Abstract
Previous studies have demonstrated that doublecortin-positive immature neurons exist predominantly in the superficial layer of the cerebral cortex of adult mammals such as guinea pigs, and these neurons exhibit very weak properties of self-proliferation during adulthood under physiological conditions. To verify whether environmental enrichment has an impact on the proliferation and maturation of these immature neurons in the prefrontal cortex of adult guinea pigs, healthy adult guinea pigs were subjected to short-term environmental enrichment. Animals were allowed to play with various cognitive and physical stimulating objects over a period of 2 weeks, twice per day, for 60 minutes each. Immunofluorescence staining results indicated that the number of doublecortin-positive cells in layer II of the prefrontal cortex was significantly increased after short-term environmental enrichment exposure. In addition, these doublecortin-positive cells co-expressed 5-bromo-2-deoxyuridine (a marker of cell proliferation), c-Fos (a marker of cell viability) and NeuN (a marker of mature neurons). Experimental findings showed that short-term environmental enrichment can induce proliferation, activation and maturation of doublecortin-positive cells in layer II of the prefrontal cortex of adult guinea pigs.
Keywords: nerve regeneration, neurogenesis, prefrontal cortex, neocortex, guinea pig, doublecortin protein, cell proliferation, neurons, 5-bromodeoxyuridine, NSFC grant, neural regeneration
Introduction
Constitutive endogenous neurogenesis is known to exist throughout life in the subgranular zone of the hippocampal dentate gyrus and the forebrain subventricular zone of the lateral ventricles of the adult mammalian brain[1]. Moreover, new neurons in the adult subgranular zone may play a role in the construction, as well as cognitive function, of the hippocampus[2]. However, it is still controversial whether adult neurogenesis takes place in the neocortex of mammals[3,4]. Previous arguments suggested that there was no obvious neurogenesis in the neocortex of adult mammals under physiological conditions[3,5]. Nonetheless, accumulating evidence indicates that a certain amount of cells located around superficial layers of the cortex express immature neuronal markers including doublecortin, which are usually expressed by developing neurons undergoing differentiation and maturation[6,7,8,9,10]. Although some scholars proposed that these cells were generated prenatally[11,12] or derived from the subventricular zone[5], our previous works demonstrated that a small number of doublecortin-positive cells in the cerebrum of adult guinea pigs colocalized with other endogenous nuclear proliferative molecules, as well as the incorporation of bromodeoxyuridine[8]. Growing evidence implies that a putative neurogenic niche probably exists in the neocortex of adult mammals[13,14,15]. However, new neurons are generated at very low levels in healthy adult animals[13,16]. Once the environment changes, such as the reappearance of certain factors during the period of development[17] or under various pathological conditions[13,15,18,19], adult neocortical neurogenesis might be accelerated.
Environmental enrichment exposure is a well-established model for increasing physiological stimulation of the nervous system, which aims to provide complicated sensory and motor stimuli, thus improving the opportunity for learning and memory[20,21,22]. Many studies have shown that exposure to environmental enrichment can induce neurogenesis of the hippocampal region, thus improving learning and memory[21,23,24,25]. We hypothesize that the newborn neurons in our experiment may also be involved in the learning and memory process associated with the prefrontal cortex. To date, few reports have investigated the regulation of neurogenesis in the neocortex of adult mammals under environmental enrichment conditions. The prefrontal cortex is the advanced nervous center of learning and memory in mammals, and is characterized as the manifested brain region of memorizing activity[26,27]. It has been widely accepted that the hippocampus[28,29] and prefrontal cortex[30,31] are essential components in the processing of novel environment exploration. Our previous studies demonstrated that doublecortin-positive immature neurons exist predominantly in layer II of the cerebral cortex of adult mammals such as guinea pigs, cats and monkeys[6,7,8]. These neurons exhibited very weak properties of self-proliferation during adulthood under physiological conditions[8,32]. As mentioned above, exposure to environmental enrichment induces hippocampal neurogenesis and improves learning and memory in adult animals. Whether environmental enrichment exposure can regulate doublecortin-positive immature neurons (possibly newly generated neurons) existing predominantly in layer II of the prefrontal cortex of adult mammals needs further exploration. Accordingly, the present study was designed to identify whether doublecortin-positive cells in layer II of the prefrontal cortex of adult guinea pigs (including proliferation, activation and maturation) are affected by environmental enrichment exposure, in an effort to explore the regulatory effect of adult neocortical neurogenesis under physiological stimulation.
First, we used the cell proliferation marker bromodeoxyuridine to double label newborn doublecortin-positive cells and found that the number of new neurons increased. The addition of new neurons following environmental enrichment led to anatomical modification of new circuits. However, it remains unclear if this anatomical modification results in the functional alteration of the prefrontal cortex. If the number of activated new neurons was not affected, the prefrontal cortex might not be functionally altered, despite anatomical modification. Next, we addressed whether neurons were activated by environmental enrichment. We examined the expression of c-Fos protein to determine the activation of neurons. c-Fos is one kind of phosphorylated protein coded by the immediate early gene c-fos. The level of c-Fos in cells is low under normal conditions. All sorts of stimuli either intra- or extra-cellular can accelerate c-fos to synthesize the phosphorylated protein c-Fos. The detection of c-Fos in neurons is often considered as an indicator of cell activation[33,34]. Finally, we examined the maturation of these doublecortin-positive cells through colocalization with NeuN.
Results
Quantitative analysis of experimental animals
Forty-eight guinea pigs were randomly and equally divided into two groups: an environmental enrichment group (exposure to environmental enrichment for 2 days) and a control group (normal feeding). All animals were injected intraperitoneally with bromodeoxyuridine (50 mg/kg; Sigma-Aldrich, St. Louis, MO, USA) once daily for 4 consecutive days, beginning on the 11th day of environmental enrichment exposure. After bromodeoxyuridine administration was over, all animals were sacrificed at 0, 10 or 20 days for subsequent trials. There were eight guinea pigs at each time point. Forty-eight guinea pigs were involved in the results analysis. No animals died during experimentation and all animals were used in the final analysis.
The number of doublecortin-positive cells in layer II of the medial prefrontal cortex of adult guinea pigs increased after environmental enrichment
To evaluate the overall impact of environmental enrichment on the morphology and survival of doublecortin-positive cells in the brain, we measured the expression of doublecortin using immunohistochemistry. All labeled profiles were confirmed by 4′, 6-diamino-2-phenylindole counterstaining.
The vast majority of doublecortin-positive cells were localized to layer II of the prefrontal cortex (Figure 1A–M) of all adult guinea pigs in this study. As previously described[10], these doublecortin-positive cells exhibited a heterogeneous morphology. The cell bodies exhibited various shapes, including round, oval, bipolar, multipolar, pyramidal-like and irregular. Neurites extending from the cell bodies were also heavily labeled. There was no difference in cell morphology between the environmental enrichment and control groups at each time point.
Figure 1.
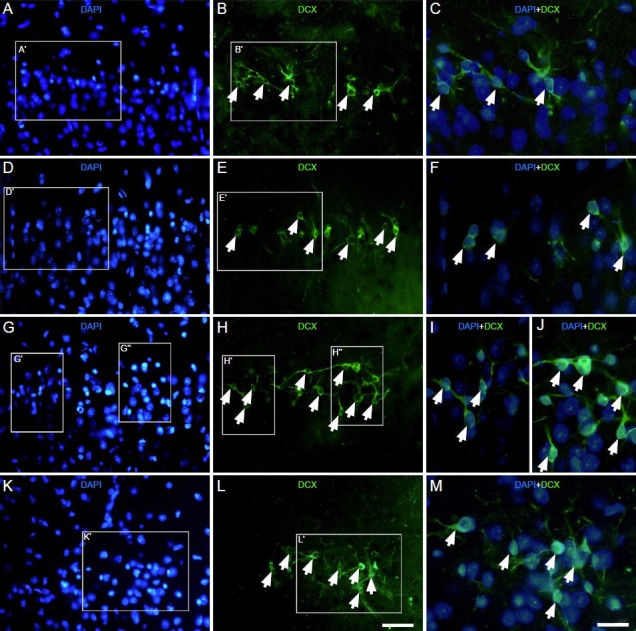
Effect of environmental enrichment exposure on doublecortin-positive cells around layer II of the medial prefrontal cortex of guinea pigs (immunofluorescence staining).
