy value represents the degree of directionality of microstructures (e.g., axons, myelin, and microtubules)[9,16,17]. Apparent diffusion coefficient value indicates the magnitude of water diffusion in tissue, which can increase with some forms of pathology, particularly vasogenic edema or accumulation of cellular debris from neuronal damage[9,16,17]. Therefore, the decrease of fractional anisotropy value with the increase of apparent diffusion coefficient value on 1-month diffusion tensor imaging suggests neural injury; in contrast, the increase of fractional anisotropy value with the decrease of apparent diffusion coefficient value indicates recovery of neural injury. The patient presented with complete weakness of the right extremities at the onset of traumatic brain injury and showed slow motor recovery, to the point of being able to move the affected extremities against some resistance at 6 months after onset, which demonstrates the nearly normal motor function at 27 months after onset. This recovery course suggests that recovery of the motor function of the affected side progresses through brain plasticity[17,18,19].
Several diffusion tensor imaging studies on a corticospinal tract injured by transtentorial herniation in patients with stroke or traumatic brain injury have been reported[5,11,12,20]. However, to the best of our knowledge, only one study on recovery of an injured corticospinal tract by transtentorial herniation has been reported[21]. Using diffusion tensor imaging and transcranial magnetic stimulation, Kwon et al[21] reported on a patient with spontaneous intracerebral hemorrhage who showed recovery of the corticospinal tract after injury by brain CT and MRI-confirmed transtentorial herniation. Their results showed that through the diffusion tensor tractography, the left corticospinal tract was disrupted below the cerebral peduncle at 3 weeks after onset, however, the disruption had recovered, with motor recovery, at 1 year after onset; through the transcranial magnetic stimulation, there was no motor-evoked potential for the affected hemisphere at 3 weeks after onset, and motor-evoked potentials compatible with a regenerated corticospinal tract were obtained from the muscle of the affected hand at 6 months after onset.
In conclusion, we demonstrated recovery of a corticospinal tract injured by transtentorial herniation in a patient with head trauma. This result has important implications for brain rehabilitation in terms of recovery of injured neural tracts following traumatic transtentorial herniation. With regard to the motor recovery mechanism in patients with traumatic brain injury, a few diffusion tensor imaging studies have reported[22,23,24]. These studies have focused on the recovery following diffuse axonal injury or traumatic hemorrhage. Therefore, this is the first diffusion tensor imaging study to demonstrate recovery of a corticospinal tract after injury by traumatic transtentorial herniation. However, because it is a case report, this study is limited. Further complementary studies involving larger case numbers are warranted.
Footnotes
Sang Seok Yeo, M.S., PT.
Funding: This study was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology, No. 2012R1A1A4A01001873.
Conflicts of interest: None declared.
(Edited by Song LP)
REFERENCES
- [1].Katz DI, Alexander MP, Klein RB. Recovery of arm function in patients with paresis after traumatic brain injury. Arch Phys Med Rehabil. 1998;79:488–493. doi: 10.1016/s0003-9993(98)90060-0. [DOI] [PubMed] [Google Scholar]
- [2].Hillier SL, Sharpe MH, Metzer J. Outcomes 5 years post-traumatic brain injury (with further reference to neurophysical impairment and disability) Brain Inj. 1997;11:661–675. doi: 10.1080/026990597123214. [DOI] [PubMed] [Google Scholar]
- [3].Walker WC, Pickett TC. Motor impairment after severe traumatic brain injury: A longitudinal multicenter study. J Rehabil Res Dev. 2007;44:975–982. doi: 10.1682/jrrd.2006.12.0158. [DOI] [PubMed] [Google Scholar]
- [4].Brown AW, Malec JF, Diehl NN, et al. Impairment at rehabilitation admission and 1 year after moderate-to-severe traumatic brain injury: A prospective multi-centre analysis. Brain Inj. 2007;21:673–680. doi: 10.1080/02699050701468925. [DOI] [PubMed] [Google Scholar]
