Abstract
In this study, we investigated whether a single nucleotide polymorphism (rs42524 G > C) in the type I alpha 2 collagen gene was associated with sporadic ruptured intracranial aneurysm or its clinical characteristics in patients from Northeast China. Genotyping of the rs42524 G > C polymorphism was carried out using a polymerase chain reaction-restriction fragment length polymorphism assay. The data showed that the frequency of the rs42524 GC + CC genotype was significantly higher than the GG genotype among intracranial aneurysm patients whose Hunt and Hess grading scale was > 3. In addition, the rs42524 G > C genotype was found to have a statistically significant association with intracranial aneurysm risk. These findings indicate that the type I alpha 2 collagen gene gene may be involved in a predisposition to intracranial aneurysm in the Northeast Chinese population. Crucially, the rs42524 C allele may be an important risk factor for increased severity of the condition in patients with ruptured intracranial aneurysms.
Keywords: neural regeneration, clinical practice, intracranial aneurysm, type I collagen gene, single nucleotide polymorphism, polymerase chain reaction-restriction fragment length polymorphism assay, susceptibility, risk factors, neuroregeneration
Research Highlights
(1) The frequency of the type I alpha 2 collagen gene single nucleotide polymorphism rs42524 GC + CC genotype was significantly higher than the GG genotype among patients with intracranial aneurysms in Northeast China, whose Hunt and Hess grading was > 3.
(2) The C allele of the type I alpha 2 collagen gene single nucleotide polymorphism rs42524 may be an important risk factor for increased pathology in patients with ruptured intracranial aneurysms.
INTRODUCTION
Intracranial aneurysm is a life-threatening condition. Not enough is known about the links between intracranial aneurysm and an individual's genetic make-up. In addition, we do not know whether the prognosis following a ruptured intracranial aneurysm is linked to an individual's genotype. In this study, we investigated the relationship between intracranial aneurysm and the type I alpha 2 collagen gene. Intracranial aneurysm is a cerebrovascular disorder characterized by abnormal local distension of the intracranial artery. Whilst the overall incidence of ruptured intracranial aneurysm is low (18–23/100 000 per year)[1], the incidence of intact intracranial aneurysm is estimated at 2.7–6.5%, according to autopsy reports and angiographic evaluations[2,3,4]. Intracranial aneurysm may occur at any age, but is more common in females than males[5,6]. Currently, intracranial aneurysm is considered a multigenic disease caused by genetic and environmental factors[7,8,9,10,11,12,13].
However, the pathogenesis of intracranial aneurysm is not clear, and this increases the difficulty in treating it. Here, we investigated a single nucleotide polymorphism (SNP) within the type I alpha 2 collagen gene gene (rs42524 G > C) in samples from 367 patients with sporadic intracranial aneurysm and 396 controls. This randomized study in the Northeast of China explored associations between genetic polymorphism and the clinical characteristics of intracranial aneurysm and compared the results with a similar study undertaken in Japan[1].
RESULTS
Quantitative analysis of subjects
A total of 367 sporadic intracranial aneurysm patients were recruited from the Department of Neurosurgery at the First Affiliated Hospital of China Medical University; these patients served as the case group. Another 396 patients exhibiting tumors of the nervous system, without intracranial aneurysm, were included in the control group. In the case group, there were 128 males and 239 females, with a median age of 52.82 ± 10.19 years, whilst the control group comprised 197 males and 199 females, with a median age of 51.61 ± 12.57 years. A significant difference in the gender composition was found between the two groups (two-sided fourfold table chi-square test, P < 0.001), but no difference in age (mean ± SD, P > 0.05). All of the subjects were included in the end-point data analysis; no patients dropped out or were lost from the study.
Electrophoresis pattern for type I alpha 2 collagen gene (single nucleotide polymorphism rs42524) PCR products from intracranial aneurysm patients
The PCR products amplified from the type I alpha 2 collagen gene locus (single nucleotide polymorphism rs42524) were 128 bp in length (Figure 1).
Figure 1.
Type I alpha 2 collagen single nucleotide polymorphism rs42524 PCR products from the case group.