Images of doublecortin (green) and 4′,6-diamino-2-phenylindole (blue) staining in the control group (A-C) at 0 day (D-F), 10 days (G-J) and 20 days (K-M) post-environmental enrichment. (C, F, I, J, M) Merged images of frames A′–B′, D′–E′, G′–H′, G”–H” and K′–L′ in A–B, D–E, G–H and K–L, respectively. White arrowheads indicate doublecortin-positive cells. The magnification of the objective lens was 10 × in A, B, D, E, G, H, K, L and 20 × in C, F, I, J, M. Samples were visualized via fluorescent microscopy. Scale bar = 100 μm in A, B, D, E, G, H, K, L; 50 μm in C, F, I, J and M.
Next, we assessed whether the number of doublecortin-positive cells in layer II of the medial prefrontal cortex was affected by environmental enrichment. The number of doublecortin-positive cells in each field was 15.0 ± 0.7, 16.5 ± 0.7 and 12.0 ± 0.6 after 0, 10, and 20 days of environmental enrichment, respectively. Compared to the control group (11.2 ± 0.3 at 0 day, 11.0 ± 0.8 at 10 days and 10.8 ± 0.2 at 20 days), the numbers increased to 33.7% (P < 0.001), 49.9% (P < 0.001) and 11.5% (P < 0.01) at 0, 10 and 20 days, respectively, in the environmental enrichment group (Figure 2). The number of doublecortin-positive cells in the environmental enrichment group showed significant differences at different time points (P < 0.01; Figure 2), while there was no obvious difference in the control group.
Figure 2.

Effect of environmental enrichment on the number of doublecortin (DCX)-positive cells in layer II of the medial prefrontal cortex of guinea pigs.
Data are represented as the number of DCX-positive cells in each high-power field. X axis is the survival time and Y axis is the mean number of DCX-positive cells in each high-power field (× 40). The difference between the environmental enrichment group and the con-trol group at the same time point was compared using unpaired t-test, while the difference between the environmental enrichment group at different survival times was compared using one-way analysis of vari-ance and Duncan's multiple range test. Data are expressed as mean ± SD of eight guinea pigs in each group at each time point. aP < 0.001, vs. control group at the same survival time point (0 and 10 days); bP < 0.01, vs. control group at the same survival time point (20 days); cP < 0.01, vs. environmental enrichment group at 0 day; dP < 0.01, vs. environ-mental enrichment group at 10 days.
The number of bromodeoxyuridine/doublecortin double labeled cells increased in layer II of the medial prefrontal cortex of adult guinea pigs after environmental enrichment
To determine if these cells were newly born after environmental enrichment, we measured the co-expression of doublecortin with bromodeoxyuridine, which labels all newly dividing cells, at various time points. Double-labeled profiles were confirmed by precise colocalization of bromodeoxyuridine with 4′,6-diamino-2-phenylindole nuclear labeling.
The results indicated that few bromodeoxyuridine/doublecortin double labeled cells were detected in layer II of the medial prefrontal cortex in the control group (Figure 3A–C). A small number of bromodeoxyuridine/doublecortin double labeled cells was observed in the environmental enrichment group at 0 and 10 days (Figure 3D–I). The percentage of double labeled cells to doublecortin-positive cells at 0 and 10 days post-environmental enrichment was significantly higher than that in the control group (P < 0.05; Figure 4). In contrast, there was no significant difference in the percentage of cells between the environmental enrichment and control groups at 20 days (P > 0.05; Figure 4).
Figure 3.
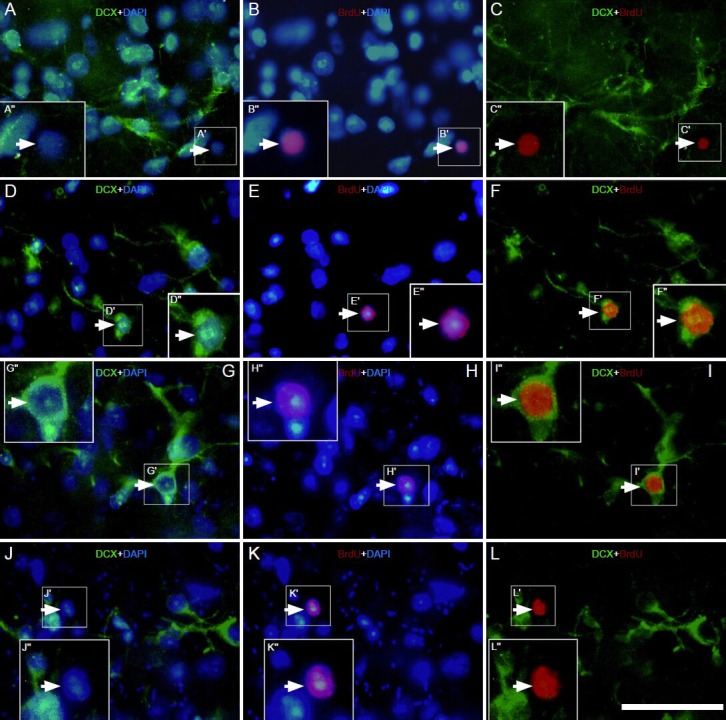
Effect of environmental enrichment on bromodeoxyuridine (BrdU)/doublecortin (DCX) double-positive cells in layer II of the medial prefrontal cortex of guinea pigs (immunofluorescence staining).
Images immunostained with DCX (green), BrdU (red) and 4′,6-diamino-2-phenylindole (blue) showing the expression of BrdU/DCX double la-beled cells in the control group at (A–C) 0 days (D–F), 10 day (G–I) and 20 day (J–L) post-environmental enrichment. The big square insert (-”) is a high magnification view of the small frame (-′) in each image. The magnification of the objective lens was 20 × in A–L. The arrowheads indicate BrdU/DCX double labeled cells; the arrows indicate BrdU-positive cells without DCX colocalization. Samples were visualized via fluorescent mi-croscopy (Nikon, Eclipse 80i). Scale bar = 25 μm.
Figure 4.
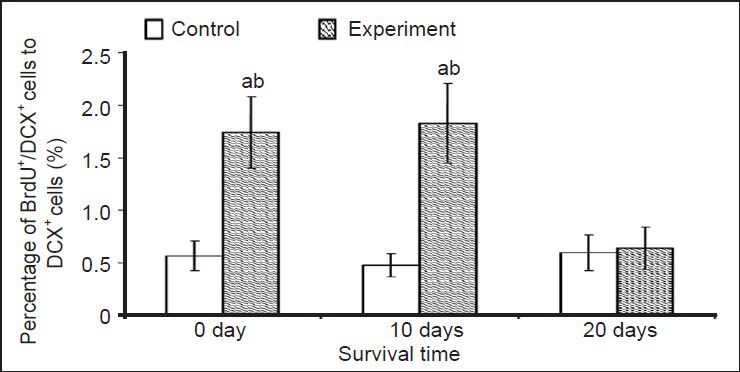
Effect of environmental enrichment on the ratio of bromodeoxyuridine (BrdU)/doublecortin (DCX) double-positive cells in layer II of the medial prefrontal cortex of guinea pigs.
