Abstract
Bone marrow mesenchymal stem cell transplantation is an effective treatment for neonatal hypoxic-ischemic brain damage. However, the in vivo transplantation effects are poor and their survival, colonization and differentiation efficiencies are relatively low. Red or near-infrared light from 600–1,000 nm promotes cellular migration and prevents apoptosis. Thus, we hypothesized that the combination of red light with bone marrow mesenchymal stem cell transplantation would be effective for the treatment of hypoxic-ischemic brain damage. In this study, the migration and colonization of cultured bone marrow mesenchymal stem cells on primary neurons after oxygen-glucose deprivation were detected using Transwell assay. The results showed that, after a 40-hour irradiation under red light-emitting diodes at 660 nm and 60 mW/cm2, an increasing number of green fluorescence-labeled bone marrow mesenchymal stem cells migrated towards hypoxic-ischemic damaged primary neurons. Meanwhile, neonatal rats with hypoxic-ischemic brain damage were given an intraperitoneal injection of 1 × 106 bone marrow mesenchymal stem cells, followed by irradiation under red light-emitting diodes at 660 nm and 60 mW/cm2 for 7 successive days. Shuttle box test results showed that, after phototherapy and bone marrow mesenchymal stem cell transplantation, the active avoidance response rate of hypoxic-ischemic brain damage rats was significantly increased, which was higher than that after bone marrow mesenchymal stem cell transplantation alone. Experimental findings indicate that 660 nm red light emitting diode irradiation promotes the migration of bone marrow mesenchymal stem cells, thereby enhancing the contribution of cell transplantation in the treatment of hypoxic-ischemic brain damage.
Keywords: nerve regeneration, stem cells, Transwell assay, red light, hypoxic-ischemic brain damage, bone marrow mesenchymal stem cells, transplantation, cell migration, learning ability, NSFC grant, neural regeneration
Introduction
The present treatment of hypoxic-ischemic brain damage includes supportive therapy, nutritional drugs, hypothermia, and hyperbaric oxygen therapy[1,2,3,4,5,6,7,8]. However, these treatments are limited in effectiveness with poor prognosis[9]. Therefore, it is very important to develop and modify new treatment measures.
As stem cell technology develops, stem cell transplantation is recognized as a novel way for treating hypoxic-ischemic brain damage and it has been confirmed to significantly improve motor behavior and reduce lesion area in animal experiments[10,11]. Preliminary clinical trials showed that muscular tension and cognitive development of neonatal children with severe hypoxic-ischemic brain damage were greatly promoted after 28-day injection of neural stem cells via ventricular puncture[12]. Bone marrow mesenchymal stem cells are characterized by their multi-differentiation potential, ease in harvesting, low immunogenicity, high amplification potential, and few ethical limitations[13,14,15]. These advantages allow their widespread application in the field of stroke, spinal cord injury and other hypoxic ischemic neurological disorders. It was reported that bone marrow mesenchymal stem cell transplantation improved nervous system damage and promoted neurological functional recovery in the treatment of hypoxic ischemic nervous system diseases[16], and was also effective for hypoxic-ischemic brain damage[17,18,19,20]. However, a series of previous studies suggested that the neuronal differentiation rate of bone marrow mesenchymal stem cells during the in vitro culture reached 78–92%, but their in vivo transplantation efficiency, and survival and differentiation rates were very low (1–17%)[21,22]. The low levels of bone marrow mesenchymal stem cell transplantation, survival, colonization and differentiation efficacy greatly restrict their therapeutic effect. An accumulating number of studies have investigated the contribution of bone marrow mesenchymal stem cell transplantation on the promotion of neurological function after hypoxic-ischemic brain damage, but their outcomes have been unsatisfactory[23,24,25,26]. Apoptosis is the key mediator of bone marrow mesenchymal stem cell migration, colonization and survival after transplantation.
The biomedical application of red or near-infrared light from 600–1,000 nm has attracted great concern in recent years[27,28,29,30]. Red or near-infrared light has been shown to promote keratinocyte migration[31] and inhibit apoptosis in PC12 cells[32]. The intrinsic mechanism is explained by the fact that light energy stimulates mitochondrial cytochrome activity, promotes reactive oxygen species production and ATP synthesis, and improves mitochondrial function, thus increasing cellular viability[28,33,34]. Although biological modulation of red or near-infrared energy increases bone marrow mesenchymal stem cell migration, colonization and survival, the combined therapy of red or near-infrared light with stem cells has been rarely studied, and whether phototherapy can promote the role of bone marrow mesenchymal stem cell transplantation in the treatment of hypoxic-ischemic brain damage remains unclear. Therefore, this study aims to observe the effect of 660 nm red light-emitting diode and bone marrow mesenchymal stem cell transplantation on neurological functional recovery after hypoxic-ischemic brain damage.
Results
Red light irradiation promoted bone marrow mesenchymal stem cell migration toward primary neurons after oxygen-glucose deprivation
After 3 hours of culture, green fluorescence-labeled GFP-bone marrow mesenchymal stem cells were visible in Transwell chambers and the number of migrated cells gradually increased as culture time proceeded (20 hours later). There were more migrating cells in the irradiation group after high-power red light-emitting diode irradiation than in the control group (P < 0.05), indicating that red light irradiation significantly promoted bone marrow mesenchymal stem cell migration and colonization to primary neurons following oxygen-glucose deprivation (Figure 1).