Restriction endonuclease analysis of the type I alpha 2 collagen gene gene (single nucleotide polymorphism rs42524) PCR products from intracranial aneurysm patients
Figure 2 shows that following restriction with Bsa JI, the rs42524 homozygous C allele was 128 bp in length. In contrast, the homozygous allele G allele produced bands of 107 bp and 21 bp, whereas bands of 128 bp, 107 bp and 21 bp were associated with heterozygotes.
Figure 2.
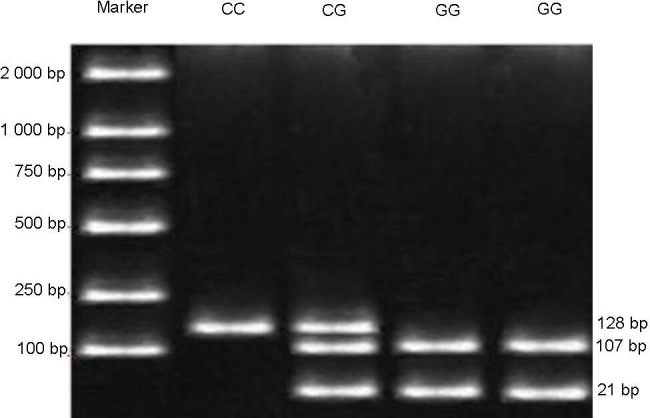
Restriction digests of the collagen type I alpha 2 gene single nucleotide polymorphism rs42524 in the case group.
Age distribution of intracranial aneurysm patients
In the 367 intracranial aneurysm patients analyzed, 236 (64.3%) of the cases were in the 40–59 year age (Figure 3).
Figure 3.
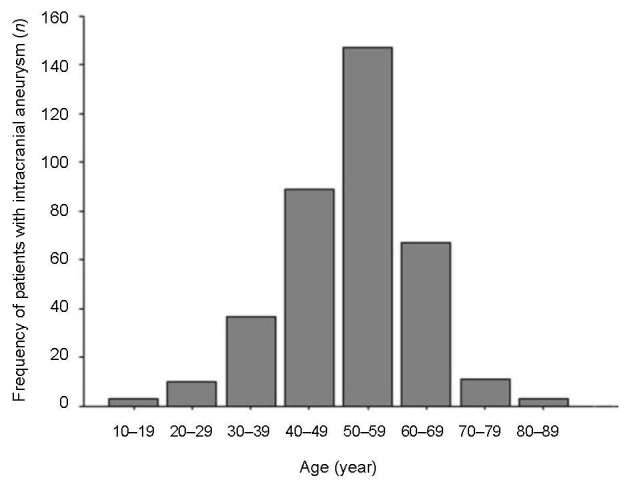
Age distribution of intracranial aneurysm patients.
Clinical characteristics of the intracranial aneurysm patients versus the control group
The characteristics of the study population and its association with intracranial aneurysm are presented in Table 1.
Table 1.
Clinical characteristics of IA patients vs. controls
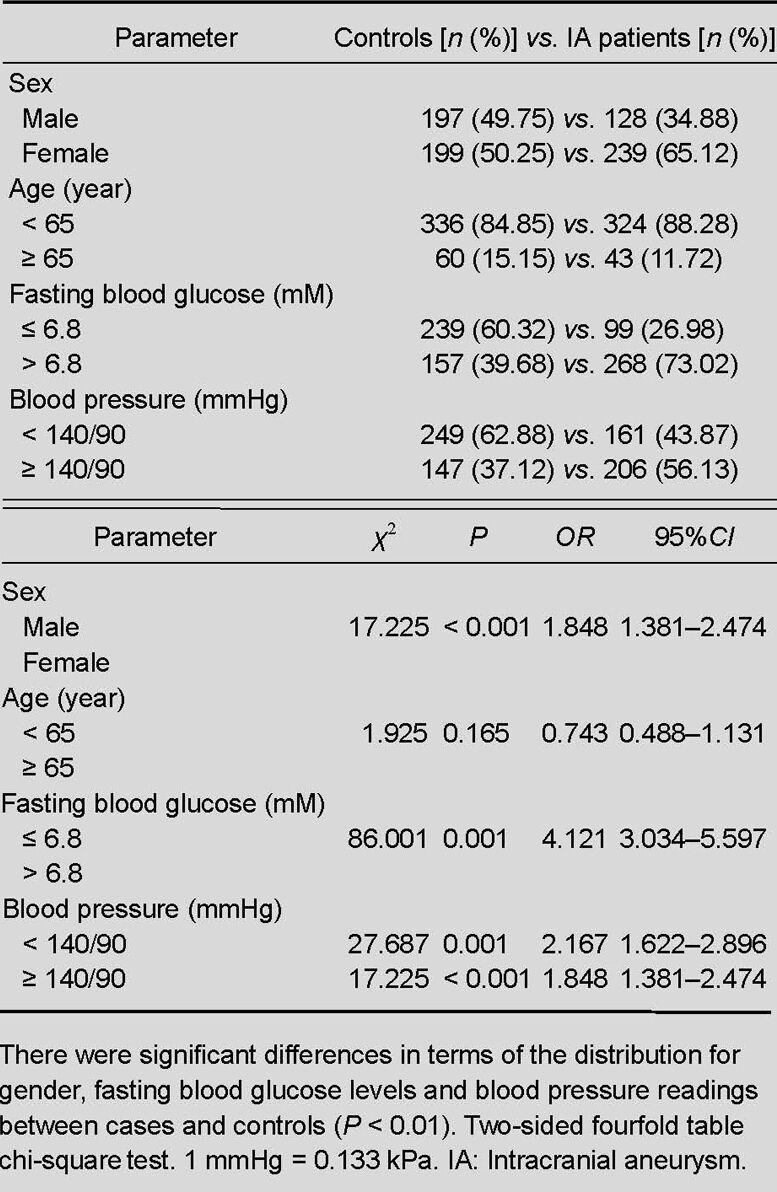
There were significant differences in terms of the gender distribution between the cases and controls (χ2 = 17.225, P < 0.001, OR = 1.848, 95%CI = 1.381–2.474). Intracranial aneurysm cases tended to have higher fasting blood glucose