Abstract
Rat bone marrow-derived mesenchymal stem cells were cultured and passaged in vitro. After induction with basic fibroblast growth factor for 24 hours, passage 3 bone marrow-derived mesenchymal stem cells were additionally induced into dopaminergic neurons using three different combinations with basic fibroblast growth factor as follows: 20% Xiangdan injection; all-trans retinoic acid + glial-derived neurotrophic factor; or sonic hedgehog + fibroblast growth factor 8. Results suggest that the bone marrow-derived mesenchymal stem cells showed typical neuronal morphological characteristics after induction. In particular, after treatment with sonic hedgehog + fibroblast growth factor 8, the expressions of nestin, neuron-specific enolase, microtubuleassociated protein 2, tyrosine hydroxylase and vesicular monoamine transporter-2 in cells were significantly increased. Moreover, the levels of catecholamines in the culture supernatant were significantly increased. These findings indicate that Xiangdan injection, all-trans retinoic acid + glial-derived neurotrophic factor, and sonic hedgehog + fibroblast growth factor 8 can all induce dopaminergic neuronal differentiation from bone marrow-derived mesenchymal stem cells. In particular, the efficiency of sonic hedgehog + fibroblast growth factor 8 was highest.
Keywords: neural regeneration, stem cells, mesenchymal stem cells, dopaminergic neuron, induction, differentiation, Xiangdan injection, all-trans retinoic acid, sonic hedgehog, fibroblast growth factor 8, catecholamine, grants-supported paper, photographs-containing paper, neuroregeneration
Research Highlights
(1) This study compared the efficiency of the induction of bone marrow-derived mesenchymal stem cells to differentiate into dopaminergic neurons using injection of the traditional Chinese medicine, Xiangdan, and a combination of inducing agents (all-trans retinoic acid + glial-derived neurotrophic factor) and cytokines (sonic hedgehog + fibroblast growth factor 8).
(2) Based on the expression of tyrosine hydroxylase and the levels of catecholamines in the induced cells, all three combinations examined could induce dopaminergic neuronal differentiation from bone marrow-derived mesenchymal stem cells, including the Xiangdan injection.
(3) The combination of sonic hedgehog + fibroblast growth factor 8 had the highest induction efficiency.
INTRODUCTION
Parkinson's disease is caused by the selective degeneration of mesencephalic dopaminergic neurons in the substantia nigra. Although advances have been achieved in the differentiation of dopaminergic neurons from embryonic stem cells in vitro, very limited success has been achieved in animal models[1], as teratoma formation, immune rejection and ethical controversy remain great concerns. Furthermore, adult neural stem/progenitor cells cannot be obtained easily and it is dangerous to harvest neural stem cells from the living body.
Mesenchymal stem cells have the capacity to differentiate into mesoderm-type cells, such as osteoblasts, chondrocytes, myocytes and adipocytes[2,3,4]. Several studies have demonstrated the ability of mesenchymal stem cells to transdifferentiate into functional cells of the nervous system[5,6]. The multipotential properties of these cells, their easy isolation and culture, absence of tumorigenic potential, as well as their high ex vivo expansive potential make them an attractive therapeutic tool[7,8]. Numerous studies have examined the differentiation of mesenchymal stem cells into dopaminergic neurons[9,10,11,12], but the majority have focused on their morphology and expression of biomarkers[13,14]. More specifically, few studies have concentrated on the secretory function of dopaminergic neurons derived from mesenchymal stem cells. Among the inducers, basic fibroblast growth factor promotes neurogenesis and enhances the differentiation and survival of dopaminergic neurons; Xiangdan injection induces mesenchymal stem cell differentiation into neuron-like cells by reducing the generation of lipid peroxides in the cells; all-trans retinoic acid can induce changes in the expression of nervous system-related genes; and glial cell line-derived neurotrophic factor can protect and repair developing and mature dopaminergic neurons. The development of dopaminergic neurons in the embryo is dependent on the interaction of two growth factors, sonic hedgehog and fibroblast growth factor 8. A cocktail containing sonic hedgehog, fibroblast growth factor 8 and basic fibroblast growth factor has been used to induce human mesenchymal stem cells to differentiate into dopaminergic neurons in vitro[9,11]. The present study harvested rat bone marrow-derived mesenchymal stem cells, and induced them to differentiate into dopaminergic neurons using different induction cocktails. The expression and changes of neural proteins, as well as the secreted molecules, such as catecholamines, of the induced bone marrow-derived mesenchymal stem cells were examined to determine an optimal method for induction and provide a theoretical evidence and experimental basis for bone marrow-derived mesenchymal stem cell transplantation for the treatment of Parkinson's disease.
RESULTS
Morphology of rat bone marrow-derived mesenchymal stem cells
Bone marrow-derived mesenchymal stem cells were isolated easily by their adherence to the plastic surface of tissue culture flasks. After plating for 24 hours, some adherent bone marrow-derived mesenchymal stem cells appeared on the flask surface, and the cells were heterogeneous in appearance. At 7 days, the adherent cells grew constantly and gathered to form obvious cell colonies. After 10–14 days, these cells developed into many clusters that spread out across the flask surface and could be used for subculture.