Figure 1.
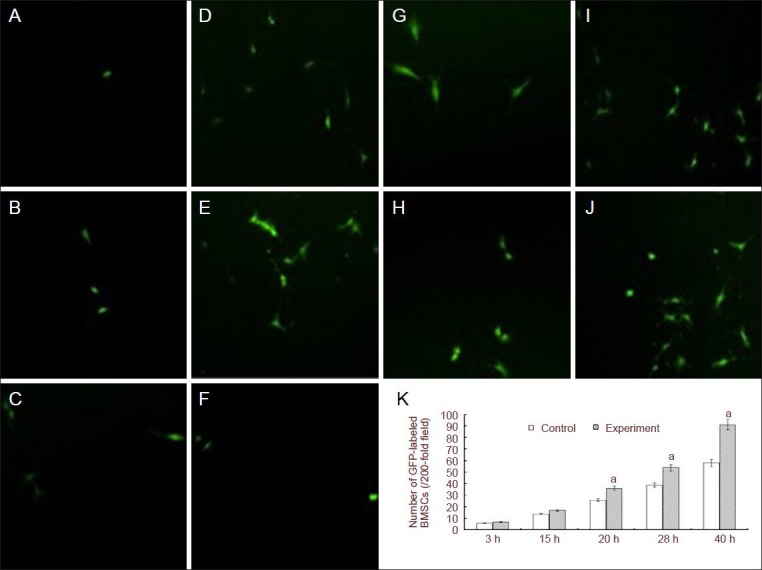
Effect of red light irradiation on bone marrow mesenchymal stem cells (BMSCs) migrating towards primary neurons after oxygen-glucose deprivation (OGD).
(A–J) Green fluorescent protein (GFP)-labeled BMSCs in OGD control and phototherapy + BMSC groups at different culture times (fluorescence microscope, × 200). After 3 hours of culture, GFP-labeled BMSCs were visible in Transwell chambers and the number of migrated cells gradually in-creased as culture time proceeded. (A–E) 3-, 15-, 20-, 28-, 40-hour OGD control groups. (F–J) Phototherapy + 3-, 15-, 20-, 28-, 40-hour OGD groups. (K) Changes of the number of GFP-labeled BMSCs in OGD control and phototherapy + OGD groups at different culture times; h: hours. Data are expressed as mean ± SD. Differences between groups were compared using one-way analysis of variance, and pairwise comparisons were performed using Student-Newman-Keuls test. aP < 0.05, vs. control group. GFP gene transfection enables labeling of rat BMSCs with green fluorescence.
Quantitative analysis of experimental animals
Eighty rats were randomly divided into four groups: normal control, model, bone marrow mesenchymal stem cell transplantation, and phototherapy + bone marrow mesenchymal stem cell transplantation. Hypoxic-ischemic brain damage was produced in rats from all but the normal control group. After the injury model was established, rats in the bone marrow mesenchymal stem cell transplantation and phototherapy + bone marrow mesenchymal stem cell transplantation groups were injected with bone marrow mesenchymal stem cells and/or given red light irradiation for 7 days. The success rate of the hypoxic-ischemic brain damage model was 90%, and the failed models and mortalities were excluded and supplemented.
Red light irradiation combined with bone marrow mesenchymal stem cell transplantation improved the learning ability of hypoxic-ischemic brain damaged rats
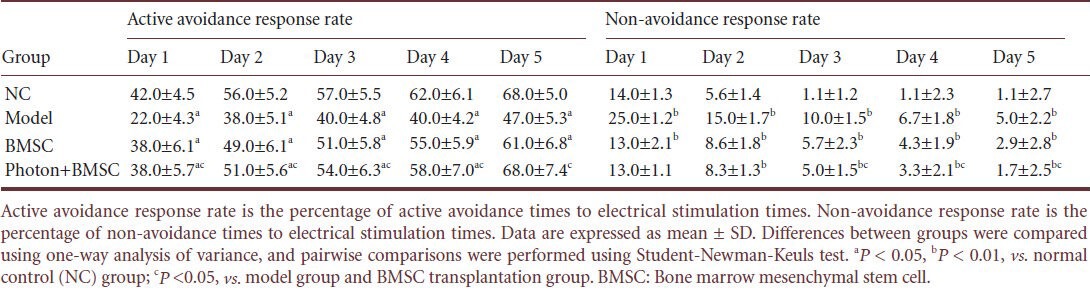
In the shuttle box test, rats in the normal control and phototherapy + bone marrow mesenchymal stem cell transplantation groups responded quickly to stimuli; while rats in the bone marrow mesenchymal stem cell transplantation and model groups had slow or no reactions. After treatment with red light irradiation for 1–5 days, the active avoidance response rate of hypoxic-ischemic brain damaged rats in the bone marrow mesenchymal stem cell transplantation group was significantly increased compared with the model group, and the increment was more apparent in the phototherapy + bone marrow mesenchymal stem cell transplantation group (P < 0.05). The non-avoidance response rate was significantly decreased compared with the model and bone marrow mesenchymal stem cell transplantation groups (P < 0.05). At days 1–4, the active avoidance response rate in the phototherapy + bone marrow mesenchymal stem cell transplantation group was lower than the normal control group (P < 0.05), but the percentage was gradually increased and became the same at day 5 between the phototherapy + bone marrow mesenchymal stem cell transplantation and normal control groups (Table 1). This illustrates that red light irradiation further promoted the restoration of learning and memory ability in rats after bone marrow mesenchymal stem cell transplantation.