levels and blood pressure readings (χ2 = 86.001, P < 0.001, OR = 4.121, 95%CI = 3.034–5.597; χ2 = 27.687, P < 0.001, OR = 2.167, 95%CI = 1.622–2.896, respectively). However, there were no significant differences regarding the age distribution between the cases and controls.
Correlation between the rs42524 G > C type I alpha 2 collagen gene single nucleotide polymorphism and intracranial aneurysm
The genotypes and allele distributions for the type I alpha 2 collagen gene rs42524 G > C polymorphism for the intracranial aneurysm cases and controls are shown in Table 2.
Table 2.
Genotypes of the type I alpha 2 collagen gene single nucleotide polymorphism rs42524 in IA patients vs. controls [n (%)]
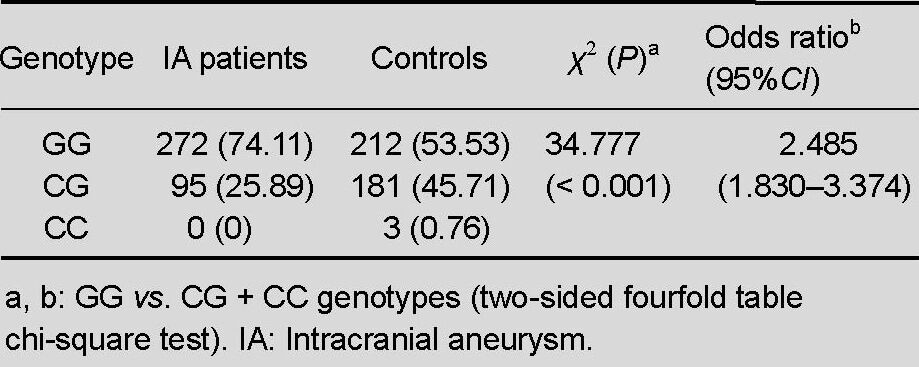
The distributions of the type I alpha 2 collagen gene rs42524 G > C polymorphism in the cases and controls were consistent with a Hardy-Weinberg equilibrium, and the frequency of the type I alpha 2 collagen gene rs42524 G > C genotype was statistically significant between both cases and controls (χ2 = 34.777, P < 0.001, OR = 2.485, 95%CI = 1.830–3.374); this finding indicates that there is an association between the rs42524 G > C polymorphism and sporadic intracranial aneurysm in Northeast China. It appears likely, therefore, that the type I alpha 2 collagen gene gene could be one of a number of genes that predispose people to intracranial aneurysm.