- [5].Choi GS, Kim OL, Kim SH, et al. Classification of cause of motor weakness in traumatic brain injury using diffusion tensor imaging. Arch Neurol. 2012;69:363–367. doi: 10.1001/archneurol.2011.1930. [DOI] [PubMed] [Google Scholar]
- [6].Andrews BT, Pitts LH. Functional recovery after traumatic transtentorial herniation. Neurosurgery. 1991;29:227–231. doi: 10.1097/00006123-199108000-00010. [DOI] [PubMed] [Google Scholar]
- [7].Skoglund TS, Nellgard B. Long-time outcome after transient transtentorial herniation in patients with traumatic brain injury. Acta Anaesthesiol Scand. 2005;49:337–340. doi: 10.1111/j.1399-6576.2005.00624.x. [DOI] [PubMed] [Google Scholar]
- [8].Cho SH, Kim SH, Choi BY, et al. Motor outcome according to diffusion tensor tractography findings in the early stage of intracerebral hemorrhage. Neurosci Lett. 2007;421:142–146. doi: 10.1016/j.neulet.2007.04.052. [DOI] [PubMed] [Google Scholar]
- [9].Mori S, Crain BJ, Chacko VP, et al. Three-dimensional tracking of axonal projections in the brain by magnetic resonance imaging. Ann Neurol. 1999;45:265–269. doi: 10.1002/1531-8249(199902)45:2<265::aid-ana21>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- [10].Kunimatsu A, Aoki S, Masutani Y, et al. The optimal trackability threshold of fractional anisotropy for diffusion tensor tractography of the corticospinal tract. Magn Reson Med Sci. 2004;3:11–17. doi: 10.2463/mrms.3.11. [DOI] [PubMed] [Google Scholar]
- [11].Cho HK, Hong JH, Kim SH, et al. Clinical usefulness of diffusion tensor imaging in patients with transtentorial herniation following traumatic brain injury. Brain Inj. 2011;25(10):1005–9. doi: 10.3109/02699052.2011.605095. [DOI] [PubMed] [Google Scholar]
- [12].Hong JH, Kim SH, Kim OL, et al. Neural tract injuries by brain herniations after head trauma. J Head Trauma Rehabil. 2012;27:154–158. doi: 10.1097/HTR.0b013e31820fc480. [DOI] [PubMed] [Google Scholar]
- [13].Demeurisse G, Demol O, Robaye E. Motor evaluation in vascular hemiplegia. Eur Neurol. 1980;19:382–389. doi: 10.1159/000115178. [DOI] [PubMed] [Google Scholar]
- [14].Smith SM, Jenkinson M, Woolrich MW, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl 1):S208–219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- [15].Yeo SS, Choi BY, Chang CH, et al. Transpontine connection fibers between corticospinal tracts in hemiparetic patients with intracerebral hemorrhage. Eur Neurol. 2010;63:154–158. doi: 10.1159/000281900. [DOI] [PubMed] [Google Scholar]
- [16].Neil JJ. Diffusion imaging concepts for clinicians. J Magn Reson Imaging. 2008;27:1–7. doi: 10.1002/jmri.21087. [DOI] [PubMed] [Google Scholar]
- [17].Assaf Y, Pasternak O. Diffusion tensor imaging (DTI)-based white matter mapping in brain research: A review. J Mol Neurosci. 2008;34:51–61. doi: 10.1007/s12031-007-0029-0. [DOI] [PubMed] [Google Scholar]
- [18].Furlan M, Marchal G, Viader F, et al. Spontaneous neurological recovery after stroke and the fate of the ischemic penumbra. Ann Neurol. 1996;40:216–226. doi: 10.1002/ana.410400213. [DOI] [PubMed] [Google Scholar]
- [19].Seitz RJ, Donnan GA. Role of neuroimaging in promoting long-term recovery from ischemic stroke. J Magn Reson Imaging. 2010;32:756–772. doi: 10.1002/jmri.22315. [DOI] [PubMed] [Google Scholar]
- [20].Yoo WK, Kim DS, Kwon YH, et al. Kernohan's notch phenomenon demonstrated by diffusion tensor imaging and transcranial magnetic stimulation. J Neurol Neurosurg Psychiatry. 2008;79:1295–1297. doi: 10.1136/jnnp.2007.138131. [DOI] [PubMed] [Google Scholar]
- [21].Kwon HG, Ahn SH, Chang CH, et al. Recovery of the corticospinal tract after injury by transtentorial herniation: A case report. NeuroRehabilitation. 2011;29:243–246. doi: 10.3233/NRE-2011-0699. [DOI] [PubMed] [Google Scholar]
- [22].Han BS, Kim SH, Kim OL, et al. Recovery of corticospinal tract with diffuse axonal injury: a diffusion tensor image study. NeuroRehabilitation. 2007;22:151–155. [PubMed] [Google Scholar]
- [23].Kim DG, Kim SH, Kim OL, et al. Long-term recovery of motor function in a quadriplegic patient with diffuse axonal injury and traumatic hemorrhage: a case report. NeuroRehabilitation. 2009;25:117–122. doi: 10.3233/NRE-2009-0506. [DOI] [PubMed] [Google Scholar]
- [24].Skoglund TS, Nilsson D, Ljungberg M, et al. Long-term follow-up of a patient with traumatic brain injury using diffusion tensor imaging. Acta Radiol. 2008;49:98–100. doi: 10.1080/02841850701561372. [DOI] [PubMed] [Google Scholar]