The subcultured cells adhered and grew quickly. They were triangular or fusiform in shape, and the speed of cell division was faster than that of the primary cultures. Passage 3 bone marrow-derived mesenchymal stem cells were arranged in parallel or whorl-like patterns (Figure 1A).
Figure 1.
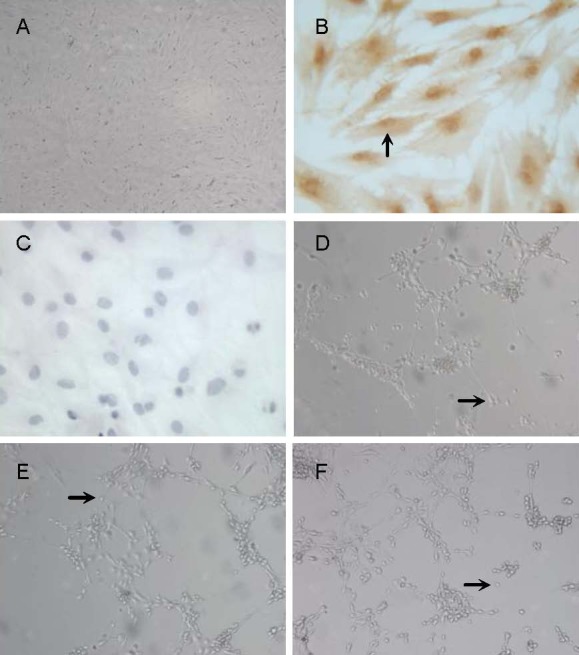
Morphology of neuron-like cells derived from rat bone marrow mesenchymal stem cells.
(A, D–F) Inverted phase-contrast microscope, × 100; (B, C) immunocytochemical staining, × 400.
(A) Passage 3 bone marrow mesenchymal stem cells.
(B) Bone marrow mesenchymal stem cells were positive for CD44 (arrow, cytoplasmic staining was brown-yellow).
(C) Bone marrow mesenchymal stem cells were negative for CD34.
(D) Neuron-like cells (arrow) derived from rat bone marrow mesenchymal stem cell induced by basic fibroblast growth factor 8 and Xiangdan injection for 3 hours.
(E) Neuron-like cells (arrow) derived from rat bone marrow mesenchymal stem cells induced by basic fibroblast growth factor 8, all-trans retinoic acid and glial cell line-derived neurotrophic factor for 6 days.
(F) Neuron-like cells (arrow) derived from rat bone marrow mesenchymal stem cells induced by basic fibroblast growth factor, sonic hedgehog and fibroblast growth factor 8 for 12 days.
Isolated bone marrow-derived mesenchymal stem cells were approximately 100% positive for known mesenchymal stem cell markers, cell surface glycoproteins CD44, but negative for CD34[15,16], indicating no contamination with hematopoietic stem cells. CD44 protein was distributed in the cytoplasm of the cell body and processes of bone marrow-derived mesenchymal stem cells (Figures 1B, C).
Differentiation of bone marrow-derived mesenchymal stem cells into dopaminergic neurons cultured with different induction cocktails
Passage 3 bone marrow-derived mesenchymal stem cells with active growth were induced into dopaminergic neurons using different cocktails. The cells of the three induction groups did not change significantly after induction by basic fibroblast growth factor. The morphology of a few cells changed after induction with Xiangdan injection (containing Danshen Root) for 0.5 hour, where most cell bodies contracted into cone or spherical shapes and became refractive after induction for 3 hours. These cells were also accompanied by bipolar and multipolar cells (Figure 1D), as well as a gradual increase in cells detaching into suspension. After 2 days of induction with all-trans retinoic acid, cells were slender, with circular cell bodies. Cell processes continued to lengthen and formed an interconnected network after induction for 6 days (Figure 1E). Up to 6 days, a few cells died but most were still alive. In the sonic hedgehog + fibroblast growth factor 8 group, neuronal induction did not occur immediately. At 6 days, the only changes observed were slight changes in cell shape; however, their morphologies were radial in appearance. At 12 days, the cell bodies of the induced bone marrow-derived mesenchymal stem cells became strongly refractive with long thin processes, and showed typical neuronal morphological characteristics (Figure 1F). The shapes of cells in the control group remained unchanged.
Expression of neural proteins of induced cells
The induced cells expressed some neuronal proteins, including nestin, neuron-specific enolase, microtubule-associated protein 2, tyrosine hydroxylase and vesicular monoamine transporter-2, in different degrees, but did not express glial fibrillary acid protein (Figure 2, supplementary Figures 1, 2 online).
Figure 2.
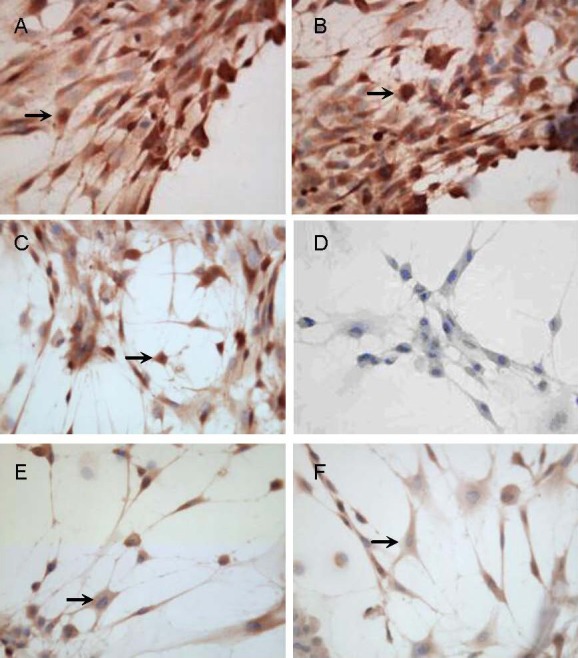
Expression of neural proteins in cells induced by sonic hedgehog and fibroblast growth factor 8 for 12 days (immunocytochemical staining, × 400).