Correlation between the type I alpha 2 collagen gene rs42524 G > C polymorphism and the clinical characteristics of intracranial aneurysm
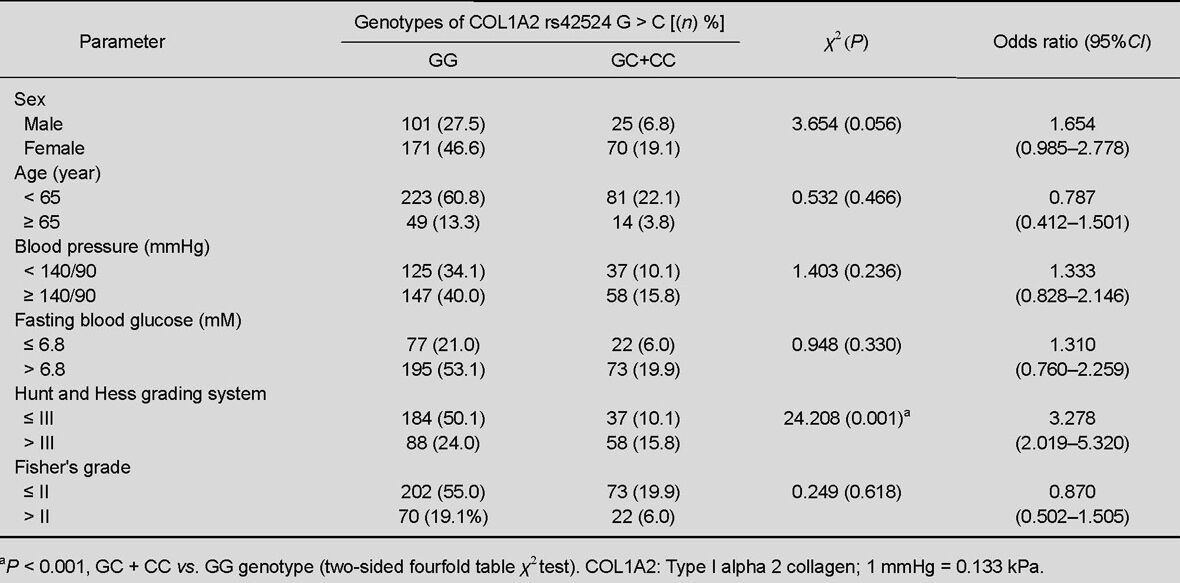
We also investigated associations between the rs42524 G > C genotype and various clinical features among the intracranial aneurysm patients (Table 3). The data showed no correlations between gender, age, blood pressure, fasting blood glucose, or Fisher scale and the rs42524 G > C genotype. However, the frequency of the type I alpha 2 collagen gene rs42524 single nucleotide polymorphism and the GC + CC genotype was significantly higher than the GG genotype among intracranial aneurysm patients whose Hunt scale score was > 3 (χ2 = 24.208, P < 0.001, OR = 3.278, 95%CI = 2.019–5.320).
Table 3.
Correlation between COL1A2 single nucleotide polymorphism rs42524 G > C genotypes and clinical features of intracranial aneurysm in patients
DISCUSSION
Single nucleotide polymorphisms are the most frequent types of polymorphisms seen in the human genome. Such polymorphisms include nucleotide substitutions, transversions, deletions and insertions.
In the human genome, a single nucleotide polymorphism occurs once every 1 000 bases and there are about 3 × 106 single nucleotide polymorphisms in total[14].
Single nucleotide polymorphisms can be used to unravel connections between genes and disease in human populations. Advances in the human genome project have allowed researchers to use single nucleotide polymorphism data to explore associations between genes, phenotypes, and diseases, especially complex diseases where more than one gene is involved. Hence, single nucleotide polymorphisms have become an important research tool in medical genetics[15,16]. There are 19 different types of collagen within the collagen protein family and variations in collagen structure can cause disease.
In the intracranial artery, type I collagen is mainly located in the arterial wall, but is also an important constituent of the extracellular matrix. Single amino acid substitutions in type I collagen are common and glycine substitution frequently results in disease. The type I alpha 2 collagen gene gene, located on chromosome 7q22.1, is 38 kb in length and has 52 exons. Various studies have shown that collagen is associated with intracranial aneurysm and its predisposition[1,13,17,18,19,20].