Table 1.
Active avoidance response rate and non-avoidance response rate of rats in shuttle box test
Discussion
Increasing studies have investigated the biological effects of red or near-infrared light at 600–1,000 nm[35,36,37]. This study was designed to explore the effect of red light energy on bone marrow mesenchymal stem cell migration and the treatment of hypoxic-ischemic brain damage. Transwell migration assays showed that 660 nm red light irradiation significantly enhanced the migration ability of bone marrow mesenchymal stem cells. Shuttle box test revealed that learning and memory ability was significantly improved in hypoxic-ischemic brain damaged rats. Experimental findings indicate that 660 nm red light irradiation enhanced the migration ability of bone marrow mesenchymal stem cells and promoted their therapeutic effect in hypoxic-ischemic brain damage.
Irradiation with a red light-emitting diode can effect the physiological characteristics of cells by the photobiomodulation effects of light energy[27]. Although the underlying mechanism remains elusive, accumulating evidence has revealed that light stimulates mitochondrial cytochrome C oxidase[27]. This light-accepting molecule is extremely sensitive to red or near-infrared light, and cytochrome C oxidase is a terminal transferase enzyme in the cell respiratory chain that is located on the inner mitochondrial membrane, where it is directly involved in ATP synthesis and energy metabolism[34], thus enhancing cell metabolism[28]. A study from University of Wisconsin found that stimulation with a 670 nm light-emitting diode red light regulated energy metabolism and accordingly enhanced nerve cell viability[37]. It also increased cytochrome C oxidase levels in normal neurons, thus promoting energy metabolism in visual cortex neurons[38]. In this study, under 660 nm red light irradiation, bone marrow mesenchymal stem cell migration ability began to increase at 3 hours and the ability was higher than the normal control group. Furthermore, the number of bone marrow mesenchymal stem cells penetrating Transwell membranes was significantly increased at 20 hours, which was also higher than the normal control group. We speculate that red light irradiation promoted bone marrow mesenchymal stem cell migration, which key for bone marrow mesenchymal stem cell transplantation in the treatment of hypoxic-ischemic brain damage. Hou et al.[39] observed bone marrow mesenchymal stem cell proliferation was significantly promoted after light-emitting diode red light irradiation at 635 nm, 60 mW/cm2. Therefore, we tentatively hypothesize that red light energy irradiation helps bone marrow mesenchymal stem cell migration and proliferation, inhibits bone marrow mesenchymal stem cell apoptosis, which contributes to bone marrow mesenchymal stem cell migration to targeted tissue.
Spatial learning and memory dysfunction is an important symptom of hypoxic-ischemic brain damage[2,40,41,42]. Shuttle box test[17,43] results revealed that bone marrow mesenchymal stem cell transplantation significantly improved learning and memory ability in hypoxic-ischemic brain damaged rats. The results of this study showed that the active avoidance response rate in rats after 660 nm red light irradiation was significantly higher than the model group. This evidence suggests that the spatial learning and memory ability of hypoxic-ischemic brain damaged rats after red light irradiation and bone marrow mesenchymal stem cell transplantation was significantly improved compared with the control group. Meanwhile, the improvement was observed soon after phototherapy and the active avoidance response rate increased sharply as the treatment proceeded. Although the biological mechanism was not studied in this study, animal behaviors indicated that light-emitting diode red light irradiation helps accelerate neurological functional restoration in hypoxic-ischemic brain damaged rats after bone marrow mesenchymal stem cell transplantation. Based on Transwell assay results, we speculate that 660 nm red light energy can be absorbed by bone marrow mesenchymal stem cell mitochondria, thus promoting bone marrow mesenchymal stem cell viability, directional migration and proliferation ability, enhancing bone marrow mesenchymal stem cell colonization and reducing cell apoptosis, thereby inhibiting neuronal apoptosis after hypoxic-ischemic brain damage and significantly improving learning and memory ability.
Although bone marrow mesenchymal stem cells have been confirmed to be effective in the treatment of hypoxic-ischemic brain damage in animal experiments and case reports[18,19]. However, low differentiation, colonization and survival rate in vivo restrict their therapeutic effect[22,44]. A series of factors are strongly linked with the improvement of the bone marrow mesenchymal stem cell environment, such as brain-derived neurotrophic factor, glial-derived neurotrophic factor, fibroblast growth factor, epidermal growth factor, and neurotrophin-3[39,45,46,47]. Appropriate vitamin A levels are also a mediator of bone marrow mesenchymal stem cell transplantation in treatment of hypoxic-ischemic brain damage[48]. It has been reported that near-infrared light energy enhances nerve cell viability[37] and bone marrow mesenchymal stem cell proliferation[39], so we suggested the combination of near-infrared light irradiation with bone marrow mesenchymal stem cells for treating hypoxic-ischemic brain damage. Preliminary experimental results suggest that 660 nm red light-emitting diode irradiation promoted bone marrow mesenchymal stem cell migration and restoration of spatial learning and memory ability in hypoxic-ischemic brain damaged rats. This provides a new technique of red light energy for bone marrow mesenchymal stem cell transplantation treatment of hypoxic-ischemic brain damage. The exact mechanism by which red light irradiation promotes bone marrow mesenchymal stem cell colonization and inhibits apoptosis following hypoxic-ischemic brain damage deserves further investigations, in particular the biological expression of molecules in the process of red light energy and anti-apoptotic effects of bone marrow mesenchymal stem cells has rarely been studied.
