Abstract
To attain the volumetric information of the optic radiation in normal human brains, we performed diffusion tensor imaging examination in 13 healthy volunteers. Simultaneously, we used a brain normalization method to reduce individual brain variation and increase the accuracy of volumetric information analysis. In addition, tractography-based group mapping method was also used to investigate the probability and distribution of the optic radiation pathways. Our results showed that the measured optic radiation fiber tract volume was a range of about 0.16% and that the fractional anisotropy value was about 0.53. Moreover, the optic radiation probability fiber pathway that was determined with diffusion tensor tractography-based group mapping was able to detect the location relatively accurately. We believe that our methods and results are helpful in the study of optic radiation fiber tract information.
Keywords: nerve regeneration, optic radiation, diffusion tensor imaging, diffusion tensor tractography, magnetic resonance imaging, volumetric analysis, probability map, group mapping, visualization, individual variation, neural regeneration
Introduction
The human visual system consists of two major components, sensory input organs, such as the retina in the eyes, and the visual pathway[1]. The object information that is captured through the eyes is transmitted by the retina and their axons, which are comprised of nerve fibers, to the optic chiasm[1,2]. The visual pathway continues to the lateral geniculate nucleus[1,2,3,4]. The optic radiation is a dense fiber tract that emerges from the lateral geniculate nucleus and continues to the occipital visual cortex[1,2,3,4]. Especially, the optic radiation is an important fiber structure that conveys visual information from the lateral geniculate nucleus to the primary visual cortex in the occipital lobe. In recent years, many studies have introduced the anatomical location and features of the optic radiation[1,2,3,4,5,6,7,8,9,10]. Knowing such above-mentioned characteristic information of the optic radiation before operation is important for surgery of the temporal lobe, longitudinal study of patients with optic neuritis, and evaluation of visual function in preterm infants[5,7,8,10,11,12]. Most of these studies have used diffusion tensor imaging, which is a magnetic resonance imaging technique that is used to examine the directional properties of the diffusion of water molecules[13,14,15,16,17,18,19,20]. In particular, several studies have been published on these topics related to the optic radiation, and these have described the characteristic information obtained from diffusion tensor tractography, which is derived from diffusion tensor imaging and is a robust technique that is used for visualizing and evaluating white matter fiber direction in the human brain[1,2,7,8,9,10,11,12,21]. However, these studies have focused on the anatomical characteristics of optic radiation fiber tracts in individual brains and on comparisons of the anatomical characteristics of the optic radiation fiber tracts between patient and control groups. Therefore, to the best of our knowledge, no diffusion tensor tractography studies of the volumetric information of optic radiation have been conducted without individual brain structure variation.
In the current study, we attempted to analyze the volumetric information of the optic radiation and to investigate the characteristics of the optic radiation in normal human brain with diffusion tensor tractography.
Results
Quantitative data
The values of the counted voxel numbers for the normalized optic radiation fiber tracts, and the percentage of the counted voxel numbers that were divided by whole voxel numbers in the Montreal Neurologic Institute (MNI) echo-planar imaging (EPI) template are shown in Table 1. These values represent the ratio of the optic radiation fiber tract volumetric information in both hemispheres of normal human brain.
Table 1.
The counted voxel numbers that were part of the extracted optic radiation fiber tracts, the calculated percentage, and the fractional anisotropy (FA) value for each subject
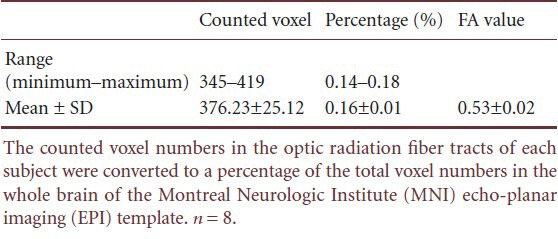
Probability map
The optic radiation probability pathway map, which represents the degree of overlapping optic radiation fiber tracts of all subjects, was overlaid onto a standard MNI EPI template (Figure 1).
Figure 1.