Sporadic intracranial aneurysms can occur at any age, but are most frequently seen in people aged 40 to 49 years[21]. In this study, the intracranial aneurysm rate increased significantly in the 40–59 year-old age group (63%), peaked in 40–49 year-olds, but decreased after aged 70 years. Other studies have indicated that advanced age can increase the pathogenicity of other risk factors associated with the disease, such as hypertension, diabetes and being female[22,23,24].
In the present study, significant differences between the gender distribution of the two groups were identified (Table 1). Consistent with this finding are the results of a different study where disease prevalence and the risk of intracranial aneurysm rupture were both more common in females than males[5]. One possible reason to account for this is that estrogen may inhibit the formation of intracranial aneurysm by maintaining the level of collagen in the cerebral vessel walls. In post-menopausal women, however, decreased levels of estrogen appear likely to increase the likelihood of suffering from intracranial aneurysm[5,6].
In this study, intracranial aneurysm patients tended to have higher fasting blood glucose levels and elevated blood pressure readings. Similarly, a Chinese study showed that hypertension and fasting blood glucose levels were associated with intracranial aneurysm[25], whilst a Japanese study showed an association between hypertension and intracranial aneurysm[26]. Data obtained from several studies support these findings[21,27,28].
Our study investigated whether an association exists between genetic polymorphism in the type I alpha 2 collagen gene rs42524 single nucleotide polymorphism G > C in the Northeast of China and sporadic intracranial aneurysm, something that has not been investigated previously. The results showed that the frequency of the type I alpha 2 collagen gene single nucleotide polymorphism rs42524 G > C genotype for intracranial aneurysm patients and controls was statistically significant, thus indicating an association between the rs42524 single nucleotide polymorphism and sporadic intracranial aneurysm in this part of China. Type I alpha 2 collagen gene gene may be one of the genes that predispose an individual to intracranial aneurysm. The association identified between rs42524 G > C polymorphism and the risk of intracranial aneurysm is consistent with a Japanese study[1].
A surprising finding was that the frequency of type I alpha 2 collagen gene single nucleotide polymorphism rs42524 GC + CC genotype was higher than that of the GG genotype among intracranial aneurysm patients whose scale of Hunt and Hess grading was > 3. This suggests that the type I alpha 2 collagen gene rs42524 C allele may increase disease severity. In addition, our study showed that there was no significant difference between the frequency of the type I alpha 2 collagen gene rs42524 GC + CC genotype and the GG genotype with respect to gender, age, blood pressure, fasting blood glucose level, or Fisher's grade among intracranial aneurysm patients. The C allele may be an important risk factor for disease severity. The increased risk of intracranial aneurysm in CC carriers of the rs42524 polymorphism may be a result of damage to or reduction of the integrity of the extracellular matrix. This could explain why the intracranial aneurysm pathology might be worse for such individuals.
The present study has some limitations. One is the relatively small sample size, which could have resulted in a less precise estimation of the association between type I alpha 2 collagen gene polymorphisms and intracranial aneurysm susceptibility. The other is that the study population was confined to the Northeast of China, thus the results may not apply to other populations.
In conclusion, our results indicate that sporadic intracranial aneurysm mostly occurs in people aged 40–59 years, but peaks at 40–49 years. Our results also showed an association between the type I alpha 2 collagen gene rs42524 G > C polymorphism and increased risk of developing intracranial aneurysm, with females being at higher risk of the condition. Fasting blood glucose and hypertension were found to be significantly associated with intracranial aneurysm. Notably, the allele C is possibly the most important risk factor for a more severe illness. Studies thus far have provided evidence for racial and ethnicity-based differences in the genes that predispose individuals towards intracranial aneurysm[29,30,31]. Further studies should be done, ideally in different populations, races and nationalities, and such results could be verified by experiments in transgenic animals.
SUBJECTS AND METHODS
Design
A case-control study.
Time and setting
Experiments were performed in the Central Laboratory of China Medical University from March 2007 to January 2008.