Data are represented as the ratio of BrdU/DCX double-positive cells to DCX-positive cells. The difference between the environmental enrich-ment exposure group and control group at the same time point was compared using unpaired t-test. Data are expressed as mean ± SD of eight guinea pigs in each group at each time point (one-way analysis of variance and Duncan's multiple range test). aP < 0.05, vs. control group at the same survival time point; bP < 0.05, vs. environmental enrich-ment exposure group at 20 days.
Activation of doublecortin-positive cells was induced in layer II of the medial prefrontal cortex of adult guinea pigs after environmental enrichment
Levels of the intracellular protein c-Fos immediately increased at 0 day and remained elevated at 20 days post-environmental enrichment when compared to the control group (Figure 5A–L). The percentages of c-Fos/doublecortin double labeled cells to doublecortin-positive cells in the environmental enrichment group at 0, 10 and 20 days was significantly increased when compared to the control group (P < 0.01; Figure 6). There were also significant differences in the environmental enrichment exposure group at different time points, but no difference was found in the control group (P < 0.01; Figure 6).
Figure 5.
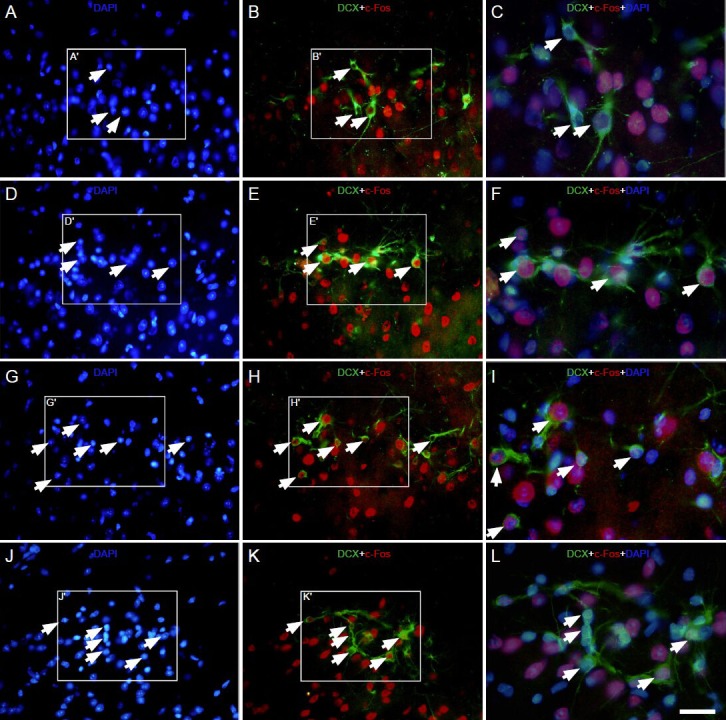
Effect of environmental enrichment exposure on c-Fos/doublecortin double-positive cells in layer II of the medial prefrontal cortex of guinea pigs (immunofluorescence staining).
Images immunostained with doublecortin (green), c-Fos (red) and DAPI (blue) showing expression of c-Fos/doublecortin double-positive cells in the control group (A–C) at 0 day (D–F), 10 days (G–I) and 20 days (J–L) post-environmental enrichment. (C, F, I, L) The merged high mag-nification images of frames A′–B′, D′–E′, G′–H′ and J′–K′ in A–B, D–E, G–H and J–K, respectively. The arrowheads indicate c-Fos/doublecortin double labeled cells; the arrows indicate doublecortin-positive cells without c-Fos colocalization. The magnification of the objective lens was 10 × in A,B, D, E, G, H, J, K and 20 × in C, F, I, L.
Samples were visualized via fluorescent microscopy (Nikon, Eclipse 80i). Scale bar = 50 μm in C, F, I, L; 100 μm in A, B, D, E, G, H, J, K.
Figure 6.
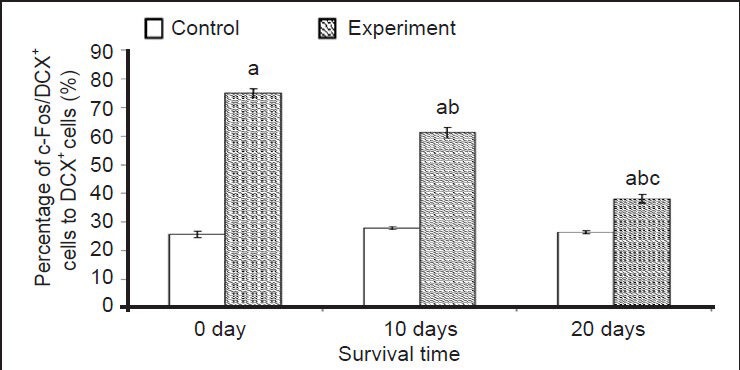
Effect of environmental enrichment exposure on the ratio of c-Fos/doublecortin (DCX) double-positive cells in layer II of the medial prefrontal cortex of guinea pigs.
Data are represented as the ratio of c-Fos/DCX double-positive cells to DCX-positive cells. The difference between the environmental enrich-ment exposure group and the control group at the same time point was compared using the unpaired t-test. Data are expressed as mean ± SD of eight guinea pigs in each group at each time point (one-way analysis of variance and Duncan's multiple range test). aP < 0.01, vs. control group at the same time point; bP < 0.01, vs. environmental enrichment exposure group at 0 day; cP < 0.01, vs. environmental enrichment group at 10 days.
The intensity of c-Fos expression in doublecortin-positive cells varied. The majority of doublecortin-positive cells in layer II of the medial prefrontal cortex strongly expressed c-Fos at 0 day post-environmental enrichment (Figure 5D–F, Figure 6). At 10 days, c-Fos expression in doublecortin-positive cells decreased (P < 0.01) and the intensity of c-Fos in some doublecortin-positive cells decreased. At 20 days, the number of cells expressing c-Fos and the intensity of c-Fos expression in doublecortin-positive cells decreased by 50% when compared to 0 days (P < 0.01; Figure 5D–F, J–L, Figure 6), which was still higher than the control group (P < 0.01).
The ratio of NeuN/doublecortin double labeled cells to doublecortin labeled cells increased in layer II of the medial prefrontal cortex of adult guinea pigs after environmental enrichment
It is well known that the maturation of new neurons is essential for functional integration into the nervous system. Therefore, we performed double immunofluorescence staining for doublecortin and NeuN (a specific marker of mature neurons) to examine the maturation of doublecortin-positive cells (Figure 7). The results showed that the maturation rate of doublecortin-positive cells in layer II of the medial prefrontal cortex at 0, 10 and 20 days after environmental enrichment was significantly increased when compared to the control group (P < 0.01; Figure 8). There were also significant differences in the environmental enrichment group at different time points (P < 0.01; Figure 8), which was consistent with our previous hypotheses.
Figure 7.
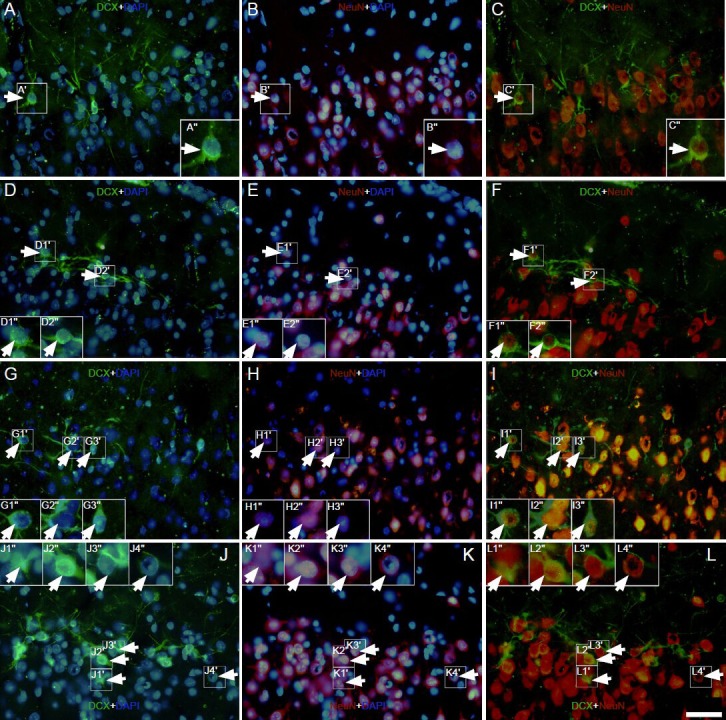
Effect of environmental enrichment on NeuN/doublecortin double-positive cells in layer II of the medial prefrontal cortex of guinea pigs (immunofluorescence staining).