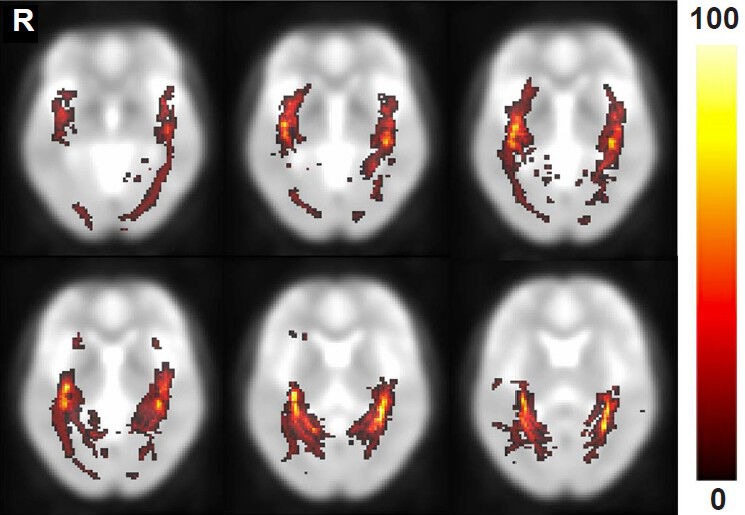
The results of the optic radiation probability pathway map in healthy subjects.
The results were overlaid and shown on the transverse plane of the Montreal Neurologic Institute (MNI) echo-planar imaging (EPI) tem-plate. The hot color scale indicates the proportional degree of overlap for all subjects and the probability of a voxel being part of the optic radiation fiber tract pathway. R: Right.
Discussion
In the current study, we analyzed the volumetric information of optic radiation fiber tracts in the human brain with brain normalization methods with a standard brain template image. In addition, we visualized a probability fiber pathway map in order to investigate the probability and distribution of the optic radiation pathway. Since the introduction of diffusion tensor imaging and fiber tracking methods, these techniques have been widely used to elucidate the anatomical structure and to perform quantitative analyses of neural fiber tracts at the subcortical level[16,17,19,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38]. Many studies have been conducted on the optic radiation, although the majority of these studies have focused on examining the anatomical structures or landmarks of surgical planning in patients[3,4,9,11,12,21,25]. Schoth et al.[21] have reported anisotropy changes in blind humans compared to healthy control subjects with diffusion tensor tractography. Winston et al.[4] have described the use of optic radiation fiber tractography in epilepsy surgical planning and anterior temporal lobe resections. Ciccarelli et al.[7] have indicated anatomical changes after optic neuritis, and they showed that optic radiation fiber tract location was different between patient and normal control groups. Moreover, some quantitative fiber tracking analyses and approaches have been used in patients with arteriovenous malformations and premature newborns in order to evaluate the optic radiation fiber tracts[5,6,26]. In the quantitative analyses, FA values, diffusivity indices, and left-to-right asymmetry indices were measured in these patients.
In this study, we measured the volumetric information of the optic radiation fiber tracts in order to determine the proportion of optic radiation fiber tracts in the whole brain of the normal human brain. The extracted optic radiation fiber tracts were normalized to a standard brain template, the MNI EPI template, in order to remove the individual differences of all of the subjects and to allow for a direct comparison of our volumetric analysis results under the same conditions. The results of this study demonstrated that the optic radiation fiber tract had a tiny volume of information that was less than 1% of visual performance compared to the volume information of the whole brain. Moreover, the optic radiation probability fiber pathway that was determined with diffusion tensor tractography-based group mapping was able to detect location with relative accuracy.