Subjects
The subjects were unrelated individuals from the Northeast of China. Case group: the trial recruited 367 sporadic intact intracranial aneurysm patients and 396 control subjects. The patients comprised 128 men and 239 women who had been diagnosed by digital subtraction angiography, three-dimensional computed tomography angiography, or magnetic resonance angiography. Diagnoses for these patients were confirmed by neurosurgical operations performed at the Department of Neurosurgery, First Affiliated Hospital of China Medical University in China. Control group: 396 controls, consisting of 197 men and 199 women, were randomly selected among people admitted to the same hospital during the same time period. These patients had tumors of the nervous system but no intracranial aneurysm as determined by digital subtraction angiography, three-dimensional computed tomography angiography or magnetic resonance angiography.
All subjects were informed about the program and its potential risks before starting and informed consent was obtained according to the Administration Regulations of Medical Institution, formulated by the State Council of the People's Republic of China[32].
All subjects agreed to ulnar vein blood samples being taken; these were collected in anticoagulant test tubes prior to analysis. Detailed information and categorization of risk factors including gender, age, blood pressure and fasting blood glucose is shown in Table 3.
Methods
Primer design
Two milliliter samples were collected from the ulnar vein of each subject, after which DNA was extracted using potassium iodide, and qualified by ultraviolet spectrophotometry. The type I alpha 2 collagen gene single nucleotide polymorphism rs42524 G > C genotypes were determined using a PCR-restriction fragment length polymorphism assay[33]. The primers were designed using Primer 5.0 (Applied Takara Biotechnology, Dalian, China), and then synthesized. The sequences of these primers are shown in Table 4.
Table 4.
Primers used in this study
Type I alpha 2 collagen gene single nucleotide polymorphism rs42524 detected by PCR-restriction fragment length polymorphism
Five nanograms of genomic DNA was amplified in a 20 μL reaction volume containing 10 × PCR buffer, 100 nM of each primer, 160 nM of each dNTP, and 0.1 U of AmpliTaq Gold DNA Polymerase (Applied Takara Biotechnology, Dalian, China). The PCR conditions were as follows: initial denaturation at 94°C for 7 minutes, followed by 35 cycles of denaturation at 94°C for 30 seconds, annealing at 53°C for 30 seconds, and extension at 72°C for 30 seconds, and a final extension at 72°C for 7 minutes. Thermocycling was conducted using the GeneAmp PCR System (model 9700, Applied Biosystems, USA). PCR products were analyzed using 2% agarose gels to determine their purity.
The National Center of Biotechnology Information database (http://www.ncbi.nlm.nih.gov) was used to determine the restriction endonuclease cutting site for the rs42524 single nucleotide polymorphism. On the basis of this information, Bsa JI was selected using the New England Biochemical Laboratory Website (http://www.neb.com). Bsa JI was obtained from Applied Takara Biotechnology (Dalian, China). Ten nanograms of each type I alpha 2 collagen gene PCR product was added to a 20 μL reaction mixture containing 10 × buffer K and 100 nM Bsa JI. Reactions were incubated in a 37°C water-bath overnight. The reaction products were run on 2% agarose gels. Samples with homozygous C alleles produced 128 bp bands; homozygous G alleles produced 107 and 21 bp bands, whilst heterozygotes produced bands of 128, 107 and 21 bp in length.
Statistical analysis
Data were analyzed with SPSS 13.0 statistical software (SPSS, Chicago, IL, USA). Numerical data were analyzed using a two-sided fourfold table chi-square test. A level of P < 0.05 was considered a statistically significant difference.
Footnotes
Pengfei Wu, M.D., Associate chief physician.
Conflicts of interest: None declared.
Ethical approval: The experiments have been approved by the Ethics Committee of the First Affiliated Hospital of China Medical University in China.
