Abstract
Carbon nanotubes can carry protein into cells to induce biological effects. Amino-functionalized carbon nanotubes are soluble and biocompatible, have high reactivity and low toxicity, and can help promote nerve cell growth. In this study, amino-functionalized ethylenediamine-treated multi-walled carbon nanotubes were used to prepare carbon nanotubes-nerve growth factor complexes by non-covalent grafting. The physicochemical properties, cytotoxicity to PC12 and chick embryo dorsal root ganglion, and biological activity of the carbon nanotubes-nerve growth factor complexes were investigated. The results showed that amino functionalization improved carbon nanotubes-nerve growth factor complex dispersibility, reduced their toxicity to PC12 cells, and promoted PC12 cell differentiation and chick embryo dorsal root ganglion.
Keywords: nerve regeneration, spinal cord injury, carbon nanotubes, amino functionalization, nerve growth factor, cytotoxicity, biological activity, dispersibility, PC12 cells, chick embryo dorsal root ganglion, NSFC grant, neural regeneration
Introduction
Carbon nanotubes (CNT) have unique one-dimensional structure. Various molecules can adsorb to their outer surface, which can be bonded to a variety of chemical groups to achieve solubilization and targeting, while their internal space can entrap ions and small molecules. CNTs can cross the cell membrane with minimal damage in certain biomedical contexts. Therefore, they are very promising for drug delivery, molecular imaging, and gene therapy applications[1,2,3,4]. The typical layered and hollow structure of CNTs is formed by carbon atoms in an aromatic, delocalized system consisting of a single layer or multilayer curled graphite sheet assemblies[5,6,7,8]. Because of this unique stable structure, CNTs are almost insoluble in any solvent, including water and common organic solvents. In solution, CNT molecules tend to aggregate, bend, entwist, and gather into bundles because of strong Van der Waals forces, making them toxic. This toxicity not only hinders the basic science research of CNTs at the molecular level, but also leads to their poor biocompatibility, which greatly limits their application in many fields[9,10]. Functional modification of CNTs can improve their solubility and biocompatibility, leading to increased utility of CNTs as drug carriers[11,12,13,14,15,16,17]. The amino group is an important modifier group in biochemistry because many chemicals, polymers, and biological molecules can be connected to carriers through an amide bond. Therefore, surface amination of CNTs is one way to make CNTs more useful in pharmaceutics. Aminization maintains the benefits of CNTs, such as solubility, biocompatibility, high reactivity, and low toxicity. The amino terminals can form covalent bonds with a variety of polymers and biologically active substances, which makes CNTs very promising in the pharmaceutical field[18,19,20,21,22]. Coccini et al.[23,24] found that aminized multiwalled CNTs (MWCNT) exhibited improved solubility and dispersity, decreased toxicity to human A549 and D384 cells, and decreased aggregation.
Nerve growth factor (NGF) is a protein that plays an important role in self-protection and repair of degenerative changes during neuronal growth, survival, and differentiation, and also protects neurons from nerve injury[25,26,27,28,29]. However, because of the rapid inactivation of NGF in aqueous solutions and its vulnerability to a variety of environmental factors, such as temperature and pH, it is difficult for NGF to penetrate the blood-brain barrier, resulting in a short half-life[30,31]. Thus, repeated administration of NGF is required to maintain an effective concentration, which seriously restricts any clinical application of NGF. Functionalized CNTs can be used as slow-release carriers for chemical drugs, DNA, and proteins[32,33,34,35,36,37,38,39,40,41]. Kam et al.[42] demonstrated that when aminized CNTs were connected to fluorescent biotin-streptavidin and the complexes were incubated with HL60 cells, strong anti-biotin streptavidin fluorescent signals were found in tumor cells, suggesting CNTs can carry macromolecular proteins into cells.
Carboxylation of CNTs before aminization increases the reactivity of CNTs compared with direct aminization, and improves the subsequent aminization[24]. Murugesan and colleagues[43] mixed MWCNTs with carboxyl MWCNTs in ethylenediamine (EDA) for 3 hours at 30°C to obtain two different aminized CNTs. Carboxylation before the condensation reaction increases the number of amino groups on the surface of CNTs and makes them more reactive. Yen et al.[44] prepared amino-MWCNTs by chloride reaction of EDA, and found that the amino-MWCNTs promoted nerve cell growth due to the positive charges on the surface amino groups. Therefore, amino-CNTs carrying NGF were created in this study to promote nerve cell growth.
Results
The morphology of MWCNTs-EDA
The morphology of MWCNTs-COOH did not change significantly after EDA modification (MWCNTs-EDA) as observed by transmission electron microscopy (Figure 1).
Figure 1.
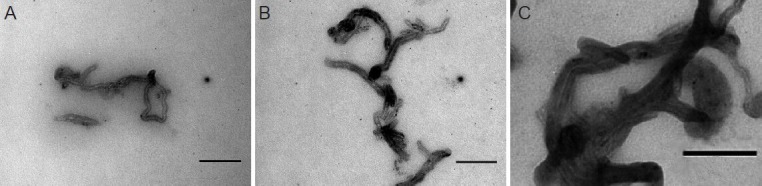
Transmission electron microscopy images of MWCNTs-COOH (A), MWCNTs-EDA (B), and MWCNTs-EDA-NGF (C).
There was no apparent differences between the morphology of MWCNTs-COOH (A) and MWCNTs-EDA (B), while the surface of the MWCNTs- EDA-NGF complex (C) is rougher and thicker, with more nanotube adhesion than on MWCNTs-EDA. Scale basr: 200 nm. MWCNTs: Multi-walled carbon nanotubes; EDA: ethylenediamine; NGF: nerve growth factor.