This study had some limitations. First, a limited number of subjects were enrolled in this study. Based on our results, the 13 healthy subjects exhibited a similar tendency for the percentage of counted voxel numbers. However, these findings are difficult to generalize to our results. In a future study, we plan to study a larger number of subjects. Second, the setting of the region of interest is a user-dependent process. This problem can be solved through fiber tracking that is combined with functional magnetic resonance imaging examinations of visual stimulation in a future study. Third, we evaluated only the optic radiation fiber tract of all of the optic fibers in the human brain. Moreover, the Fuzzy art with Add Clustering Technique (FACT) algorithm, which was used in this study, is used for limited fiber tracking and reconstruction. The FACT algorithm was considered for determining the dominant fiber direction, and the largest eigenvalue components in each voxel were used as an indicator of fiber orientation. Therefore, a fiber-crossing region or a voxel that was eligible for various fiber directions might have resulted in some erroneous points in the FACT algorithm-based fiber tracking. Further study is needed with other fiber tracking and reconstruction algorithms, such as probabilistic approaches or with an advanced fast marching algorithm. The probabilistic fiber tracking algorithm allows for the direction of the tensor in the voxel to be multidirectional, but it is not limited to a dominant direction, as is the FACT algorithm. The advanced fast marching takes into account all of the information that is contained in the diffusion tensor[2]. Thereby, every tensor is classified as linear, planar, or spherical ellipsoid[2,27]. In other words, the method that was described above is able to evaluate brain areas that contain fiber-crossing regions, such as the optic chiasm. They have important implications for more accurate fiber tracking for all of the optic nerve that is not limited to the optic radiation, which is a topic for future research.
In conclusion, we analyzed and measured the volume information of the optic radiation fiber tract in the normal human brain and found that the optic radiation fiber tract had a relatively small range of volume information compared to that of the whole brain. To the best of our knowledge, this is the first volumetric analysis study of the optic radiation fiber tract with diffusion tensor tractography and of whole-brain volume information. We believe that our approaches provide preliminary data for researchers who are studying treatments for patients with diseases that are related to the visual pathway in the brain.
Subjects and Methods
Design
An observational neuroimaging study.
Time and setting
This experiment was performed at the Department of Physical Medicine and Rehabilitation, Yeungnam University Hospital, Republic of Korea in June 2009.
Subjects
Thirteen healthy subjects (men, 6; age, 35 ± 2.16 years) were recruited into this study through advertisements. They had no previous history of neurological disease, optic nerve pathology, head injury, or physical disease. All subjects understood the purpose of the study and provided written informed consents prior to their participation. This study was approved by the Institutional Review Board of Yeungnam University Hospital in Republic of Korea.
Methods
Diffusion tensor imaging acquisition
All diffusion tensor imaging datasets were acquired with a 1.5-T magnetic resonance imaging system (Gyroscan Intera, Philips Healthcare, Best, the Netherlands) with a six-channel phased-array sensitivity-encoding (SENSE) head coil by using single-shot echo-planar imaging (EPI) with parallel acquisition in the transverse plane. The imaging parameters were as follows: repetition time/echo time, 10,726/76 ms; matrix, 128 × 128; field of view, 221 mm; slice thickness, 2.3 mm; and reduction factor for SENSE, 2. Diffusion weighting was applied along 32 non-collinear diffusion-sensitizing gradients with a b-value of 1,000 s/mm2. We obtained 63–67 contiguous transverse slices that covered the entire brain with no slice gaps. During the acquisition, radiologists routinely checked for gross head movement or other motion in real time[25].
Diffusion tensor imaging analysis
Eddy current corrections of the diffusion tensor imaging data were performed with the tool in FSL 4.0.1 (Analysis Group, FMRIB, Oxford, UK) with a 12-parameter affine registration[4,5,12,25,39]. Each diffusion-weighted image was registered to non-diffusion weighted images (b = 0). The diffusion tensor imaging datasets were analyzed with DtiStudio 3.0.3 (Department of Radiology, Johns Hopkins University, Baltimore, MD, USA), which was based on the fiber assignment by the continuous tracking (FACT) algorithm[22,40,41,42,43]. Propagation in each fiber tract was terminated if a voxel with a fractional anisotropy (FA) value less than 0.25 was reached or if the turning angles of 2 consecutive vectors were over 70° during tracking[10]. An FA threshold of 0.25–0.35 for fiber tract reconstruction has been recommended by Mori et al. and Stieltjes et al.[10,23,44]. A relatively large angle threshold was used so that the optic radiation course in acute angles could be examined[10,26]. The region of interest was manually drawn in both lateral geniculate nucleus on the transverse plane at the level of the transition from the posterior limb of the internal capsule to the cerebral peduncle on a color-coded FA map, and the volume of the region of interest was standardized for all subjects (9 voxels)[3,7]. The color-coded FA map showed the fiber pathway directions with 3 colors as follows: red (left-right direction), green (anterior-posterior), and blue (superior-inferior)[45,46].