(Edited by Chen B/Li JT/Yang Y/Song LP)
REFERENCES
- [1].Yoneyama T, Kasuya H, Onda H, et al. Collagen type I alpha 2 (COL1A2) is the susceptible gene for intracranial aneurysms. Stroke. 2004;35(2):443–448. doi: 10.1161/01.STR.0000110788.45858.DC. [DOI] [PubMed] [Google Scholar]
- [2].Farnham JM, Camp NJ, Neuhausen SL, et al. Confirmation of chromosome 7q11 locus for predisposition to intracranial aneurysm. Hum Genet. 2004;114(3):250–255. doi: 10.1007/s00439-003-1044-z. [DOI] [PubMed] [Google Scholar]
- [3].Iwamoto H, Kiyohara Y, Fujishima M, et al. Prevalence of intracranial saccular aneurysms in a Japanese community based on a consecutive autopsy series during a 30-year observation period: the hisayama study. Stroke. 1999;30(7):1390–1395. doi: 10.1161/01.str.30.7.1390. [DOI] [PubMed] [Google Scholar]
- [4].Nakagawa T, Hashi K. The incidence and treatment of asymptomatic, unruptured cerebral aneurysms. J Neurosurg. 1994;80(2):217–223. doi: 10.3171/jns.1994.80.2.0217. [DOI] [PubMed] [Google Scholar]
- [5].Rinkel GJ, Djibuti M, Algra A, et al. Prevalence and risk of rupture of intracranial aneurysms: a systematic review. Stroke. 1998;29(1):251–256. doi: 10.1161/01.str.29.1.251. [DOI] [PubMed] [Google Scholar]
- [6].Foroud T, Sauerbeck L, Brown R, et al. Genome screen in familial intracranial aneurysm. BMC Med Genet. 2009;10(3):1186–1196. doi: 10.1186/1471-2350-10-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Onda H, Yoneyama T, Akagawa H, et al. Genetic dissection of intracranial aneurysm. Brain Nerve. 2008;60(11):1245–1260. [PubMed] [Google Scholar]
- [8].Hashikata H, Liu W, Inoue K, et al. Confirmation of an association of single-nucleotide polymorphism rs1333040 on 9p21 with familial and sporadic intracranial aneurysms in japanese patients. Stroke. 2010;41(6):1138–1144. doi: 10.1161/STROKEAHA.109.576694. [DOI] [PubMed] [Google Scholar]
- [9].Zhang G, Tu Y, Feng W, et al. Association of interleukin-6-572G/C gene polymorphisms in the Cantonese population with intracranial aneurysms. J Neurol Sci. 2011;306(1-2):94–97. doi: 10.1016/j.jns.2011.03.036. [DOI] [PubMed] [Google Scholar]
- [10].Ruigrok YM, Rinkel GJ, Wijmenga C, et al. Anticipation and phenotype in familial intracranial aneurysms. Neurol Neurosurg Psych. 2004;75(10):1436–1442. doi: 10.1136/jnnp.2003.025098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Nahed BV, Bydon M, Ozturk AK, et al. Genetics of intracranial aneurysms. Neurosurgery. 2007;60(2):213–226. doi: 10.1227/01.NEU.0000249270.18698.BB. [DOI] [PubMed] [Google Scholar]
- [12].Onda H, Kasuya H, Yoneyama T, et al. Genomewide-linkage and haplotype-association studies map intracranial aneurysm to chromosome 7q11. Am J Hum Genet. 2001;69(4):804–819. doi: 10.1086/323614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Ruigrok YM, Rinkel GJ, van’t Slot R, et al. Evidence in favor of the contribution of genes involved in the maintenance of the extracellular matrix of the arterial wall to the development of intracranial aneurysms. Hum Mol Genet. 2006;15(22):3361–3368. doi: 10.1093/hmg/ddl412. [DOI] [PubMed] [Google Scholar]
- [14].Altshuler D, Pollara VJ, Cowles CR, et al. An SNP map of the human genome generated by reduced representation shotgun sequencing. Nature. 2000;407(6803):513–516. doi: 10.1038/35035083. [DOI] [PubMed] [Google Scholar]
- [15].Chakravarti A. To a future of genetic medicine. Nature. 2001;409(6822):822–823. doi: 10.1038/35057281. [DOI] [PubMed] [Google Scholar]
- [16].Low SK, Zembutsu H, Takahashi A, et al. Impact of LIMK1, MMP2 and TNF-α variations for intracranial aneurysm in Japanese population. J Hum Genet. 2011;56(3):211–216. doi: 10.1038/jhg.2010.169. [DOI] [PubMed] [Google Scholar]