Materials and Methods
Design
An in vitro comparative observation and randomized controlled animal experiment concerning cell biology.
Time and setting
Experiments were performed at the Animal Laboratory, Children's Hospital of Chongqing, China from December 2010 to March 2011.
Materials
Animals
(1) Twelve Sprague-Dawley rats, of clean grade, aged 3 months, weighing 230–250 g, four males and eight females, were provided by Animal Center, Daping Hospital, the Third Military Medical University in China (license No. SCXK (Yu) 2007-0005). All rats were housed in the Animal Center of Children's Hospital of Chongqing, China (specific pathogen free level, 21–23°C, 55 ± 5% humidity). Male and female rats were randomly mated in the same cage, leaving 9–11 neonatal rats in each delivery. Eighty postnatal day 7 rats were randomly selected, irrespective of gender, weighing 10–12 g, for in vivo evaluation of rat behavior. (2) One Sprague-Dawley rat, of clean grade, aged 4–8 weeks, weighing 100–300 g, was provided by the Animal Center, Daping Hospital, the Third Military Medical University, China for the extraction of bone marrow mesenchymal stem cells. (3) One postnatal day 1 Sprague-Dawley rat was used to obtain primary neurons. All experimental disposals of animals were in strict accordance with the Guidance Suggestions for the Care and Use of Laboratory Animals, issued by the Ministry of Science and Technology of China[49].
Instruments
A red photon instrument was developed by the staff at Chongqing University, China. It was composed of a micro-controller, current driver module, and high-power 660 nm light-emitting diode red lights (XL001WP01NRC, Hiran Optical Company, Shenzhen, Guangdong Province, China). STC89C52 Micro Control Unit was used for real-time control and to regulate light brightness, enabling real-time adjustment of light output power density. The range of output power density was 0–100 mW/cm2.
Methods
Harvesting primary neurons
Newborn Sprague-Dawley rats within 24 hours after birth were killed by decapitation, and the hippocampus and cortex tissue were isolated on ice[50]. Brain tissue was digested with TryPle (Gibco, Carlsbad, CA, USA) at 200 r/min to obtain a clear suspension. The suspension was centrifuged at 1,000 r/min for 5 minutes to obtain precipitates. The cells were cultured and suspended with Neurobasal + B27 mixed medium (Gibco, Carlsbad, CA, USA) and seeded onto polylysine (Sigma, Ronkonkoma, NY, USA)-coated 6-well plates. Half of the culture medium was changed after 1 day and completely replenished 2 days later, then changed every 3 days until day 8. The cultured cells were determined as the neurons, and not glia, upon the appearance of positive neuron-specific enolase and negative glial fibrillary acidic protein expression[50].
Establishing oxygen-glucose deprivation models
As previously reported[51], primary neurons were cultured with non-glucose balanced salt solution (11.6 mmol/L NaCl, 5.4 mmol/L KCl, 0.8 mmol/L MgSO4, 10 mmol/L NaH2 PO4, 26.2 mmol/L NaHCO3, 1.8 mmol/L CaCl2 and 10 mg/L phenol red, pH 7.4) for 2 hours to simulate ischemia. Then, neurons were cultured with 95% N2 + 5% O2 gas in a Tri-Gas incubator (Thermo Corporation, Shanghai, China) for 2 hours, to simulate hypoxia.
Bone marrow mesenchymal stem cell isolation and culture
Bone marrow mesenchymal stem cells were isolated and cultured according to previous studies[52]. In brief, after rats were killed under anesthesia, the femur and tibia were separated under sterile conditions, and the medullary canal was rinsed with DMEM/F12 medium and centrifuged. Subsequently, the fat and supernatant were discarded, and the resultant cell suspension at a density of 3 × 106/mL was incubated in a 50 mL culture flask with DMEM/F12 medium containing 10% fetal bovine serum. The flask was placed in a CO2 incubator (Thermo Corporation) with 5% CO2 at 80% humidity and 37°C for 48 hours. After culture medium was changed, non-adherent cells were removed, and the medium was changed every 2–3 days. When the cultured cells reached 80% confluence, cells were digested with 0.25% trypsin and subcultured for 4–6 generations. Prior to the experiment, the rat bone marrow mesenchymal stem cells were transfected with a GFP gene to label cells[53].
Bone marrow mesenchymal stem cell migration in vitro
Green fluorescence-labeled bone marrow mesenchymal stem cells were randomly divided into control and irradiation groups. The irradiation was performed using red light-emitting diodes at 660 nm and 60 mW/cm2 (Figure 2). In the cell incubator, light-emitting diode lights were placed close to the petri dish outer wall so that the light source irradiated the bone marrow mesenchymal stem cells within the incubator. Bone marrow mesenchymal stem cell migration and colonization on primary neurons after oxygen-glucose deprivation were tested by Transwell migration assay[54]. Briefly, the 6-well culture plates were covered with neurons after oxygen-glucose deprivation, then a Transwell petri dish (PIHT30R48, Millipore, Billerica, MA, USA) was placed into the 6-well plate. GFP-bone marrow mesenchymal stem cells were incubated in the Transwell petri dish (transparent membrane pore size was 0.4 μm). Six visual fields (200 × magnification) were randomly selected at 3, 15, 20, 28, and 40 hours to count the number of green fluorescently-labeled bone marrow mesenchymal stem cells.