Quantitative measurements and probability map of the optic radiation
After both optic radiation fiber tracts were extracted with the region of interest described above, we performed quantitative measurements and created a probability pathway map of the optic radiation. These methods were conducted based on the brain normalization method in order to avoid individual brain variation. The procedure is shown in Figure 2 and is described as follows. (1) Each subject's non-diffusion image (b = 0 image) was co-registered to the standard Montreal Neurologic Institute (MNI) space with an EPI template with SPM2 (Wellcome Department of Cognitive Neurology, London, UK). (2) The transformation matrices for each subject that were created with the coregistration process in (1) were then applied to the extracted optic radiation fiber tracts of each subject for normalization to the MNI space. (3) For the quantitative measurements, the voxels that the optic radiation fiber tract passed through in the normalized brain of each subject were counted with ImageJ (US National Institutes of Health, Bethesda, MD, USA) software. We calculated the percentage of the optic radiation fiber tracts with the counted voxel numbers divided by the whole-brain voxel numbers in the MNI EPI template. (4) In order to assess optic radiation fiber tracts quantitatively, the FA values in the extracted optic radiation fiber tracts for each subject were measured. (5) Finally, each individual normalized optic radiation was averaged pixel-by-pixel and overlaid on the MNI EPI template in order to obtain and investigate the optic radiation probability pathway map with MRIcro (Chris Rorden, USA, http://www.mricro.com) software.
Figure 2.
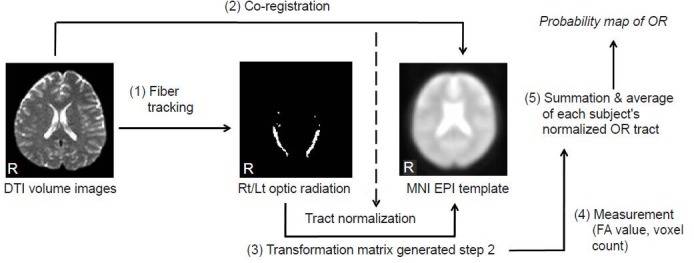
The procedure for the quantitative measurement and probability pathway map reconstruction of the optic radiation (OR) fiber tract.
The OR fiber tracts from all subjects were extracted with diffusion tensor imaging (DTI) datasets, and brain normalization processes were per-formed in order to reduce individual variation and to allow for a comparison of the results under the same conditions without individual variation and to increase accuracy. The Montreal Neurologic Institute (MNI) echo-planar imaging (EPI) template was used to normalize the individual brains and extracted optic radiation fiber tracts. The volumetric analysis was performed with a voxel-count technique. The voxels through which the optic radiation fiber tract passed were counted and a percentage of their counted numbers according to whole-brain voxel numbers in the MNI EPI template was calculated. With the extracted optic radiation fiber tracts, the fractional anisotropy (FA) values were calculated, and a probability pathway map was acquired by averaging the normalized data for each subject for the OR fiber tract. Rt/Lt: Right/left; R: right.
Footnotes
Conflicts of interest: None declared.
Peer review: This study was designed to provide information about the average size and degree of diffusion anisotropy of the optic radiation in addition to a probability map describing the likelihood of optic radiation pathway. The proposed analysis was applied to 13 healthy subjects with a mean age of 35 years and the results were demonstrated according to the analysis. This type of analysis and results are considered to be an addition to the field of diffusion tensor imaging because of inaccurate diffusion tensor imaging measurement, approximate tensor model and relatively low resolution diffusion tensor imaging. The difficulties are to verify the segmented optic radiation using various tractography segmentation algorithms. Quantitative measurements of optic radiation probability map and the estimated optic radiation size and anisotropy can help identify healthy subjects and be used as a reference for comparing healthy to pathological subjects.