Cells were positive for nestin (A), neuron-specific enolase (B), microtubule-associated protein 2 (C), tyrosine hydroxylase (E) and vesicular monoamine transporter-2 (F), but negative for glial fibrillary acid protein (D) after induction with sonic hedgehog + fibroblast growth factor 8 for 12 days. Arrows represent positive cells.
Compared with the control, the percentage of positive cells was significantly higher in three induction groups (P < 0.05). In particular, the expressions of nestin, neuron-specific enolase, microtubule-associated protein 2, tyrosine hydroxylase and vesicular monoamine transporter-2 were the highest in cells induced with sonic hedgehog + fibroblast growth factor 8. There were also differences between the Xiangdan injection and all-trans retinoic acid + glial cell line-derived neurotrophic factor groups (P < 0.05; Figure 3, supplementary Table 1 online).
Figure 3.
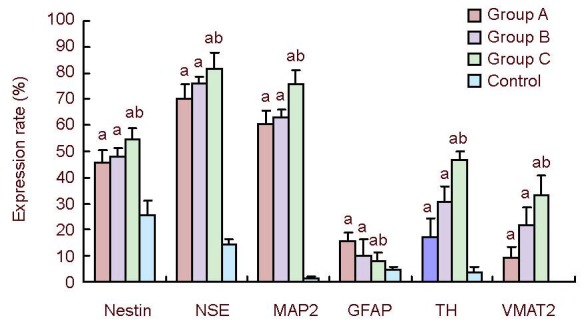
Overall expression of nestin, NSE, MAP2, GFAP, TH and VMAT2 in induced rat bone marrow-derived mesenchymal stem cells under different conditions.
Expression rate (%) = number of positive cells/total number of cells × 100%. Data were determined by immunocytochemistry and expressed as mean ± SEM. Statistical differences were tested using F-test. aP < 0.05, vs. control group; bP < 0.05, vs. Xiangdan injection group and ATRA + GDNF group.
NSE: Neuron-specific enolase; MAP2: microtubule- associated protein 2; GFAP: glial fibrillary acid protein; TH: tyrosine hydroxylase; VMAT2: vesicular monoamine transporter-2; group A: Xiangdan injection; group B: all-trans retinoic acid + glial cell line-derived neurotrophic factor; group C: sonic hedgehog + fibroblast growth factor 8.
The co-expression of neuron-specific enolase and tyrosine hydroxylase in cells from the sonic hedgehog + fibroblast growth factor 8 group was determined using double immunofluorescent staining and represented by tyrosine hydroxylase expression. The efficiency of induction was determined to be 46.7% in the sonic hedgehog + fibroblast growth factor 8 group (Figure 4).
Figure 4.
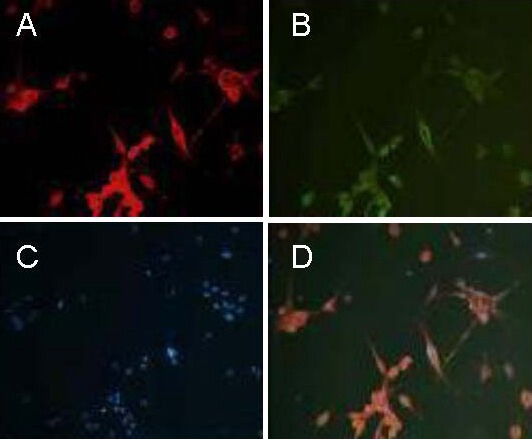
Co-expression of neuron-specific enolase and tyrosine hydroxylase in cells induced by sonic hedgehog and fibroblast growth factor 8 for 12 days (immunofluorescence staining, × 200).
The cytoplasm of the cells induced by sonic hedgehog and fibroblast growth factor 8 for 12 days are represented by red (A) and green (B) fluorescence, respectively. (A) Neuron-specific enolase; (B) tyrosine hydroxylase; (C) Hoechst 33258 stained nucleus; (D) merge of neuron-specific enolase and tyrosine hydroxylase co-expression. Results indicate that bone marrow-derived mesenchymal stem cells can differentiate into neurons expressing neuron-specific enolase and the dopaminergic neuronal marker, tyrosine hydroxylase.
Catecholamine levels in each group
Fluorescence chromatography showed that the amount of catecholamines was 2.487 ± 0.431, 2.731 ± 0.812, 2.977 ± 0.162 and 0.986 ± 0.113 μg/L, in the supernatant of the Xiangdan injection, all-trans retinoic acid + glial cell line-derived neurotrophic factor, sonic hedgehog + fibroblast growth factor 8 and control groups, respectively. The catecholamine level was significantly higher in each of the three induction groups compared with control group (P < 0.01), and was the highest in the sonic hedgehog + fibroblast growth factor 8 group.