Images immunostained with doublecortin (green), NeuN (red) and DAPI (blue) showing the expression of NeuN in doublecortin-positive cells in the control group (A–C) at 0 day (D–F), 10 days (G–I) and 20 days (J–L) post-environmental enrichment. The big square insert (-”) is a high mag-nification view of the small frame (-′) in each image. The arrowheads indicate NeuN/doublecortin double-positive cells. The magnification of the objective lens was 10 ×. Samples were visualized via fluorescent microscopy (Nikon, Eclipse 80i). Scale bar = 100 μm.
Figure 8.
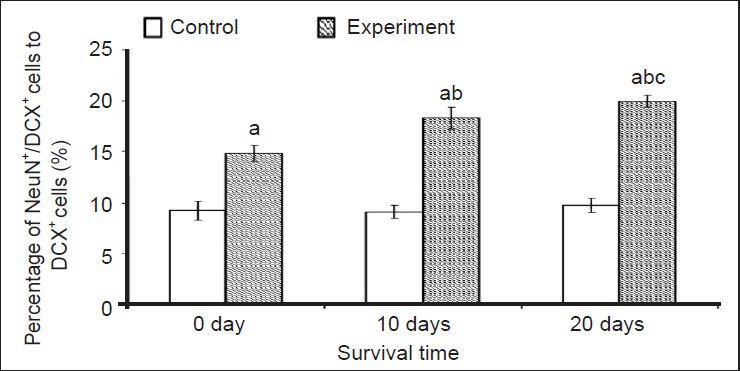
Effect of environmental enrichment exposure on the ratio of NeuN/doublecortin (DCX) double-positive cells in layer II of the medial prefrontal cortex in guinea pigs.
Data are represented as the ratio of NeuN/DCX double-positive cells to DCX-positive cells. The difference between the environmental enrichment group and the control group at the same time point was compared using unpaired t-test. Data are expressed as mean ± SD of eight guinea pigs in each group at each time point (one-way analysis of variance and Duncan's multiple range test). aP < 0.01, vs. control group at the same time point; bP < 0.01, vs. environmental enrichment expo-sure group at 0 day; cP < 0.01, vs. environmental enrichment exposure group at 10 days.
Discussion
Why did we choose a short-term environmental enrichment model?
It is generally accepted that environmental enrichment is an important physiological stimulation involved in neurogenesis of the brain. In terms of weight and cell density of the neocortex, animals under environmental enrichment exhibit a noticeable advantage over normal feeding animals[35]. In addition, diversity in cellular morphology was detected. The number of neurons in the neocortex of environmental enrichment animals was greater and appeared larger, which implied higher metabolic properties[36,37]. In addition to promoting cell survival and reducing apoptosis, which consequently improves learning and memory, profound studies found that environmental enrichment could modulate the proliferation and differentiation of neurons in the hippocampus and other related brain regions[21,24,25]. Because models for environmental enrichment exhibit various patterns, researchers have made some modifications on the content and time of exercise[22,38]. The effects on neurogenesis, learning and memory are different depending on the environmental enrichment conditions and models[39]. Generally, long-term environmental enrichment lasts for 4 months or even longer, which is principally applied to animal models of disease such as Alzheimer's disease, while proliferation of cells has been seldom detected under these conditions[40,41].
In contrast, short-term environmental enrichment is identified to be beneficial for adult neurogenesis[39]. The present study attempted to focus on the impact of environmental enrichment on neurogenesis in the prefrontal cortex of adult guinea pigs. It is therefore feasible to adopt a short-term environmental enrichment model. Consistent with aforementioned hypothesis, our results demonstrated that short-term environmental enrichment induced obvious proliferation of doublecortin-positive immature neurons in layer II of the prefrontal cortex of the adult guinea pig. Our findings suggest that short-term environmental enrichment might have a significant impact on adult neocortical neurogenesis.
Short-term environmental enrichment induces neurogenesis and maturation of doublecortin-positive cells in layer II of the prefrontal cortex of adult guinea pigs
In addition to the hippocampus, the prefrontal cortex also correlates with learning and memory function[26,27,42]. The cortex undergoes development and maturation late, yet the prefrontal cortex plays an important role in learning, memory, thinking and other advanced cognitive functions. Evidence from anatomy and electrophysiology indicates circuitries between the hippocampus and prefrontal cortex. The prefrontal-hippocampal circuitries play an important role in the communication of these two brain areas and are also essential for cognitive function[28,29,30,31]. In contrast to the hippocampus, the prefrontal cortex displays a closer relationship with exploration of novel environments[43]. Evidence indicates that the medial prefrontal cortex[31] and the prefrontal-hippocampal circuitry[28,29] are involved in novel environment detection. It is well accepted that hippocampal neurogenesis associates closely to the hippocampal-dependent learning and memory function of adult mammals[20,44,45,46]. Physiological or pathological aging, including Alzheimer's disease, may be related to abnormalities in adult hippocampal neurogenesis and the occurrence of cognitive disorders[20,47,48]. Therefore, increasing hippocampal neurogenesis may improve learning and memory function[39,42]. Thus, we hypothesized that doublecortin-positive immature neurons in the adult prefrontal cortex may be involved in the process of novel environment detection, and relevant changes may occur when they exposed to novel environments.
Our present study revealed that doublecortin-positive cells in the early period post short-term environmental enrichment (0 day) exhibited a predominant increase in layer II of the medial prefrontal cortex in adult guinea pigs. Meanwhile, the proportion of these cells concurrently expressing bromodeoxyuridine was improved to some extent, suggesting that environmental enrichment exposure might induce neurogenesis of the prefrontal cortex.
The origin of bromodeoxyuridine/doublecortin-positive cells remains unclear. Several hypotheses are available to elucidate this issue: (1) new immature neurons may be derived from the well-accepted neurogenic niche, the subventricular zone. It was observed that neurons of the prefrontal cortex actually arose from the ependymal zone and subventricular zone during neural development[49,50]. Moreover, neuroblasts from the subventricular zone were identified to migrate to the focal cortex under some pathological conditions[51,52,53]; (2) it was speculated that these newly generated neurons were either derived from the nearby pia mater[54]; or (3) were locally generated in situ from neuroblasts of the prefrontal cortex. It is worth mentioning that under normal circumstance, neuroblasts from the subventricular zone normally migrate to the olfactory bulb and nearby regions confined to a 5 mm radius at slow speed[55]. However, in our experiment, we immediately observed an increase in the number of doublecortin-positive cells in the early period after short-term environmental enrichment (0 day); furthermore, some cells exhibited just accomplished mitotic division morphology. These results suggest that the increasing doublecortin-positive cells under environmental enrichment partly arose from the local cortex in situ.