Figure 2.
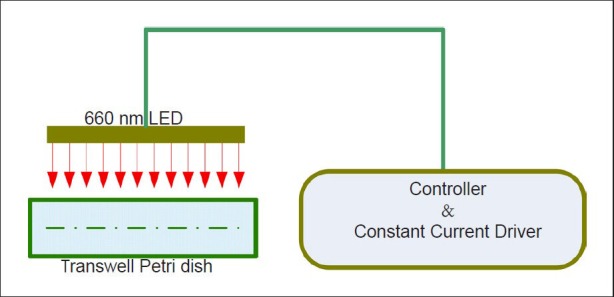
Diagram of bone marrow mesenchymal stem cells migration in vitro.
LED: Light-emitting diode.
Establishing hypoxic-ischemic brain damage model
Newborn Sprague-Dawley rats at 7 days after birth were used to establish the hypoxic-ischemic brain damage models according to the Rice method[55,56] with minor modifications. In brief, a median incision was made on the neck, subcutaneous fat was separated, and the left common carotid artery was isolated from the deep sternocleidomastoid muscle and ligated for 30 minutes using 4-0 sutures, producing ischemia. Then, rats were placed in an anoxic box for 2.5 hours, which was filled with 8% O2 + 92% N2, 3 L/min, to produce hypoxia. The normal control group was only subjected to neck skin incision and suture. After the damage was successfully produced, newborn rats were returned to the cage for maternal feeding. The success of hypoxic-ischemic brain damage models is defined upon the appearance of arterial transection scars and brain tissue atrophy by histological findings.
Bone marrow mesenchymal stem cell transplantation and phototherapy
After hypoxic-ischemic brain damage models were established for 24 hours, rats in the bone marrow mesenchymal stem cell transplantation group and phototherapy + bone marrow mesenchymal stem cell transplantation group were given intraperitoneal injection of 100 μL D-hanks solution containing 1 × 106 bone marrow mesenchymal stem cells. While rats in model group were treated with intraperitoneal PBS. In the phototherapy + bone marrow mesenchymal stem cell trans-plantation group, rats received irradiation under red light- emitting diodes at 660 nm and 60 mW/cm2 immediately after bone marrow mesenchymal stem cell transplantation, for 30 minutes per day. Rats in the supine position were fixed in a foil tank, light-emitting diode lights penetrated through the hole and directly irradiated the rat heads, but the rat body was blocked with opaque foil paper. Rats in other groups were fixed in the same way, but the light was turned off. After the irradiation is complete, the rats were returned to the cage with their mother (Figure 3).
Figure 3.
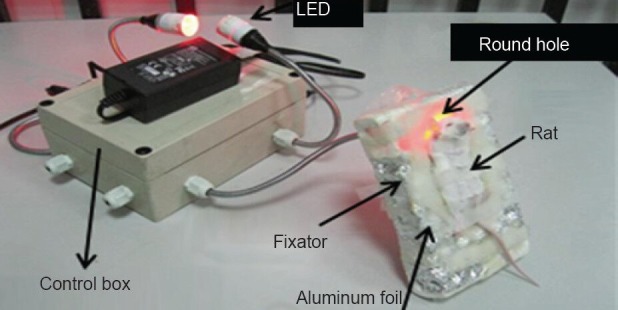
Irradiation in a rat.
LED: Light-emitting diode.
Shuttle box test of learning ability in rats
All rats were evaluated by shuttle box test after phototherapy was performed[46]. In brief, rats were placed in the shuttle box to adapt to the training for 3 minutes. After the light source was turned on, there was a buzzing sound, one side of the shuttle box bottom was energized, and the rat was stimulated with light on the other side (passive avoidance). After the training was completed, when the rats heard the buzzing sound, they immediately gave a response, running from the energized region to the non-energized region, and this was recorded as active avoidance. If rats failed to reach non-energized regions, the result was recorded as non-avoidance. The times of active avoidance and non-avoidance responses of 20 rats in each group were recorded. Active avoidance response rate is the percentage of active avoidance response times to total electrical stimuli times. Non-avoidance response rate is the percentage of non-avoidance response times to total electrical stimuli times. The percentages in 20 tests were calculated to reflect the active learning ability.
Statistical analysis
Data are expressed as mean ± SD and analyzed using SAS 8.1 software (SAS Institute Inc., Cary, NC, USA). Differences among groups were compared with one-way analysis of variance and pairwise comparisons were performed using Student-Newman-Keuls test. A P < 0.05 was considered statistically significant.
Footnotes
Funding: This study was supported by the National Natural Science Foundation of China, No. 30970758, 31271060; the National Science and Technology Support Program of China, No. 2011BAI14B04, 2012BAI16B02; the Natural Science Foundation of Chongqing in China, No. cstc2012jjA10103.
Conflicts of interest: None declared.