Copyedited by Ng WH, El-Rafei A, Li CH, Song LP, Liu WJ, Zhao M
References
- [1].El-Rafei A, Engelhorn T, Wärntges S, et al. A framework for voxel-based morphometric analysis of the optic radiation using diffusion tensor imaging in glaucoma. Magn Reson Imaging. 2011;29:1076–1087. doi: 10.1016/j.mri.2011.02.034. [DOI] [PubMed] [Google Scholar]
- [2].Staempfli P, Rienmueller A, Reischauer C, et al. Reconstruction of the human visual system based on diffusion tensor imaging fiber tracking. J Magn Reson Imaging. 2007;26:886–893. doi: 10.1002/jmri.21098. [DOI] [PubMed] [Google Scholar]
- [3].Sherbondy AJ, Dougherty RF, Napel S, et al. Identifying the human optic radiation using diffusion imaging and fiber tractography. J Vis. 2008;8:12.1–1211. doi: 10.1167/8.10.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Winston GP, Mancini L, Stretton J, et al. Diffusion tensor imaging tractography of the optic radiation for epilepsy surgical planning: a comparison of two methods. Epilepsy Res. 2011;97:124–132. doi: 10.1016/j.eplepsyres.2011.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Bassi L, Ricci D, Volzone A, et al. Probabilistic diffusion tractography of the optic radiations and visual function in preterm infants at term equivalent age. Brain. 2008;131:573–582. doi: 10.1093/brain/awm327. [DOI] [PubMed] [Google Scholar]
- [6].Berman JI, Glass HC, Miller SP, et al. Quantitative fiber tracking analysis of the optic radiation correlated with visual performance in premature newborns. Am J Neuroradiol. 2009;30:120–124. doi: 10.3174/ajnr.A1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Ciccarelli O, Toosy AT, Hickman SJ, et al. Optic radiation changes after optic neuritis detected by tractography-based group mapping. Hum Brain Mapp. 2005;25:308–316. doi: 10.1002/hbm.20101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Kolbe S, Bajraszewski C, Chapman C, et al. Egan, Diffusion tensor imaging of the optic radiations after optic neuritis. Hum Brain Mapp. 2012;33:2047–2061. doi: 10.1002/hbm.21343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Nilsson D, Starck G, Ljungberg M, et al. Intersubject variability in the anterior extent of the optic radiation assessed by tractography. Epilepsy Res. 2007;77:11–16. doi: 10.1016/j.eplepsyres.2007.07.012. [DOI] [PubMed] [Google Scholar]
- [10].Yamamoto A, Miki Y, Urayama S, et al. Diffusion tensor fiber tractography of the optic radiation: analysis with 6-, 12-, 40-, and 81-directional motion-probing gradients, a preliminary study. Am J Neuroradiol. 2007;28:92–96. [PMC free article] [PubMed] [Google Scholar]
- [11].Powell HW, Parker GJ, Alexander DC, et al. MR tractography predicts visual field defects following temporal lobe resection. Neurology. 2005;65:596–599. doi: 10.1212/01.wnl.0000172858.20354.73. [DOI] [PubMed] [Google Scholar]
- [12].Winston GP, Daga P, Stretton J, et al. Optic radiation tractography and vision in anterior temporal lobe resection. Ann Neurol. 2012;71:334–341. doi: 10.1002/ana.22619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Basser PJ, Mattiello J, LeBihan D. Estimation of the effective self-diffusion tensor from the NMR spin echo. J Magn Reson B. 1994;103:247–254. doi: 10.1006/jmrb.1994.1037. [DOI] [PubMed] [Google Scholar]
- [14].Basser PJ, Mattiello J, LeBihan D. MR diffusion tensor spectroscopy and imaging. Biophys J. 1994;66:259–267. doi: 10.1016/S0006-3495(94)80775-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Beaulieu C. The basis of anisotropic water diffusion in the nervous system-a technical review. NMR Biomed. 2002;15:435–455. doi: 10.1002/nbm.782. [DOI] [PubMed] [Google Scholar]