However, not all newborn neurons are fortunate enough to survive. Generally, newly generated neurons without maturation and functional integration into the pre-existing neuronal network die within 4 weeks[55]. Moreover, immature neurons (newly born or not) continue to undergo development and form extensive synaptic contact among mature neurons, in addition to functionally integrating into the nervous system. Kempermann et al.[55,56] demonstrated that generation of new neurons and functional integration into the nervous system occur during the process of learning and memory. The present study revealed that after short-term environmental enrichment, doublecortin-positive cells in shallow layers of the adult guinea pig prefrontal cortex increased and exhibited an improved ability on developing into mature neurons (NeuN/doublecortin double labeled cells), which suggested that environmental enrichment could induce the survival and maturation of neocortical newborn neurons in adult guinea pigs. Other related experiments also suggested a similar phenomenon. For instance, Kee et al.[45] and Tashiro et al.[34] discovered that environmental enrichment promoted the survival of 1- to 3-week-old newborn neurons in the subgranular zone. All together, short-term environmental enrichment could induce the proliferation, survival and maturation of new neurons in the prefrontal cortex of adult guinea pigs.
Although bromodeoxyuridine/doublecortin double labeled cells were detected in both the environmental enrichment and control groups, they still displayed significant discrepancies following statistical analysis. It was reasonable to speculate that the factors that potentially contribute to the aforementioned results may include (1) the solubility of bromodeoxyuridine was relatively low, making it difficult for proliferating cells to incorporate equal amounts; (2) bromodeoxyuridine has certain toxic effects, which may affect neurogenesis to some extent[57].
Short-term environmental enrichment induces the activation of doublecortin-positive cells in layer II of the prefrontal cortex of adult guinea pigs
Immediate early genes are a class of genes that can be rapidly and transiently activated when cells undergo some sort of stimuli. The immediate early gene c-fos is widely used as a marker of brain neuronal activity[58] and the activation of c-fos has been associated with learning processes[59]. The phosphate nucleoprotein c-Fos is a product encoded by c-fos and the expression of c-Fos is generally considered to be a symbol of neural functional activity and cellular activation[34]. Multiple stimulations in vivo and in vitro can induce prompt expression of c-Fos. Novel environmental stimuli have been considered as an important factor in triggering c-Fos protein expression[60,61,62]. In the hippocampus, c-Fos protein expression has been proposed to be a marker of successful environmental enrichment[61]. In the present study, the expression of c-Fos was dramatically up-regulated in doublecortin-positive cells in the prefrontal cortex in animals that underwent environmental enrichment exposure. These results suggest that the doublecortin-positive cells in layer II of the prefrontal cortex were reactive to novel environmental exposure. These cells were effectively activated after exposure to short-term environmental enrichment and this activation state may play an essential role in the evolution of these cells. Some experiments also support our hypothesis that DNA-binding of c-Fos with the other immediate early gene c-jun forms the transcriptional activator AP-1, which actives gene transcription and hence accelerates cell proliferation and differentiation[63]. In our study, the increased number of doublecortin-positive cells was accompanied by the dramatic up-regulation of c-Fos expression at early stages of post-environmental enrichment. This evidence suggested that an increase in c-Fos may contribute to the proliferation of these doublecortin-positive cells located in the prefrontal cortex after environmental enrichment. The expression of c-Fos was still higher than the control group at later stages post-environmental enrichment (20 days), however, it was markedly reduced when compared to early stages, implying that c-Fos participates in other neuronal functions, such as the maturation of cells and neuronal circuitry integration[18].
In summary, short-term environmental enrichment is an efficient method for improving adult neocortical neurogenesis in the prefrontal cortex. Novel environmental stimuli may induce the activation, survival and maturation of newly generated neurons in the prefrontal cortex. These alterations may be involved in learning and memory associated with the prefrontal cortex.
Materials and Methods
Design
A randomized controlled animal experiment.
Time and setting
All experiments were performed at the laboratory of the Department of Anatomy and Neurobiology, Xiangya School of Medicine, Central South University, China, between June 2011 and April 2012.
Materials
Forty-eight adult male guinea pigs (aged 3 months, weighing 200–240 g) were obtained from the Animal Center of Xiangya School of Medicine, Central South University, China (license No. SCXK (Xiang) 2009-0012). All animals were housed in standard cages upon arrival and allowed 1 week to acclimate to the house environment. They were given tap water and food in an environmentally controlled room at 25°C and 50–60% relative humidity with a 12-hour light-dark cycle.
Animal use was in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals. All procedures on animal use were approved by the Animal Ethics Committee of Xiangya School of Medicine, Central South University in China. All experimental procedures were carried out in accordance with the Guidance Suggestions for the Care and Use of Laboratory Animals[69].
Methods
Establishment of environmental enrichment model and bromodeoxyuridine administration
One week after the animals arrived, half of the guinea pigs (n = 24) were randomly assigned to environmental enrichment exposure over a period of 2 weeks, twice per day for 60 minutes each, while the other 24 were housed individually in standard cages. The environmental enrichment boxes were equipped with different cognitive and physical stimulating objects such as horizontal platforms, a small running wheel, tunnels, wooden ladder, wooden blocks, glass balls, jars, a bridge and maze. The spatial arrangement of the objects was changed daily, and some of the toys were replaced with new ones twice a week (on Monday and Thursday) to create novel environmental stimulation. The environmental enrichment was set according to Puurunen and Sale[64,65]. To detect proliferating cells, all animals were injected intraperitoneally with bromodeoxyuridine (50 mg/kg; Sigma-Aldrich, St. Louis, MO, USA) dissolved in 0.9% (w/v) normal saline once a day for 4 consecutive days, beginning on the 11th day of environmental enrichment exposure. After bromodeoxyuridine administration was over, eight animals from each group were sacrificed at 0, 10 or 20 days respectively. Animals were divided into six groups and each group contained eight animals. Animals were grouped into post-environmental enrichment exposure 0 day, 10 days, and 20 days and matched to 0 day, 10 days, and 20 days controls.
Tissue preparation
At 0, 10 or 20 days after environmental enrichment exposure, guinea pigs were deeply anesthetized and perfused transcardially with 0.9% (w/v) normal saline, followed by 4% (w/v) paraformaldehyde in 0.1 mol/L phosphate buffer (pH 7.4). Subsequently, brains were post-fixed for 24 hours in the same fixative and cryoprotected in 30% (w/v) sucrose dissolved in 0.1 mol/L phosphate buffer at 4°C. Coronal sections of the prefrontal cortex were cut throughout the rostrocaudal on a freezing microtome at 12 μm thick. According to Luparello's paper concerning the stereotaxic coordinates of the forebrain of the guinea pig[66], a 5 mm thick section of the rostral cortex was removed and the sections were collected from the front of the prefrontal cortex. All serial sections of the prefrontal cortex were collected and stored in cryoprotectant at −20°C until ready for use.
Immunohistochemistry of doublecortin, bromodeoxyuridine, c-Fos and NeuN in the prefrontal cortex
The primary antibodies used in this study included goat anti-doublecortin (1:1,000; Santa Cruz Biotechnology, Santa Cruz, CA, USA)[67], rat anti-bromodeoxyuridine (1:2,000; Serotec, MCA2060, CA, USA), rabbit anti-c-Fos (1:1,000; Santa Cruz Biotechnology), and mouse anti-NeuN (1:4,000; Chemicon, MAB377, CA, USA) antibodies. The secondary antibodies included Alexa Fluor 488 donkey anti-goat IgG, Alexa Fluor 594 conjugated donkey anti-rabbit, rat and mouse IgG (1:200; Invitrogen, Carlsbad, CA, USA).
For routine immunofluorescence staining, sections were rinsed extensively in 0.01 mol/L PBS (pH 7.4) and blocked with 10% (v/v) normal donkey serum (Sigma-Aldrich) and 0.5% (v/v) Triton X-100 (Sigma-Aldrich) in 0.01 mol/L PBS for 1 hour at room temperature. Then primary antibodies in 0.01 mol/L PBS diluent (containing 5% (v/v) donkey serum and 0.5% (v/v) Triton X-100) were applied over 48 hours at 4°C on a rotator. Sections were then rinsed four times for 10 minutes in 0.01 mol/L PBS, and further incubated in secondary antibodies plus DAPI (1:500; Sigma-Aldrich) in 0.01 mol/L PBS diluents for 2 hours at room temperature. Sections were given a further rinse with 0.01 mol/L PBS before being coverslipped using Fluoromount Medium (Sigma-Aldrich). Sections were stored at 4°C in the dark until fluorescence light microscope observation.