Copyedited by Wallace M, Wei JZ, Lv J, Yu J, Yang Y, Li CH, Song LP, Zhao M
References
- [1].Parer JT, King T. Fetal heart rate monitoring: is it salvageable? Am J Obstet Gynecol. 2000;182(4):982–987. doi: 10.1016/s0002-9378(00)70358-9. [DOI] [PubMed] [Google Scholar]
- [2].Almli CR, Levy TJ, Han BH, et al. BDNF protects against spatial memory deficits following neonatal hypoxia-ischemia. Exp Neurol. 2000;166(1):99–114. doi: 10.1006/exnr.2000.7492. [DOI] [PubMed] [Google Scholar]
- [3].Hosono T, Kamo A, Hakotani S, et al. Effect of hypothermia on motor function of adult rats after neonatal hyperthermic hypoxic-ischemic brain insult. Eur J Appl Physiol. 2010;109(1):35–39. doi: 10.1007/s00421-009-1156-9. [DOI] [PubMed] [Google Scholar]
- [4].Glass HC, Ferriero DM. Treatment of hypoxic-ischemic encephalopathy in newborns. Curr Treat Options Neurol. 2007;9(6):414–423. doi: 10.1007/s11940-007-0043-0. [DOI] [PubMed] [Google Scholar]
- [5].Bona E, Hagberg H, Løberg EM, et al. Protective effects of moderate hypothermia after neonatal hypoxia-ischemia: short- and long-term outcome. Pediatr Res. 1998;43(6):738–745. doi: 10.1203/00006450-199806000-00005. [DOI] [PubMed] [Google Scholar]
- [6].Eicher DJ, Wagner CL, Katikaneni LP, et al. Moderate hypothermia in neonatal encephalopathy: efficacy outcomes. Pediatr Neurol. 2005;32(1):11–17. doi: 10.1016/j.pediatrneurol.2004.06.014. [DOI] [PubMed] [Google Scholar]
- [7].Adachi M, Sohma O, Tsuneishi S, et al. Combination effect of systemic hypothermia and caspase inhibitor administration against hypoxic-ischemic brain damage in neonatal rats. Pediatr Res. 2001;50(5):590–595. doi: 10.1203/00006450-200111000-00010. [DOI] [PubMed] [Google Scholar]
- [8].Al-Waili NS, Butler GJ, Beale J, et al. Hyperbaric oxygen in the treatment of patients with cerebral stroke, brain trauma, and neurologic disease. Adv Ther. 2005;22(6):659–678. doi: 10.1007/BF02849960. [DOI] [PubMed] [Google Scholar]
- [9].Zhu CL, Xu FL. Progress in treatment of neonatal hypoxic-ischemic encephalopathy. Shiyong Erke Linchuang Zazhi. 2008;23(14):1062–1064. [Google Scholar]
- [10].van Velthoven CT, Kavelaars A, van Bel F, et al. Mesenchymal stem cell treatment after neonatal hypoxic-ischemic brain injury improves behavioral outcome and induces neuronal and oligodendrocyte regeneration. Brain Behav Immun. 2010;24(3):387–393. doi: 10.1016/j.bbi.2009.10.017. [DOI] [PubMed] [Google Scholar]
- [11].Yan XH, Yu Z, Liu W, et al. Effects of intravenous transplantation of human umbilical blood mesenchymal stem cells on the improvement of brain function after hypoxic-ischemic brain damage in neonatal rats. Zhongguo Zuzhi Gongcheng Yanjiu. 2012;16(45):8445–8452. [Google Scholar]
- [12].Luan Z, Yin GC, Hu XH, et al. Treatment of an infant with severe neonatal hypoxic-ischemic encephalopathy sequelae with transplantation of human neural stem cells into cerebral ventricle. Zhonghua Er Ke Za Zhi. 2005;43(8):580–583. [PubMed] [Google Scholar]
- [13].Pittenger MF, Martin BJ. Mesenchymal stem cells and their potential as cardiac therapeutics. Circ Res. 2004;95(1):9–20. doi: 10.1161/01.RES.0000135902.99383.6f. [DOI] [PubMed] [Google Scholar]
- [14].Jiang Y, Jahagirdar BN, Reinhardt RL, et al. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418(6893):41–49. doi: 10.1038/nature00870. [DOI] [PubMed] [Google Scholar]
- [15].Borlongan CV, Glover LE, Tajiri N, et al. The great migration of bone marrow-derived stem cells toward the ischemic brain: therapeutic implications for stroke and other neurological disorders. Prog Neurobiol. 2011;95(2):213–228. doi: 10.1016/j.pneurobio.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Lindvall O, Kokaia Z. Stem cells for the treatment of neurological disorders. Nature. 2006;441(7097):1094–1096. doi: 10.1038/nature04960. [DOI] [PubMed] [Google Scholar]
- [17].Liu Y, Zhang X, Dai Y, et al. Effects of bone marrow mesenchymal stem cells on learning and memory functional recovery in neonatal rats with hypoxic-ischemic brain damage. Zhonghua Erke Zazhi. 2008;46(9):648–653. [PubMed] [Google Scholar]