- [16].Mori S, Crain BJ, Chacko VP, et al. Three-dimensional tracking of axonal projections in the brain by magnetic resonance imaging. Ann Neurol. 1999;45:265–269. doi: 10.1002/1531-8249(199902)45:2<265::aid-ana21>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- [17].Mori S, van Zijl PC. Fiber tracking: principles and strategies-a technical review. NMR Biomed. 2002;15:468–480. doi: 10.1002/nbm.781. [DOI] [PubMed] [Google Scholar]
- [18].Pierpaoli C, Basser PJ. Toward a quantitative assessment of diffusion anisotropy. Magn Reson Med. 1996;36:893–906. doi: 10.1002/mrm.1910360612. [DOI] [PubMed] [Google Scholar]
- [19].Pierpaoli C, Jezzard P, Basser PJ, et al. Diffusion tensor MR imaging of the human brain. Radiology. 1996;201:637–648. doi: 10.1148/radiology.201.3.8939209. [DOI] [PubMed] [Google Scholar]
- [20].Wakana S, Jiang H, Nagae-Poetscher LM, et al. Fiber tract-based atlas of human white matter anatomy. Radiology. 2004;230:77–87. doi: 10.1148/radiol.2301021640. [DOI] [PubMed] [Google Scholar]
- [21].Schoth F, Burgel U, Dorsch R, et al. Diffusion tensor imaging in acquired blind humans. Neurosci Lett. 2006;398:178–182. doi: 10.1016/j.neulet.2005.12.088. [DOI] [PubMed] [Google Scholar]
- [22].Jiang H, van Zijl PC, Kim J, et al. DtiStudio: resource program for diffusion tensor computation and fiber bundle tracking. Comput Methods Programs Biomed. 2006;81:106–116. doi: 10.1016/j.cmpb.2005.08.004. [DOI] [PubMed] [Google Scholar]
- [23].Mori S, Kaufmann WE, Davatzikos C, et al. Imaging cortical association tracts in the human brain using diffusion-tensor-based axonal tracking. Magn Reson Med. 2002;47:215–223. doi: 10.1002/mrm.10074. [DOI] [PubMed] [Google Scholar]
- [24].White ML, Zhang Y. Three-tesla diffusion tensor imaging of Meyer's loop by tractography, color-coded fractional anisotropy maps, and eigenvectors. Clin Imaging. 2010;34:413–417. doi: 10.1016/j.clinimag.2009.11.010. [DOI] [PubMed] [Google Scholar]
- [25].Yogarajah M, Focke NK, Bonelli S, et al. Defining Meyer's loop-temporal lobe resections, visual field deficits and diffusion tensor tractography. Brain. 2009;132:1656–1668. doi: 10.1093/brain/awp114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Okada T, Miki Y, Kikuta K, et al. Diffusion tensor fiber tractography for arteriovenous malformations: quantitative analyses to evaluate the corticospinal tract and optic radiation. Am J Neuroradiol. 2007;28:1107–1113. doi: 10.3174/ajnr.A0493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Westin CF, Maier SE, Mamata H, et al. Processing and visualization for diffusion tensor MRI. Med Image Anal. 2002;6:93–108. doi: 10.1016/s1361-8415(02)00053-1. [DOI] [PubMed] [Google Scholar]
- [28].Seo JP, Lee MY, Kwon YH, et al. Delayed gait recovery in a stroke patient. Neural Regen Res. 2013;8:1514–1518. doi: 10.3969/j.issn.1673-5374.2013.16.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Li J, Chen X, Zhang J, et al. Intraoperative diffusion tensor imaging predicts the recovery of motor dysfunction after insular lesions. Neural Regen Res. 2013;8:1400–1409. doi: 10.3969/j.issn.1673-5374.2013.15.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Kuhnt D, Bauer MH, Sommer J, et al. Optic radiation fiber tractography in glioma patients based on high angular resolution diffusion imaging with compressed sensing compared with diffusion tensor imaging-initial experience. PLoS One. 2013;8:e70973. doi: 10.1371/journal.pone.0070973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Surova Y, Szczepankiewicz F, Latt J, et al. Assessment of global and regional diffusion changes along white matter tracts in Parkinsonian disorders by MR tractography. PLoS One. 2013;8:e66022. doi: 10.1371/journal.pone.0066022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Seo JP, Jang SH. Traumatic thalamic injury demonstrated by diffusion tensor tractography of the spinothalamic pathway. Brain Inj. 2013;27:749–753. doi: 10.3109/02699052.2013.771794. [DOI] [PubMed] [Google Scholar]