For bromodeoxyuridine immunolabeling, sections were rinsed extensively in 0.01 mol/L PBS (pH 7.4). Afterwards, sections were pretreated with 50% (v/v) formamide (Sigma-Aldrich) in 2 × SSC (Sigma-Aldrich) at 65°C for 1 hour, followed by incubation in 2 mol/L HCl (Sigma-Aldrich) for 30 minutes at 37°C to denature DNA. Sections were neutralized in 0.01 mol/L borate buffer (pH 8.0) for 10 minutes at room temperature. The next administrations were the same as routine immunofluorescence staining.
For double immunolabeling of bromodeoxyuridine/doublecortin, sections were rinsed extensively in 0.01 mol/L PBS, followed by incubation in 50% (v/v) formamide in 2 × SSC solution at 65°C for 60 minutes and subsequently incubated in 2 mol/L HCl for 30 minutes at 37°C and neutralized in 0.01 mol/L borate buffer for 10 minutes. After extensive washing in PBS, slices were blocked in PBS plus 10% (v/v) normal donkey serum for 60 minutes at room temperature, followed by incubation in primary antibody (rat anti-bromodeoxyuridine monoclonal antibody, 1:1,000 and goat anti-doublecortin monoclonal antibody, 1:500) mix diluted in PBS plus 5% (v/v) normal donkey serum for 48 hours at 4°C. Next, sections were again extensively rinsed in PBS and incubated in secondary antibody (Alexa Fluor 594 donkey anti-rat; Alexa Fluor 488 donkey anti-goat) mix diluted in PBS plus 5% (v/v) normal donkey serum for 2 hours, followed by extensive rinsing in 0.01 mol/L PBS and coverslipped.
In this study, omission of the primary antibodies in the incubation buffer or substitution of a given antibody with normal serum from its host animal species yielded no specific labeling. Controls for binding of the secondary antibody were performed where one primary antibody was omitted.
Imaging acquisition and cell quantification
The prefrontal cortex of rodents, including guinea pigs, has two subfields, one is the medial prefrontal region which lies along the anterior medial wall of the hemispheres and the other is the orbital frontal cortex that lies along the rhinal fissure and forms the anterior insular area[68]. The medial prefrontal cortex in the rat is analogous to the dorsolateral and medial frontal cortex in primates, including humans, and the rodent orbital frontal cortex is analogous to the primate orbital regions. In this study, we focused on the medial prefrontal cortex for several experiments to determine the contribution of the medial prefrontal cortex in the process of novelty detection[31].
Sections were imaged on a Nikon microscope (Nikon, Eclipse 80i, Tokyo, Japan) equipped with a digital imaging system. Quantification of immunoreactive cells was performed on the full rostrocaudal extent of the medial prefrontal cortex. The method of quantification of positive cells is briefly described as follows: The stained positive cells in layer II of the medial prefrontal cortex were counted in each cortical segment. Images were captured continuously using a 40 × objective along the pia mater. Analyses of doublecortin-positive cells as well as the percentage of c-Fos/bromodeoxyuridine/NeuN colocalization with doublecortin to doublecortin-positive cell were performed at the level of the comparable rostra-caudal level and cell counts were carried out on at least five coronal sections per brain. Images of the same cellular profile were taken using green (doublecortin labeling), red (other immunolabel) and blue (DAPI stain) fluorescent filters. Among them, the number of doublecortin-positive cells was calculated as the total cell number/the number of the total images in each section. For the fluorescent double staining, we judged doublecortin to be co-localized with c-Fos, bromodeoxyuridine or NeuN when more than two-thirds of the cell body was double stained. Although the overlap in staining was not obvious for all cells, given that the thickness of sections was only 12 μm and our exclusion criteria were consistent, the comparison of co-localization between the two groups appeared to be reasonable.
Statistical analysis
Means of cell numbers and percentages were calculated for individual and group of animals. The software used was SPSS 12.0 software (SPSS, Chicago, IL, USA). Statistical comparisons were conducted by the unpaired t-test to compare between enriched and paired control animals at the same time point; or were analyzed by one-way analysis of variance between the different environmental enrichment survival time groups. Duncan's multiple range test was applied to analyze differences. Data are presented as mean ± SD. Significant interactions were followed with a minimum level of P < 0.05 to determine group differences. Analysis was performed under blind conditions.
Footnotes
Funding: This study was supported by the National Natural Science Foundation of China, No. 30900773; the Natural Science Foundation of Hunan Province in China, No. 11JJ2020; and Young Teachers Training Program of University of Hunan Province.
Conflicts of interest: None declared.
Peer review: Previous studies have confirmed that environmental enrichment exposure significantly promotes neural regeneration in the hippocampus, thus participating in the learning and memory. Therefore this study adopted environmental enrichment on adult guinea pigs in an effort to observe the changes of these immature neurons in the prefrontal cortex. Experimental findings indicate that these neurons were similar to new hippocampal neurons, and were possibly involved in the learning and memory.
Copyedited by Diwakarla S, Chen A, Huo X, Wang J, Yang Y, Li CH, Song LP, Zhao M
References
- [1].Eriksson PS, Perfilieva E, Bjork-Eriksson T, et al. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4(11):1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- [2].Deng W, Aimone JB, Gage FH. New neurons and new memories: how does adult hippocampal neurogenesis affect learning and memory? Nat Rev Neurosci. 2010;11(5):339–350. doi: 10.1038/nrn2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Rakic P. Neuroscience. No more cortical neurons for you. Science. 2006;313(5789):928–929. doi: 10.1126/science.1131713. [DOI] [PubMed] [Google Scholar]
- [4].Gould E. How widespread is adult neurogenesis in mammals? Nat Neurosci. 2007;8(6):481–488. doi: 10.1038/nrn2147. [DOI] [PubMed] [Google Scholar]
- [5].Shapiro LA, Ng K, Zhou QY, et al. Subventricular zone-derived, newly generated neurons populates several olfactory and limbic forebrain regions. Epilepsy Behav. 2009;14(Suppl 1):74–80. doi: 10.1016/j.yebeh.2008.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Xiong K, Luo DW, Patrylo PR, et al. Doublecortin-expressing cells are present in layer II across the adult guinea pig cerebral cortex: partial colocalization with mature interneuron markers. Exp Neurol. 2008;211(1):271–282. doi: 10.1016/j.expneurol.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Cai Y, Xiong K, Chu YP, et al. Doublecortin expression in adult cat and primate cerebral cortex relates to immature neurons that develop into GABAergic subgroups. Exp Neurol. 2009;216(2):342–356. doi: 10.1016/j.expneurol.2008.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Xiong K, Cai Y, Zhang XM, et al. Layer I as a putative neurogenic niche in young adult guinea pig cerebrum. Mol Cell Neurosci. 2010;45(2):180–191. doi: 10.1016/j.mcn.2010.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Kutsuna N, Suma T, Takada Y, et al. Decrease in doublecortin expression without neuronal cell death in rat retrosplenial cortex after stress exposure. Neuroreport. 2012;23(4):211–215. doi: 10.1097/WNR.0b013e32834fca3a. [DOI] [PubMed] [Google Scholar]