- [18].van Velthoven CT, Kavelaars A, van Bel F, et al. Repeated mesenchymal stem cell treatment after neonatal hypoxia-ischemia has distinct effects on formation and maturation of new neurons and oligodendrocytes leading to restoration of damage, corticospinal motor tract activity, and sensorimotor function. J Neurosci. 2010;30(28):9603–9611. doi: 10.1523/JNEUROSCI.1835-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Baksh D, Song L, Tuan RS. Adult mesenchymal stem cells: characterization, differentiation, and application in cell and gene therapy. J Cell Mol Med. 2004;8(3):301–316. doi: 10.1111/j.1582-4934.2004.tb00320.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Chen J, Li Y, Wang L, et al. Therapeutic benefit of intravenous administration of bone marrow stromal cells after cerebral ischemia in rats. Stroke. 2001;32(4):1005–1011. doi: 10.1161/01.str.32.4.1005. [DOI] [PubMed] [Google Scholar]
- [21].Li Y, Chen J, Wang L, et al. Treatment of stroke in rat with intracarotid administration of marrow stromal cells. Neurology. 2001;56(12):1666–1672. doi: 10.1212/wnl.56.12.1666. [DOI] [PubMed] [Google Scholar]
- [22].Shen LH, Li Y, Chen J, et al. One-year follow-up after bone marrow stromal cell treatment in middle-aged female rats with stroke. Stroke. 2007;38(7):2150–2156. doi: 10.1161/STROKEAHA.106.481218. [DOI] [PubMed] [Google Scholar]
- [23].Kurozumi K, Nakamura K, Tamiya T, et al. Mesenchymal stem cells that produce neurotrophic factors reduce ischemic damage in the rat middle cerebral artery occlusion model. Mol Ther. 2005;11(1):96–104. doi: 10.1016/j.ymthe.2004.09.020. [DOI] [PubMed] [Google Scholar]
- [24].McCarty RC, Gronthos S, Zannettino AC, et al. Characterisation and developmental potential of ovine bone marrow derived mesenchymal stem cells. J Cell Physiol. 2009;219(2):324–333. doi: 10.1002/jcp.21670. [DOI] [PubMed] [Google Scholar]
- [25].Zhang W, Yan Q, Zeng YS, et al. Implantation of adult bone marrow-derived mesenchymal stem cells transfected with the neurotrophin-3 gene and pretreated with retinoic acid in completely transected spinal cord. Brain Res. 2010;1359:256–271. doi: 10.1016/j.brainres.2010.08.072. [DOI] [PubMed] [Google Scholar]
- [26].Zhao LX, Zhang J, Cao F, et al. Modification of the brain-derived neurotrophic factor gene: a portal to transform mesenchymal stem cells into advantageous engineering cells for neuroregeneration and neuroprotection. Exp Neurol. 2004;190(2):396–406. doi: 10.1016/j.expneurol.2004.06.025. [DOI] [PubMed] [Google Scholar]
- [27].Desmet KD, Paz DA, Corry JJ, et al. Clinical and experimental applications of NIR-LED photobiomodulation. Photomed Laser Surg. 2006;24(2):121–128. doi: 10.1089/pho.2006.24.121. [DOI] [PubMed] [Google Scholar]
- [28].Yu W, Naim JO, McGowan M, et al. Photomodulation of oxidative metabolism and electron chain enzymes in rat liver mitochondria. Photochem Photobiol. 1997;66(6):866–871. doi: 10.1111/j.1751-1097.1997.tb03239.x. [DOI] [PubMed] [Google Scholar]
- [29].Szundi I, Liao GL, Einarsdóttir O. Near-infrared time-resolved optical absorption studies of the reaction of fully reduced cytochrome c oxidase with dioxygen. Biochemistry. 2001;40(8):2332–2339. doi: 10.1021/bi002220v. [DOI] [PubMed] [Google Scholar]
- [30].Karu TI, Kolyakov SF. Exact action spectra for cellular responses relevant to phototherapy. Photomed Laser Surg. 2005;23(4):355–361. doi: 10.1089/pho.2005.23.355. [DOI] [PubMed] [Google Scholar]
- [31].Yu HS, Wu CS, Yu CL, et al. Helium-neon laser irradiation stimulates migration and proliferation in melanocytes and induces repigmentation in segmental-type vitiligo. J Invest Dermatol. 2003;120(1):56–64. doi: 10.1046/j.1523-1747.2003.12011.x. [DOI] [PubMed] [Google Scholar]
- [32].Duan R, Zhu L, Liu TC, et al. Light emitting diode irradiation protect against the amyloid beta 25-35 induced apoptosis of PC12 cell in vitro. Lasers Surg Med. 2003;33(3):199–203. doi: 10.1002/lsm.10216. [DOI] [PubMed] [Google Scholar]
- [33].Hamblin MR, Demidova TN. Mechanisms of low level light therapy. Proc SPIE. 2006;6140:614001. [Google Scholar]
- [34].Medeiros DM, Jennings D. Role of copper in mitochondrial biogenesis via interaction with ATP synthase and cytochrome c oxidase. J Bioenerg Biomembr. 2002;34(5):389–395. doi: 10.1023/a:1021206220851. [DOI] [PubMed] [Google Scholar]
- [35].Kamali F, Bayat M, Torkaman G, et al. The therapeutic effect of low-level laser on repair of osteochondral defects in rabbit knee. J Photochem Photobiol B. 2007;88(1):11–15. doi: 10.1016/j.jphotobiol.2007.04.010. [DOI] [PubMed] [Google Scholar]
- [36].AlGhamdi KM, Kumar A, Moussa NA. Low-level laser therapy: a useful technique for enhancing the proliferation of various cultured cells. Lasers Med Sci. 2012;27(1):237–249. doi: 10.1007/s10103-011-0885-2. [DOI] [PubMed] [Google Scholar]