DISCUSSION
The present study compared the efficiencies of three different induction cocktails for bone marrow-derived mesenchymal stem cell differentiation into dopaminergic neurons in vitro. Results showed that the sonic hedgehog + fibroblast growth factor 8 combination was an efficient and easy method for differentiating bone marrow-derived mesenchymal stem cells into dopaminergic neurons based on tyrosine hydroxylase expression and catecholamine content in the supernatant fluid.
Some antioxidants and cytokines act as inducers for cell differentiation in vitro. Combination of various inducing factors is one of the most common methods for inducing bone marrow-derived mesenchymal stem cells to differentiate into dopaminergic neurons in vitro. Retinoic acid and brain-derived neurotrophic factors have been used as inducers to bone marrow-derived mesenchymal stem cells into nerve cells, producing both neurons and astrocytes[17,18]. Antioxidants, such as β-mercaptoethanol, dimethyl sulfoxide and butylated hydroxyanisole, have also been used for this purpose. Within a few hours, most bone marrow-derived mesenchymal stem cells (80%) transform into neuron-like shapes and express neuron-specific markers[19].
For the growth, development and differentiation of dopaminergic neurons, various cytokines play different roles. Basic fibroblast growth factor promotes neurogenesis, and enhances the differentiation and survival of dopaminergic neurons. It may act as a mitogen to expand the initial development of transdifferentiated neural progenitors, while maintaining cell survival for further lineage progression[20,21]. The exact mechanism that the bone marrow-derived mesenchymal stem cells differentiate into neuron-like cells induced by Xiangdan injection, widely used as a traditional Chinese medicine to treat stroke, remains unclear, although the reduction of lipid peroxide generation is a possibility[22,23]. Retinoic acid is an active derivative of vitamin A and is widely expressed in the nervous system during development. Its role as a potent inducer of cell differentiation, and the wide expression of its receptors and binding proteins in the brain suggest an important role of retinoic acid in brain development and function[24]. As a strong inducer of differentiation, all-trans retinoic acid can cause changes in the expression of nervous system-related genes and induce embryonic stem cells to differentiate into dopaminergic neurons[25]. Glial cell line-derived neurotrophic factor is particularly important for the survival and function of dopaminergic neurons[26,27]; it can promote their differentiation and can protect and repair both developing and mature neurons. The development of dopaminergic neurons in the embryo is dependent on the interaction of two growth factors, sonic hedgehog and fibroblast growth factor 8. This combination could also induce embryonic stem cells and neuronal precursors to differentiate into dopaminergic neurons in vitro[28,29]. It was previously reported that human bone marrow-derived mesenchymal stem cells could be induced to differentiate into dopaminergic neurons with sonic hedgehog, fibroblast growth factor 8 and basic fibroblast growth factor. The efficiency of induction was determined to be around 67%[9].
Based on the functions of these inducing factors, we designed three different methods of induction as follows: Xiangdan injection; all-trans retinoic acid + glial cell line-derived neurotrophic factor; and sonic hedgehog + fibroblast growth factor 8. Most of the induced rat bone marrow-derived mesenchymal stem cells showed typical neuronal morphological characteristics. Xiangdan injection has been used to induce neural stem cells or bone marrow-derived mesenchymal stem cells to differentiate into neuronal cells[30,31]. To confirm that Xiangdan injection has effects on proliferation, neurite outgrowth and differentiation of bone marrow-derived mesenchymal stem cells into dopaminergic neurons, we designed the Xiangdan injection group. Results showed that this combination could induce bone marrow-derived mesenchymal stem cells to express tyrosine hydroxylase very early following induction (after 3 hours), but the expression was low (17.2%) and most cells died soon thereafter. As all-trans retinoic acid and glial cell line-derived neurotrophic factor are involved in differentiation, survival and function, we also used a cocktail of all-trans retinoic acid + glial cell line-derived neurotrophic factor. In this group, the expression of tyrosine hydroxylase was 30.7%, which was in the mid-range between the Xiangdan injection and sonic hedgehog + fibroblast growth factor 8 groups. In the sonic hedgehog + fibroblast growth factor 8 group, the morphology of bone marrow-derived mesenchymal stem cells changed slowly and only a few cells died. The expression of tyrosine hydroxylase was also highest (46.7%) in the sonic hedgehog + fibroblast growth factor 8 group among the three induction groups. In view of these results, we believe that sonic hedgehog and fibroblast growth factor 8 are the most important inducers of dopaminergic neuronal differentiation, as sonic hedgehog plays a pivotal role in ventral midbrain patterning and dopaminergic neuron fate specification during development[32], and fibroblast growth factor signaling has multiple roles during the induction and maintenance of the telencephalon[33].
The induced cells expressed different levels of neuronal proteins, such as nestin, neuron-specific enolase, microtubule-associated protein 2, tyrosine hydroxylase and vesicular monoamine transporter-2, which suggests that these neuron-like cells express neuronal antigens quite early after induction. The induced cells co-expressed both neuron-specific enolase and tyrosine hydroxylase by double-immunofluorescence microscopy, which further indicates that these cells differentiated into dopaminergic neurons. The rate of tyrosine hydroxylase expression in the sonic hedgehog + fibroblast growth factor 8 group was the highest, and was determined to be 46.7%.