- [10].Klempin F, Kronenberg G, Cheung G, et al. Properties of doublecortin (DCX) expressing cells in the piriform cortex compared to the neurogenic dentate gyrus of adult mice. PLoS One. 2011;6(10):e25760. doi: 10.1371/journal.pone.0025760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Gómez-Climent MA, Castillo-Gómez E, Varea E, et al. A population of prenatally generated cells in the rat paleocortex maintains an immature neuronal phenotype into adulthood. Cereb Cortex. 2008;18(10):2229–2240. doi: 10.1093/cercor/bhm255. [DOI] [PubMed] [Google Scholar]
- [12].Bonfanti L, Nacher J. New scenarios for neuronal structural plasticity in non-neurogenic brain parenchyma: The case of cortical layer II immature neurons. Prog Neurobiol. 2012;98(1):1–15. doi: 10.1016/j.pneurobio.2012.05.002. [DOI] [PubMed] [Google Scholar]
- [13].Magavi SS, Leavitt BR, Macklis JD. Induction of neurogenesis in the neocortex of adult mice. Nature. 2000;405(6789):951–955. doi: 10.1038/35016083. [DOI] [PubMed] [Google Scholar]
- [14].Magavi SS, Macklis JD. Induction of neuronal type-specific neurogenesis in the cerebral cortex of adult mice: manipulation of neural precursors in situ. Brain Res Dev Brain Res. 2002;134(1-2):57–76. doi: 10.1016/s0165-3806(01)00316-9. [DOI] [PubMed] [Google Scholar]
- [15].Tonchev AB, Yamashima T, Sawamoto K, et al. Enhanced proliferation of progenitor cells in the subventricular zone and limited neuronal production in the striatum and neocortex of adult macaque monkeys after global cerebral ischemia. J Neurosci Res. 2005;81(6):776–788. doi: 10.1002/jnr.20604. [DOI] [PubMed] [Google Scholar]
- [16].Cameron HA, Dayer AG. New interneurons in the adult neocortex: small, sparse, but significant? Biol Psychiatry. 2008;63(7):650–655. doi: 10.1016/j.biopsych.2007.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Sohur US, Arlotta P, Macklis JD. Developmental controls are re-expressed during induction of neurogenesis in the neocortex of young adult mice. Front Neurosci. 2012;6:12. doi: 10.3389/fnins.2012.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Ohira K, Furuta T, Hioki H, et al. Ischemia-induced neurogenesis of neocortical layer 1 progenitor cells. Nat Neurosci. 2010;13(2):173–179. doi: 10.1038/nn.2473. [DOI] [PubMed] [Google Scholar]
- [19].Ohira K. Injury-induced neurogenesis in the mammalian forebrain. Cell Mol Life Sci. 2011;68(10):1645–1656. doi: 10.1007/s00018-010-0552-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Drapeau E, Mayo W, Aurousseau C, et al. Spatial memory performances of aged rats in the water maze predict levels of hippocampal neurogenesis. Proc Natl Acad Sci USA. 2003;100(24):14385–14390. doi: 10.1073/pnas.2334169100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Van Praag H, Kempermann G, Gage FH. Neural consequences of environmental enrichment. Nat Rev Neurosci. 2000;1(3):191–198. doi: 10.1038/35044558. [DOI] [PubMed] [Google Scholar]
- [22].Will B, Galani R, Kelche C, et al. Recovery from brain injury in animals: relative efficacy of environmental enrichment, physical exercise or formal training (1990-2002) Prog Neurobiol. 2004;72(3):167–182. doi: 10.1016/j.pneurobio.2004.03.001. [DOI] [PubMed] [Google Scholar]
- [23].Frick KM, Benoit JD. Use it or lose it: environmental enrichment as a means to promote successful cognitive aging. ScientificWorldJournal. 2010;10:1129–1141. doi: 10.1100/tsw.2010.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Brown J, Cooper-Kuhn CM, Kempermann G, et al. Enriched environment and physical activity stimulate hippocampal but not olfactory bulb neurogenesis. Eur J Neurosci. 2003;17(10):2042–2046. doi: 10.1046/j.1460-9568.2003.02647.x. [DOI] [PubMed] [Google Scholar]
- [25].Di GA, Mainardi M, Chillemi S, et al. Environmental enrichment modulates cortico-cortical interactions in the mouse. PLoS One. 2011;6(9):e25285. doi: 10.1371/journal.pone.0025285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Funahashi S. Prefrontal cortex and working memory processes. Neuroscience. 2006;139(1):251–261. doi: 10.1016/j.neuroscience.2005.07.003. [DOI] [PubMed] [Google Scholar]
- [27].Watanabe M, Hikosaka K, Sakagami M, et al. Reward expectancy-related prefrontal neuronal activities: are they neural substrates of “affective” working memory? Cortex. 2007;43(1):53–64. doi: 10.1016/s0010-9452(08)70445-3. [DOI] [PubMed] [Google Scholar]
- [28].Knight R. Contribution of human hippocampal region to novelty detection. Nature. 1996;383(6597):256–259. doi: 10.1038/383256a0. [DOI] [PubMed] [Google Scholar]
- [29].Yamaguchi S, Hale LA, D’Esposito M, et al. Rapid prefrontal-hippocampal habituation to novel events. J Neurosci. 2004;24(23):5356–5363. doi: 10.1523/JNEUROSCI.4587-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Daffner KR, Mesulam MM, Scinto LF, et al. The central role of the prefrontal cortex in directing attention to novel events. Brain. 2000;123:927–939. doi: 10.1093/brain/123.5.927. [DOI] [PubMed] [Google Scholar]
- [31].Dias R, Honey RC. Involvement of the rat medial prefrontal cortex in novelty detection. Behav Neurosci. 2002;116(3):498–503. doi: 10.1037//0735-7044.116.3.498. [DOI] [PubMed] [Google Scholar]
- [32].Feliciano DM, Bordey A. Newborn cortical neurons: only for neonates? Trends Neurosci. 2013;36(1):51–61. doi: 10.1016/j.tins.2012.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Lehner M, Taracha E, Skorzewska A, et al. Sensitivity to pain and c-Fos expression in brain structures in rats. Neurosci Lett. 2004;370(1):74–79. doi: 10.1016/j.neulet.2004.07.089. [DOI] [PubMed] [Google Scholar]
- [34].Tashiro A, Makino H, Gage FH. Experience-specific functional modification of the dentate gyrus through adult neurogenesis: a critical period during an immature stage. J Neurosci. 2007;27(12):3252–3259. doi: 10.1523/JNEUROSCI.4941-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Schrijver NC, Pallier PN, Brown VJ, et al. Double dissociation of social and environmental stimulation on spatial learning and reversal learning in rats. Behav Brain Res. 2004;152(2):307–314. doi: 10.1016/j.bbr.2003.10.016. [DOI] [PubMed] [Google Scholar]
- [36].Rasin MR, Darmopil S, Petanjek Z, et al. Effect of environmental enrichment on morphology of deep layer III and layer V pyramidal cells of occipital cortex in oldest-old rat-A quantitative golgi cox study. Coll Antropol. 2011;35(Suppl 1):253–258. [PubMed] [Google Scholar]
- [37].Gelfo F, De Bartolo P, Giovine A, et al. Layer and regional effects of environmental enrichment on the pyramidal neuron morphology of the rat. Neurobiol Learn Mem. 2009;91(4):353–365. doi: 10.1016/j.nlm.2009.01.010. [DOI] [PubMed] [Google Scholar]