- [37].Liang HL, Whelan HT, Eells JT, et al. Near-infrared light via light-emitting diode treatment is therapeutic against rotenone- and 1-methyl-4-phenylpyridinium ion-induced neurotoxicity. Neuroscience. 2008;153(4):963–974. doi: 10.1016/j.neuroscience.2008.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Wong-Riley MT, Liang HL, Eells JT, et al. Photobiomodulation directly benefits primary neurons functionally inactivated by toxins: role of cytochrome c oxidase. J Biol Chem. 2005;280(6):4761–4771. doi: 10.1074/jbc.M409650200. [DOI] [PubMed] [Google Scholar]
- [39].Hou JF, Zhang H, Yuan X, et al. In vitro effects of low-level laser irradiation for bone marrow mesenchymal stem cells: proliferation, growth factors secretion and myogenic differentiation. Lasers Surg Med. 2008;40(10):726–733. doi: 10.1002/lsm.20709. [DOI] [PubMed] [Google Scholar]
- [40].Arteni NS, Salgueiro J, Torres I, et al. Neonatal cerebral hypoxia-ischemia causes lateralized memory impairments in the adult rat. Brain Res. 2003;973(2):171–178. doi: 10.1016/s0006-8993(03)02436-3. [DOI] [PubMed] [Google Scholar]
- [41].Balduini W, De Angelis V, Mazzoni E, et al. Long-lasting behavioral alterations following a hypoxic/ischemic brain injury in neonatal rats. Brain Res. 2000;859(2):318–325. doi: 10.1016/s0006-8993(00)01997-1. [DOI] [PubMed] [Google Scholar]
- [42].Wang LS, Zhou J, Shao XM, et al. Huperzine A attenuates cognitive deficits and brain injury after hypoxia-ischemic brain damage in neonatal rats. Zhonghua Er Ke Za Zhi. 2003;41(1):42–45. [PubMed] [Google Scholar]
- [43].Liu LP, Na HR. Effects of bone marrow mesenchymal stem cells on memory functional in neonatal rats with hypoxic-ischemic brain damage. Zhongguo Laonian Xue Zazhi. 2011;31(5):2910–2911. [Google Scholar]
- [44].Li Y, Chen J, Wang L, et al. Treatment of stroke in rat with intracarotid administration of marrow stromal cells. Neurology. 2001;56(12):1666–1672. doi: 10.1212/wnl.56.12.1666. [DOI] [PubMed] [Google Scholar]
- [45].Parekkadan B, Milwid JM. Mesenchymal stem cells as therapeutics. Annu Rev Biomed Eng. 2010;12:87–117. doi: 10.1146/annurev-bioeng-070909-105309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Huang YY, Chen AC, Carroll JD, et al. Biphasic dose response in low level light therapy. Dose Response. 2009;7(4):358–383. doi: 10.2203/dose-response.09-027.Hamblin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Liu W, Wu AQ, Li WQ, et al. Effect of He-Ni Laser on brain injury after hypoxia-ischemia in newborn rats. Zhongguo Linchuang Kangfu. 2004;8(16):3058–3060. [Google Scholar]
- [48].Jiang W, Yu Q, Gong M, et al. Vitamin A deficiency impairs postnatal cognitive function via inhibition of neuronal calcium excitability in hippocampus. J Neurochem. 2012;121(6):932–943. doi: 10.1111/j.1471-4159.2012.07697.x. [DOI] [PubMed] [Google Scholar]
- [49].The Ministry of Science and Technology of the People's Republic of China. Guidance Suggestions for the Care and Use of Laboratory Animals. 2006-09-30 [Google Scholar]
- [50].Jiang W. Chongqing: Chongqing Medical University; 2011. Effect and mechanism of vitamin A in the rats with ischemic brain injury. [Google Scholar]
- [51].Singh G, Siddiqui MA, Khanna VK, et al. Oxygen glucose deprivation model of cerebral stroke in PC-12 cells: glucose as a limiting factor. Toxicol Mech Methods. 2009;19(2):154–160. doi: 10.1080/15376510802355216. [DOI] [PubMed] [Google Scholar]
- [52].Kang XQ, Zang WJ, Song TS, et al. Isolation, culture and morphology observation of rat bone marrow mesenchymal stem cells. Xi’an Jiaotong Daxue Xuebao: Yixue Ban. 2003;24(5):518–519. [Google Scholar]
- [53].Zhou GD, Wang XY, Liu DL, et al. In vivo tracing of bone marrow stromal cell differentiating into chondrocytes by green fluorescent protein gene transfection. Xibao yu Fenzi Mianyi Xue Zazhi. 2004;20(1):27–30. [PubMed] [Google Scholar]
- [54].Shen MQ. Tianjin: Tianjin Medical University; 2010. In vitro study of mesenchymal stem cell therapy in neonatal rats with hypoxic-ischemic encephalopathy. [Google Scholar]
- [55].Sveinsson OA, Gudjonsson T, Petersen PH. The application of stem cells for research and treatment of neurological disorders. Laeknabladid. 2008;94(2):117–122. [PubMed] [Google Scholar]
- [56].Tuby H, Maltz L, Oron U. Low-level laser irradiation (LLLI) promotes proliferation of mesenchymal and cardiac stem cells in culture. Lasers Surg Med. 2007;39(4):373–378. doi: 10.1002/lsm.20492. [DOI] [PubMed] [Google Scholar]