As an important neurotransmitter, catecholamines include epinephrine, norepinephrine and dopamine[34,35]. The present study also analyzed the supernatant fluid from the three induction groups and found that they contained secreted catecholamines, with the sonic hedgehog + fibroblast growth factor 8 group secreting the highest concentration, thereby indicating that this combination of cytokines is more effective in the induction of bone marrow-derived mesenchymal stem cells into dopaminergic neuron-like cells.
In conclusion, bone marrow-derived mesenchymal stem cells have the potential to differentiate into dopaminergic neurons using a number of inducer cocktails in vitro. The dopaminergic neurons derived from bone marrow-derived mesenchymal stem cells expressed several neuronal-specific proteins. Furthermore, neurons derived from bone marrow-derived mesenchymal stem cells secreted catecholamines, indicating that they become functional neurons. Finally, the sonic hedgehog + fibroblast growth factor 8 cocktail was the most effective in inducing bone marrow-derived mesenchymal stem cell differentiation into dopaminergic neuron-like cells.
MATERIALS AND METHODS
Design
An in vitro, comparative, observational study of cytology.
Time and setting
The experiment was performed at the Laboratory of Human Anatomy and Histology and Embryology, Weifang Medical University, China from 2009 to 2011.
Materials
Twelve 6-week-old Sprague-Dawley rats, of either gender, weighing 150–180 g, of clean grade, were provided by the Animal Research Center, Weifang Medical University, license No. SYXK (Lu) 2005 0043. Animals were housed with illumination periods from 7:00–19:00, at 20 ± 2°C, with a relative humidity of 45–55%, and allowed free access to food and water. Rats were handled in accordance with the Guidance Suggestions for the Care and Use of Laboratory Animals, formulated by the Ministry of Science and Technology of China[36].
Methods
Culture of rat bone marrow-derived mesenchymal stem cells
Rat bone marrow-derived mesenchymal stem cells were cultured using their physical characteristic of adhering to a plastic surface[37]. Rats were sacrificed by cervical dislocation, following which tibias and femurs were obtained under sterile conditions. After cutting away the ends of the bones, the marrow (5 mL) was extracted using a needle and syringe. Cells were suspended in Dulbecco's-modified Eagle's medium (Gibco, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (Hangzhou Sijiqing Biological Engineering Materials Co., Ltd., Hangzhou, China) at 37°C in a humidified-atmosphere of 95% air and 5% CO2. After 24 hours, nonadherent cells were removed by replacing the medium. The medium was changed every 3 days. Once more than 80% of adherent cells converged, they were detached with trypsin (Sigma, St. Louis, MO, USA). To expand the culture, the cells were diluted at 1:2 per passage. Passage 3 cells were used for neuronal induction and subsequent determination of their biological identity.
Dopaminergic neuronal induction of bone marrow-derived mesenchymal stem cells
Passage 3 bone marrow-derived mesenchymal stem cells were trypsinized and subcultured onto polylysine-coated coverslips in multiwell plates. Each plate contained 1 × 105 cells. At approximately 80% confluence, bone marrow-derived mesenchymal stem cells were induced into dopaminergic neurons using different inducers as follows: in the Xiangdan injection group, cells were pre-induced in L-Dulbecco's-modified Eagle's medium containing 10% fetal bovine serum and 25 ng/mL basic fibroblast growth factor (Peprotech, Rocky Hill, NJ, USA) for 24 hours, followed by L-Dulbecco's-modified Eagle's medium and 20% Xiangdan injection (containing 1 000 g/L salvia miltiorrhiza, 1 000 g/L dalbergia odorifera (No. Z13021387), Hebei Tiancheng Pharmaceutical Co., Ltd., China) for 3 hours; in the all-trans retinoic acid + glial cell line-derived neurotrophic factor group, cells were induced in neurobasal medium containing 25 ng/mL basic fibroblast growth factor and 2% B27 (Gibco) for 24 hours, followed by neurobasal medium containing 2% B27, 1 μM all-trans retinoic acid (Sigma) and 50 ng/mL glial cell line-derived neurotrophic factor (Peprotech) for 6 days; in the sonic hedgehog + fibroblast growth factor 8 group, cells were induced in neurobasal medium containing 25 ng/mL basic fibroblast growth factor and 2% B27 for 24 hours, followed by neurobasal medium containing 2% B27, 250 ng/mL sonic hedgehog (Peprotech) and 100 ng/mL fibroblast growth factor 8 (Peprotech) for 12 days; in the control group, cells were cultured without any inducers.