- [33].Cauley KA, Filippi CG. Diffusion-tensor imaging of small nerve bundles: cranial nerves, peripheral nerves, distal spinal cord, and lumbar nerve roots- clinical applications. Am J Roentgenol. 2013;201:W326–335. doi: 10.2214/AJR.12.9230. [DOI] [PubMed] [Google Scholar]
- [34].Yeo SS, Jang SH. Corticospinal tract recovery in a patient with traumatic transtentorial herniation. Neural Regen Res. 2013;8:469–473. doi: 10.3969/j.issn.1673-5374.2013.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].El-Rafei A, Engelhorn T, Warntges S, et al. Glaucoma classification based on visual pathway analysis using diffusion tensor imaging. Magn Reson Imaging. 2013;31:1081–1091. doi: 10.1016/j.mri.2013.01.001. [DOI] [PubMed] [Google Scholar]
- [36].Seo JP, Choi BY, Chang CH, et al. Diffusion tensor imaging findings of optic radiation in patients with putaminal hemorrhage. Eur Neurol. 2013;69:236–241. doi: 10.1159/000345271. [DOI] [PubMed] [Google Scholar]
- [37].Wang S, Qiu D, So KF, et al. Radiation induced brain injury: assessment of white matter tracts in a pre-clinical animal model using diffusion tensor MR imaging. J Neurooncol. 2013;112:9–15. doi: 10.1007/s11060-012-1031-0. [DOI] [PubMed] [Google Scholar]
- [38].Yeo SS, Kim SH, Kim OL, et al. Optic radiation injury in a patient with traumatic brain injury. Brain Inj. 2012;26:891–895. doi: 10.3109/02699052.2012.661119. [DOI] [PubMed] [Google Scholar]
- [39].Smith SM, Jenkinson M, Woolrich MW, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl 1):S208–219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- [40].Krishnan AP, Asher IM, Davis D, et al. Evidence that MR diffusion tensor imaging (tractography) predicts the natural history of regional progression in patients irradiated conformally for primary brain tumors. Int J Radiat Oncol Biol Phys. 2008;71:1553–1562. doi: 10.1016/j.ijrobp.2008.04.017. [DOI] [PubMed] [Google Scholar]
- [41].Kim CH, Koo BB, Chung CK, et al. Thalamic changes in temporal lobe epilepsy with and without hippocampal sclerosis: a diffusion tensor imaging study. Epilepsy Res. 2010;90:21–27. doi: 10.1016/j.eplepsyres.2010.03.002. [DOI] [PubMed] [Google Scholar]
- [42].Lima M, Yamamoto A, Brion V, et al. Reduced-distortion diffusion MRI of the craniovertebral junction. Am J Neuroradiol. 2012;33:1321–1325. doi: 10.3174/ajnr.A2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Wiltshire K, Concha L, Gee M, et al. Corpus callosum and cingulum tractography in Parkinson's disease. Can J Neurol Sci. 2010;37:595–600. doi: 10.1017/s0317167100010751. [DOI] [PubMed] [Google Scholar]
- [44].Stieltjes B, Kaufmann WE, van Zijl PC, et al. Diffusion tensor imaging and axonal tracking in the human brainstem. Neuroimage. 2001;14:723–735. doi: 10.1006/nimg.2001.0861. [DOI] [PubMed] [Google Scholar]
- [45].Lee SK, Kim DI, Kim J, et al. Diffusion-tensor MR imaging and fiber tractography: a new method of describing aberrant fiber connections in development CNS anomalies. Radiographics. 2005;25:53–65. doi: 10.1148/rg.251045085. [DOI] [PubMed] [Google Scholar]
- [46].Huang H, Yamamoto Akria, Hossain MA, et al. Quantitative Cortical Mapping of Fractional Anisotropy in Developing Rat Brains. J Neurosci. 2008;28:1427–1433. doi: 10.1523/JNEUROSCI.3194-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]