- [38].Toth LA, Kregel K, Leon L, et al. Environmental enrichment of laboratory rodents: the answer depends on the question. Comp Med. 2011;61(4):314–321. [PMC free article] [PubMed] [Google Scholar]
- [39].Valero J, España J, Parra-Damas A, et al. Short-term environmental enrichment rescues adult neurogenesis and memory deficits in APP(Sw, Ind) transgenic mice. PLoS One. 2011;6(2):e16832. doi: 10.1371/journal.pone.0016832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Nygren J, Wieloeh T, Pesic J, et al. Enriched environment attenuates cell genesis in subventrieular zone after focal isehemia in mice and decreases migration of newborn cells to the striatum. Stroke. 2006;37(11):2824–2829. doi: 10.1161/01.STR.0000244769.39952.90. [DOI] [PubMed] [Google Scholar]
- [41].Catlow BJ, Rowe AR, Clearwater CR, et al. Effects of environmental enrichment and physical activity on neurogenesis in transgenic PS1/APP mice. Brain Res. 2009;1256:173–179. doi: 10.1016/j.brainres.2008.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Schwindel CD, McNaughton BL. Hippocampal-cortical interactions and the dynamics of memory trace reactivation. Prog Brain Res. 2011;193:163–177. doi: 10.1016/B978-0-444-53839-0.00011-9. [DOI] [PubMed] [Google Scholar]
- [43].Petrides M. The orbitofrontal cortex: novelty, deviation from expectation, and memory. Ann NY Acad Sci. 2007;1121:33–53. doi: 10.1196/annals.1401.035. [DOI] [PubMed] [Google Scholar]
- [44].Shors TJ, Miesegaes G, Beylin A, et al. Neurogenesis in the adult is involved in the formation of trace memories. Nature. 2001;410(6826):372–376. doi: 10.1038/35066584. [DOI] [PubMed] [Google Scholar]
- [45].Kee N, Teixeira CM, Wang AH, et al. Preferential incorporation of adult-generated granule cells into spatial memory networks in the dentate gyrus. Nat Neurosci. 2007;10(3):355–362. doi: 10.1038/nn1847. [DOI] [PubMed] [Google Scholar]
- [46].Dupret D, Revest JM, Koehl M, et al. Spatial relational memory requires hippocampal adult neurogenesis. PLoS One. 2008;3(4):e1959. doi: 10.1371/journal.pone.0001959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Zhao CS, Puurunen K, Schallert T, et al. Effect of cholinergicm edication, before and after focal photothrombotic ischemic cortical injury, on histological and functional outcome in aged and young adult rats. Behav Brain Res. 2005;156(1):85–94. doi: 10.1016/j.bbr.2004.05.011. [DOI] [PubMed] [Google Scholar]
- [48].Kaneko N, Sawamoto K. Adult neurogenesis and its alteration under pathological conditions. Neurosci Res. 2009;63(3):155–164. doi: 10.1016/j.neures.2008.12.001. [DOI] [PubMed] [Google Scholar]
- [49].Rakic P. Evolution of the neocortex: Perspective from developmental biology. Nat Rev Neurosci. 2009;10(10):724–735. doi: 10.1038/nrn2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Lui JH, Hansen DV, Kriegstein AR. Development and evolution of the human neocortex. Cell. 2011;146(1):18–36. doi: 10.1016/j.cell.2011.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Arvidsson A, Collin T, Kirik D, et al. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med. 2002;8(9):963–970. doi: 10.1038/nm747. [DOI] [PubMed] [Google Scholar]
- [52].Darsalia V, Heldmann U, Lindvall O, et al. Stroke-induced neurogenesis in aged brain. Stroke. 2005;36(8):1790–1795. doi: 10.1161/01.STR.0000173151.36031.be. [DOI] [PubMed] [Google Scholar]
- [53].Kokaia Z, Thored P, Arvidsson A, et al. Regulation of stroke-induced neurogenesis in adult brain-recent scientific progress. Cereb Cortex. 2006;16(Suppl 1):i162–i167. doi: 10.1093/cercor/bhj174. [DOI] [PubMed] [Google Scholar]
- [54].Nakagomi T, Molnár Z, Nakano-Doi A, et al. Ischemia-induced neural stem/progenitor cells in the pia mater following cortical infarction. Stem Cells Dev. 2011;20(12):2037–2051. doi: 10.1089/scd.2011.0279. [DOI] [PubMed] [Google Scholar]
- [55].Zhao C, Deng W, Gage FH. Mechanisms and functional implications of adult neurogenesis. Cell. 2008;132(4):645–660. doi: 10.1016/j.cell.2008.01.033. [DOI] [PubMed] [Google Scholar]
- [56].Kempermann G. Why new neurons? Possible functions for adult hippocampal neurogenesis. J Neurosci. 2002;22(3):635–638. doi: 10.1523/JNEUROSCI.22-03-00635.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Lehner B, Sandner B, Marschallinger J, et al. The dark side of BrdU in neural stem cell biology: detrimental effects on cell cycle, differentiation and survival. Cell Tissue Res. 2011;345(3):313–328. doi: 10.1007/s00441-011-1213-7. [DOI] [PubMed] [Google Scholar]
- [58].Farifar R, Zangenehpour S, Chaudhuri A. Cellular-resolution activity mapping of the brain using immediate-early gene expression. Front Biosci. 2004;9:104–109. doi: 10.2741/1198. [DOI] [PubMed] [Google Scholar]
- [59].Aggleton JP, Brown MW. Contrasting hippocampal and perirhinal cortex function using immediate early gene imaging. Q J Exp Psychol B. 2005;58(3-4):218–233. doi: 10.1080/02724990444000131. [DOI] [PubMed] [Google Scholar]
- [60].Yochiy A, Britto LR, Hunziker MH. Novelty, but not operant aversive learning, enhances Fos and Egr-1 expression in the medial prefrontal cortex and hippocampal areas of rats. Behav Neurosci. 2012;126(6):826–834. doi: 10.1037/a0030721. [DOI] [PubMed] [Google Scholar]
- [61].Rinaldi A, Romeo S, Agustín-Pavón C, et al. Distinct patterns of Fos immunoreactivity in striatum and hippocampus induced by different kinds of novelty in mice. Neurobiol Learn Mem. 2010;94(3):373–381. doi: 10.1016/j.nlm.2010.08.004. [DOI] [PubMed] [Google Scholar]
- [62].Radulovic J, Tronson NC. Molecular specificity of multiple hippocampal processes governing fear extinction. Rev Neurosci. 2010;21(1):1–17. doi: 10.1515/revneuro.2010.21.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Hess J, Angel P, Schorpp-Kistner M. AP-1 subunits: quarrel and harmony among siblings. J Cell Sci. 2004;117(Pt 25):5965–5973. doi: 10.1242/jcs.01589. [DOI] [PubMed] [Google Scholar]
- [64].Puurunen K, Sivenius J. Influence of enriched environment on spatial learning following cerebral insult. Rev Neurosci. 2002;13(4):347–364. doi: 10.1515/revneuro.2002.13.4.347. [DOI] [PubMed] [Google Scholar]
- [65].Sale A, Putignano E, Cancedda L, et al. Enriched environment and acceleration of visual system development. Neuropharmacology. 2004;47(5):649–660. doi: 10.1016/j.neuropharm.2004.07.008. [DOI] [PubMed] [Google Scholar]
- [66].Luparello TJ, Stein M, Park CD. A stereotaxic atlas of hypothalamus of the guinea pig. J Comp Neurol. 1964;122:201–217. doi: 10.1002/cne.901220206. [DOI] [PubMed] [Google Scholar]
- [67].Huang JF, Huang K, Shang L, et al. Chronic lead exposure reduces doublecortin-expressing immature neurons in young adult guinea pig cerebral cortex. BMC Neurosci. 2012;13:82. doi: 10.1186/1471-2202-13-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Kolb B, Pellis S, Robinson TE. Plasticity and functions of the orbital frontal cortex. Brain Cogn. 2004;55(1):104–15. doi: 10.1016/S0278-2626(03)00278-1. [DOI] [PubMed] [Google Scholar]
- [69].The Ministry of Science and Technology of the People's Republic of China. Guidance Suggestions for the Care and Use of Laboratory Animals. 2006-09-30 [Google Scholar]