Immunocytochemistry and immunofluorescence for detection of neural proteins
Cells were fixed with acetone at 4°C for 10 minutes, incubated in 3% H2O2-methanol for 30 minutes and washed three times with 0.01 M PBS. For blocking nonspecific immune reaction, the cells were treated with goat serum at room temperature for 30 minutes. Cells were incubated overnight at 4°C with the following antibodies: rabbit anti-CD34 polyclonal antibody (1:300; Wuhan Boster, Wuhan, China); rabbit anti-CD44 polyclonal antibody (1:300; Wuhan Boster); rabbit anti-nestin polyclonal antibody (1:100; Wuhan Boster); mouse anti-rat microtubule-associated protein 2 monoclonal antibody (1:100; Wuhan Boster); rabbit anti-neuron specific enolase polyclonal antibody (1:100; Beijing Zhongshan Golden Bridge Biological Technology Co., Ltd. Beijing, China); rabbit anti-glial fibrillary acid protein polyclonal antibody (1:100; Beijing Zhongshan Golden Bridge Biological Technology Co., Ltd.); mouse anti-rat tyrosine hydroxylase monoclonal antibody (1:500; Sigma); goat anti-vesicular monoamine transporter-2 polyclonal antibody (1:100; Santa Cruz Biotechnology, Santa Cruz, CA, USA). After three washes in 0.01 M PBS, cells were incubated with biotinylated goat anti-mouse or anti-rabbit IgG (General SP Kit working fluid, Beijing Zhongshan Golden Bridge Biological Technology Co., Ltd.) at 37°C for 30 minutes, followed by 30 minutes of incubation in avidin-biotinylated peroxidase complex at 37°C, except for the vesicular monoamine transporter-2. For vesicular monoamine transporter-2, the cells were treated with the polymer auxiliary agent at 37°C for 30 minutes, and then mixed with horseradish enzyme labeled rabbit anti-goat IgG dimmer at 37°C for 30 minutes. After washing with 0.01 M PBS, diaminobenzidine (Zhongshan Golden Bridge Biological Technology Co., Ltd.) was used as a chromagen, with reactions sustained at room temperature and in the dark for 10 minutes. After decoloration with distilled water, cells were counterstained with hematoxylin, dehydrated with a gradient alcohol series, cleared with dimethyl benzene and mounted with neutral balsam. The primary antibodies in the negative control group were replaced by PBS. The expression of each antigen was examined in separate experiments at least three times. Ten nonrepetitive visual fields were selected randomly under a light microscope in each group (× 100). Expression rate (%) = the number of positive cells/(the number of positive cells + the number of negative cells) × 100%.
For immunofluorescence, cells were incubated overnight at 4°C with the following antibodies: mouse anti-rat tyrosine hydroxylase monoclonal antibody (1:250) regularly mixed with rabbit anti-neuron-specific enolase polyclonal antibody (1:50). After rinsing with 0.01 M PBS for three times, cells were incubated with secondary antibodies: fluorescein isothiocyanate-goat anti-mouse IgG (1:100, Zhongshan Golden Bridge Biological Technology Co., Ltd.) regularly mixed with Cy3-goat anti-rabbit IgG (1:500, Beyotime, Shanghai, China) at 37°C for 60 minutes in the dark. Cell nuclei were counterstained with Hoechst 33258 (Beyotime) at room temperature for 10 minutes in the dark. Labeled cells were observed under a fluorescent microscope (Leica, Solms, Germany) with the appropriate fluorescence filters.
Measurement of catecholamine levels in supernatant fluid
After induction, the supernatant fluid was collected, centrifuged at 2 000 r/min and 0.25 g EDTA-Na2 was then added, shaken, and boiled for 2 minutes. After cooling, they were placed in 0.75 g Al2O3, shaken for 2 minutes, mixed with 10 M NaOH until the pH reached 8.0–8.6, and shaken for 5 minutes. The pH was re-adjusted between 8.0–8.5, and the product was shaken for 5 minutes again. Al2O3 was transferred to the column, washed with sodium acetate and double distilled water, followed by 2 mL of 0.5 M H2SO4. The eluent in burettes was gathered after elution twice and then formulated for testing. The catecholamine level was detected using the fluorescence spectrophotometer RF-5301PC (Shimadzu, Kyoto, Japan), with excitation and emission wavelengths of 410 nm and 510 nm, respectively.
Statistical analysis
The SPSS 16.0 software (SPSS, Chicago, IL, USA) was used to analyze data, which were expressed as mean ± SEM. Statistical differences were calculated using F-test. P values < 0.05 were considered statistically significant.
Footnotes
Wenyu Fu, M.D., Professor.
Funding: This study was supported by the Scientific Research Foundation for the Returned Overseas, No. [2009]1001; the Natural Science Foundation of Shandong Province, No. Y2008C129.
Conflicts of interest: None declared.
Ethical approval: The experiments were approved by the Animal Ethics Committee of Weifang Medical University in China.
Supplementary information: Supplementary data associated with this article can be found, in the online version, by visiting www.nrronline.org.