- [17].Lanfranconi S, Markus HS. COL4A1 mutations as a monogenic cause of cerebral small vessel disease: a systematic review. Stroke. 2010;41(8):e513–518. doi: 10.1161/STROKEAHA.110.581918. [DOI] [PubMed] [Google Scholar]
- [18].Ruigrok YM, Rinkel GJ. Genetics of intracranial aneurysms. Stroke. 2008;39(3):1049–1055. doi: 10.1161/STROKEAHA.107.497305. [DOI] [PubMed] [Google Scholar]
- [19].Grond-Ginsbach C, Schnippering H, Hausser I, et al. Ultrastructural connective tissue aberrations in patients with intracranial aneurysms. Stroke. 2002;33(9):2192–2196. doi: 10.1161/01.str.0000026863.51751.de. [DOI] [PubMed] [Google Scholar]
- [20].Hua T, Zhang D, Zhao YL, et al. Correlation of COL3A1 gene with type III collagen stability in intracranial aneurysm. Zhonghua Yi Xue Za Zhi. 2008;88(7):445–448. [PubMed] [Google Scholar]
- [21].Broderick JP, Viscoli CM, Brott T, et al. Major risk factors for aneurysmal subarachnoid hemorrhage in the young are modifiable. Stroke. 2003;34(6):1375–1381. doi: 10.1161/01.STR.0000074572.91827.F4. [DOI] [PubMed] [Google Scholar]
- [22].Juvela S. Risk factors for multiple intracranial aneurysms. Stroke. 2000;31(2):392–397. doi: 10.1161/01.str.31.2.392. [DOI] [PubMed] [Google Scholar]
- [23].Juvela S. Natural history of unruptured intracranial aneurysms: risks for aneurysm formation, growth, and rupture. Acta Neurochir Suppl. 2002;82:27–30. doi: 10.1007/978-3-7091-6736-6_5. [DOI] [PubMed] [Google Scholar]
- [24].McColgan P, Thant KZ, Sharma P. The genetics of sporadic ruptured and unruptured intracranial aneurysms: a genetic meta-analysis of 8 genes and 13 polymorphisms in approximately 20,000 individuals. J Neurosurg. 2010;112(4):714–721. doi: 10.3171/2009.8.JNS092. [DOI] [PubMed] [Google Scholar]
- [25].Gu YX, Chen XC, Song DL, et al. Risk factors for intracranial aneurysm in a Chinese ethnic population. Chin Med J (Engl) 2006;119(16):1359–1364. [PubMed] [Google Scholar]
- [26].Inoue K, Mineharu Y, Inoue S, et al. Search on chromosome 17 centromere reveals tnfrsf13b as a susceptibility gene for intracranial aneurysm: a preliminary study. Circulation. 2006;113(16):2002–2010. doi: 10.1161/CIRCULATIONAHA.105.579326. [DOI] [PubMed] [Google Scholar]
- [27].Nahed BV, DiLuna ML, Morgan T, et al. Hypertension, age, and location predict rupture of small intracranial aneurysms. Neurosurg. 2005;57(4):676–683. [PubMed] [Google Scholar]
- [28].Penn DL, Komotar RJ, Sander Connolly E. Hemodynamic mechanisms underlying cerebral aneurysm pathogenesis. J Neurol Sci. 2011;18(11):1435–1438. doi: 10.1016/j.jocn.2011.05.001. [DOI] [PubMed] [Google Scholar]
- [29].Krex D, König IR, Ziegler A, et al. Extended single nucleotide polymorphism and haplotype analysis of the elastin gene in Caucasians with intracranial aneurysms provides evidence for racially/ethnically based differences. Cerebrovasc Dis. 2004;18(2):104–110. doi: 10.1159/000079257. [DOI] [PubMed] [Google Scholar]
- [30].Semmler A, Linnebank M, Krex D, et al. Polymorphisms of homocysteine metabolism are associated with intracranial aneurysms. Cerebrovasc Dis. 2008;26(4):425–429. doi: 10.1159/000155638. [DOI] [PubMed] [Google Scholar]
- [31].Akiyama K, Narita A, Nakaoka H, et al. Genome-wide association study to identify genetic variants present in Japanese patients harboring intracranial aneurysms. J Hum Genet. 2010;55(10):656–661. doi: 10.1038/jhg.2010.82. [DOI] [PubMed] [Google Scholar]
- [32].State Council of the People's Republic of China. Administrative Regulations on Medical Institution 1994-09-01 [Google Scholar]
- [33].Llamas-Martínez S, Esparza-Gómez G, Campo-Trapero J, et al. Genotypic determination by PCR-RFLP of human papillomavirus in normal oral mucosa, oral leukoplakia and oral squamous cell carcinoma samples in Madrid (Spain) Anticancer Res. 2008;28(6A):3733–3741. [PubMed] [Google Scholar]