(Edited by Huang YL, Wang DF/Su LL/Song LP)
REFERENCES
- [1].Cho EG, Zaremba JD, McKercher SR, et al. MEF2C enhances dopaminergic neuron differentiation of human embryonic stem cells in a parkinsonian rat model. PLoS One. 2011;6(8):e24027. doi: 10.1371/journal.pone.0024027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Xu J, Wang W, Ludeman M, et al. Chondrogenic differentiation of human mesenchymal stem cells in three- dimensional alginate gels. Tissue Eng Part A. 2008;14(5):667–680. doi: 10.1089/tea.2007.0272. [DOI] [PubMed] [Google Scholar]
- [3].Scheideler M, Elabd C, Zaragosi LE, et al. Comparative transcriptomics of human multipotent stem cells during adipogenesis and osteoblastogenesis. BMC Genomics. 2008;9:340. doi: 10.1186/1471-2164-9-340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Zhang Y, Khan D, Delling J, et al. Mechanisms underlying the osteo- and adipo-differentiation of human mesenchymal stem cells. ScientificWorldJournal 2012. 2012 doi: 10.1100/2012/793823. 793823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Alexanian AR. Epigenetic modifiers promote efficient generation of neural-like cells from bone marrow-derived mesenchymal cells grown in neural environment. J Cell Biochem. 2007;100(2):362–371. doi: 10.1002/jcb.21029. [DOI] [PubMed] [Google Scholar]
- [6].Liu J, Song L, Jiang C, et al. Electrophysiological properties and synaptic function of mesenchymal stem cells during neurogenic differentiation: a mini-review. Int J Artif Organs. 2012;35(5):323–337. doi: 10.5301/ijao.5000085. [DOI] [PubMed] [Google Scholar]
- [7].Krampera M, Pasini A, Pizzolo G, et al. Regenerative and immunomodulatory potential of mesenchymal stem cells. Curr Opin Pharmacol. 2006;6(4):435–441. doi: 10.1016/j.coph.2006.02.008. [DOI] [PubMed] [Google Scholar]
- [8].Rodríguez R, García-Castro J, Trigueros C, et al. Multipotent mesenchymal stromal cells: clinical applications and cancer modeling. Adv Exp Med Biol. 2012;741:187–205. doi: 10.1007/978-1-4614-2098-9_13. [DOI] [PubMed] [Google Scholar]
- [9].Trzaska KA, Kuzhikandathil EV, Rameshwar P. Specification of a dopaminergic phenotype from adult human mesenchymal stem cells. Stem Cells. 2007;25(11):2797–2808. doi: 10.1634/stemcells.2007-0212. [DOI] [PubMed] [Google Scholar]
- [10].Tio M, Tan KH, Lee W, et al. Roles of db-cAMP, IBMX and RA in aspects of neural differentiation of cord blood derived mesenchymal-like stem cells. PLoS One. 2010;5(2):e9398. doi: 10.1371/journal.pone.0009398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Trzaska KA, Rameshwar P. Dopaminergic neuronal differentiation protocol for human mesenchymal stem cells. Methods Mol Biol. 2011;698:295–303. doi: 10.1007/978-1-60761-999-4_22. [DOI] [PubMed] [Google Scholar]
- [12].Park S, Kim E, Koh SE, et al. Dopaminergic differentiation of neural progenitors derived from placental mesenchymal stem cells in the brains of Parkinson's disease model rats and alleviation of asymmetric rotational behavior. Brain Res. 2012;1466:158–166. doi: 10.1016/j.brainres.2012.05.032. [DOI] [PubMed] [Google Scholar]
- [13].Tondreau T, Lagneaux L, Dejeneffe M, et al. Bone marrow-derived mesenchymal stem cells already express specific neural proteins before any differentiation. Differentiation. 2004;72(7):319–326. doi: 10.1111/j.1432-0436.2004.07207003.x. [DOI] [PubMed] [Google Scholar]
- [14].Nadri S, Soleimani M, Mobarra Z, et al. Expression of dopamine-associated genes on conjunctiva stromal-derived human mesenchymal stem cells. Biochem Biophys Res Commun. 2008;377(2):423–428. doi: 10.1016/j.bbrc.2008.09.148. [DOI] [PubMed] [Google Scholar]
- [15].Deans RJ, Moseley AB. Mesenchymal stem cells: biology and potential clinical uses. Exp Hematol. 2000;28(8):875–884. doi: 10.1016/s0301-472x(00)00482-3. [DOI] [PubMed] [Google Scholar]
- [16].Hussain I, Magd SA, Eremin O, et al. New approach to isolate mesenchymal stem cell (MSC) from human umbilical cord blood. Cell Biol Int. 2012;36(7):595–600. doi: 10.1042/CBI20110336. [DOI] [PubMed] [Google Scholar]
- [17].Sanchez-Ramos J, Song S, Cardozo-Pelaez F, et al. Adult bone marrow stromal cells differentiate into neural cells in vitro. Exp Neurol. 2000;164(2):247–256. doi: 10.1006/exnr.2000.7389. [DOI] [PubMed] [Google Scholar]
- [18].Mammadov B, Karakas N, Isik S. Comparison of long-term retinoic acid-based neural induction methods of bone marrow human mesenchymal stem cells. In Vitro Cell Dev Biol Anim. 2011;47(7):484–491. doi: 10.1007/s11626-011-9425-4. [DOI] [PubMed] [Google Scholar]
- [19].Woodbury D, Schwarz EJ, Prockop DJ, et al. Adult rat and human bone marrow stromal cells differentiate into neurons. J Neurosci Res. 2000;61(4):364–370. doi: 10.1002/1097-4547(20000815)61:4<364::AID-JNR2>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- [20].Sun D, Bullock MR, McGinn MJ, et al. Basic fibroblast growth factor-enhanced neurogenesis contributes to cognitive recovery in rats following traumatic brain injury. Exp Neurol. 2009;216(1):56–65. doi: 10.1016/j.expneurol.2008.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Timmer M, Cesnulevicius K, Winkler C, et al. Fibroblast growth factor (FGF)-2 and FGF receptor 3 are required for the development of the substantia nigra, and FGF-2 plays a crucial role for the rescue of dopaminergic neurons after 6-hydroxydopamine lesion. J Neurosci. 2007;27(3):459–471. doi: 10.1523/JNEUROSCI.4493-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Zhang HA, Gao M, Zhang L, et al. Salvianolic acid A protects human SH-SY5Y neuroblastoma cells against H2O2-induced injury by increasing stress tolerance ability. Biochem Biophys Res Commun. 2012;421(3):479–483. doi: 10.1016/j.bbrc.2012.04.021. [DOI] [PubMed] [Google Scholar]
- [23].Liu CS, Chen NH, Zhang JT. Protection of PC12 cells from hydrogen peroxide-induced cytotoxicity by salvianolic acid B, a new compound isolated from Radix Salviae miltiorrhizae. Phytomedicine. 2007;14(7-8):492–497. doi: 10.1016/j.phymed.2006.11.002. [DOI] [PubMed] [Google Scholar]
- [24].Maden M. Retinoic acid in the development, regeneration and maintenance of the nervous system. Nat Rev Neurosci. 2007;8(10):755–765. doi: 10.1038/nrn2212. [DOI] [PubMed] [Google Scholar]
- [25].Dinsmore J, Ratliff J, Jacoby D, et al. Embryonic stem cells as a model for studying regulation of cellular differentiation. Theriogenology. 1998;49(1):145–151. doi: 10.1016/s0093-691x(97)00409-3. [DOI] [PubMed] [Google Scholar]
- [26].Lin LF, Doherty DH, Lile JD, et al. GDNF: a glial cell line-derived neurotrophic factor for midbrain dopaminergic neurons. Science. 1993;260(5111):1130–1132. doi: 10.1126/science.8493557. [DOI] [PubMed] [Google Scholar]
- [27].Glavaski-Joksimovic A, Virag T, Mangatu TA, et al. Glial cell line-derived neurotrophic factor-secreting genetically modified human bone marrow-derived mesenchymal stem cells promote recovery in a rat model of Parkinson's disease. J Neurosci Res. 2010;88(12):2669–2681. doi: 10.1002/jnr.22435. [DOI] [PubMed] [Google Scholar]
- [28].Kim DW. Efficient induction of dopaminergic neurons from embryonic stem cells for application to Parkinson's disease. Yonsei Med J. 2004;45(Suppl):23–27. doi: 10.3349/ymj.2004.45.Suppl.23. [DOI] [PubMed] [Google Scholar]
- [29].Kim JH, Auerbach JM, Rodríguez-Gómez JA, et al. Dopamine neurons derived from embryonic stem cells function in an animal model of Parkinson's disease. Nature. 2002;418(6893):50–56. doi: 10.1038/nature00900. [DOI] [PubMed] [Google Scholar]
- [30].Guo G, Li B, Wang Y, et al. Effects of salvianolic acid B on proliferation, neurite outgrowth and differentiation of neural stem cells derived from the cerebral cortex of embryonic mice. Sci China Life Sci. 2010;53(6):653–662. doi: 10.1007/s11427-010-3106-5. [DOI] [PubMed] [Google Scholar]
- [31].Hu L, Yu J, Li F, et al. Effects of Salvia miltorrhiza in neural differentiation of rat mesenchymal stem cells with optimized protocol. J Ethnopharmacol. 2011;136(2):334–340. doi: 10.1016/j.jep.2011.04.043. [DOI] [PubMed] [Google Scholar]
- [32].Ye W, Shimamura K, Rubenstein JL, et al. FGF and Shh signals control dopaminergic and serotonergic cell fate in the anterior neural plate. Cell. 1998;93(5):755–766. doi: 10.1016/s0092-8674(00)81437-3. [DOI] [PubMed] [Google Scholar]
- [33].Paek H, Gutin G, Hébert JM. FGF signaling is strictly required to maintain early telencephalic precursor cell survival. Development. 2009;136(14):2457–2465. doi: 10.1242/dev.032656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Peterson G, Kumar A, Gart E, Narayanan S. Catecholamines increase conjugative gene transfer between enteric bacteria. Microb Pathog. 2011;51(1-2):1–8. doi: 10.1016/j.micpath.2011.03.002. [DOI] [PubMed] [Google Scholar]
- [35].Tsunoda M, Funatsu T. Catecholamine analysis with strong cation exchange column liquid chromatography-peroxyoxalate chemiluminescence reaction detection. Anal Bioanal Chem. 2012;402(3):1393–1397. doi: 10.1007/s00216-011-5542-x. [DOI] [PubMed] [Google Scholar]
- [36].The Ministry of Science and Technology of the People's Republic of China. Guidance Suggestions for the Care and Use of Laboratory Animals 2006-09-30 [Google Scholar]
- [37].Kuznetsov SA, Friedenstein AJ, Robey PG. Factors required for bone marrow stromal fibroblast colony formation in vitro. Br J Haematol. 1997;97(3):561–570. doi: 10.1046/j.1365-2141.1997.902904.x. [DOI] [PubMed] [Google Scholar]