The chemical structure of MWCNTs-EDA
Figure 2 displays the thermogravimetric analysis (TGA) curves of EDA, MWCNTs-COOH, and MWCNTs-EDA. The weight loss temperature of EDA was between 150–500°C, showing a characteristic weight loss stage with 94.02% change in mass. The temperature at the fastest weight loss rate was 119.074°C, and no apparent change occurred after 200°C. The weight loss stages of EDA and the physical mixing product of EDA and MWCNTs-COOH were nearly the same. MWCNTs-COOH showed no evident weight loss at temperatures ranging from 150 to 500°C (Figure 2A, B), while MWCNTs-EDA exhibited a weight loss stage in the same temperature range. Furthermore, the weight loss temperature of MWCNTs-EDA was significantly higher than that of the physical mixture of EDA and MWCNTs-COOH. These results verify that MWCNTs-COOH were modified by EDA.
Figure 2.
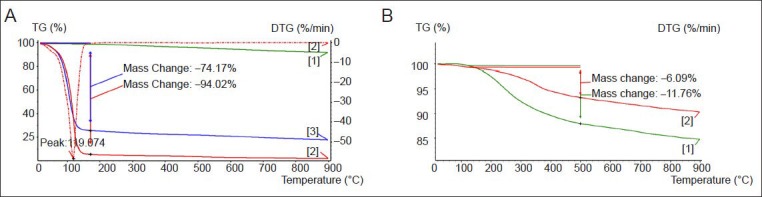
Thermogravimetric analysis (TGA) curves.
(A) MWCNTs-COOH [1], EDA [2], and MWCNTs mixed with EDA [3]. (B) Dialyzed [1] and undialyzed [2] MWCNTs-EDA. The weight loss temperature of MWCNTs-COOH is significantly higher than that of the physical mixing product of EDA and MWCNTs-COOH. MWCNTs: Multi-walled carbon nanotubes; EDA: ethylenediamine; TG: thermogravimetric analysis; DTG: differential thermogravimetry.
Infrared spectroscopy of MWCNTs-COOH and MWCNTs-EDA also showed that EDA was successfully grafted to the surface of CNTs[45] (Figure 3).
Figure 3.
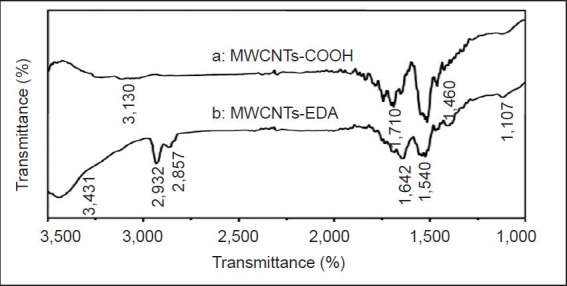
Fourier transform infrared spectroscopy of the MWCNTs- COOH (curve a) and MWCNTs-EDA (curve b).
(A) Infrared spectroscopic characterization of carboxylic CNTs shows that MWCNT surfaces have functional groups, including carbonyl groups at 710/cm and carboxyl groups at 3,130 and 1460/cm. (B) A shift of the band center from 1,710/cm to 1,642/cm indicates that the carboxylic acid groups in MWCNTs completely reacted with the amino groups in EDA to form amide groups. Infrared absorption of the free amino groups in EDA appears at 3,431/cm because of the stretching mode of the N-H bond and at 1,540 and 1,107/cm because of the in-plane vibration mode of the N-H and C-N bonds. The bands at 2,932 and 2,857/cm result from the C-H stretch vibration. MWCNTs: Multi-walled carbon nanotubes; EDA: ethylenediamine.
Cytotoxicity of MWCNTs-COOH and MWCNTs-EDA
Figure 4 shows the effects of MWCNTs-EDA and MWCNTs-COOH on the viability of PC12 cells as determined by the MTT method. PC12 cells were incubated with CNTs at different concentrations (3.125, 6.25, 12.5, 25, 50, 100 and 200 μg/mL; 100 μL/well) in PBS for over 48 hours.
Figure 4.
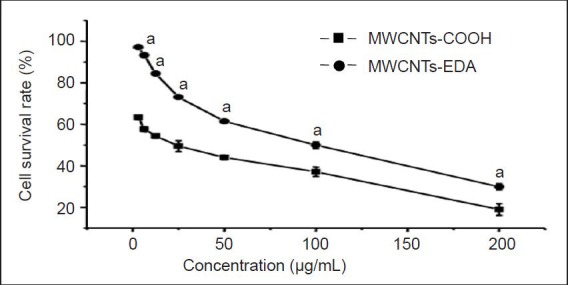
Viability of PC12 cells at different concentrations of complexes after incubation for 48 hours (MTT).
The MTT assay shows that the number of living cells gradually decreases with the increase in MWCNT complex concentration after culture for 48 hours. The experimental data are expressed as mean ± SD (n= 8), assessed by one-way analysis of variance. aP < 0.05, vs. MWCNTs-COOH. MWCNTs: Multi-walled carbon nanotubes; EDA: ethylenediamine.
The survival rate of the PC12 cells incubated with both types of CNTs decreased with increasing CNT concentration in the range of 3.125–200 μg/mL. Cell viability was significantly lower (P < 0.05) after exposure to MWCNTs-COOH compared with MWCNTs-EDA, suggesting that the biocom-patibility of the covalently modified MWCNTs-COOH was improved.
Figure 5 shows the morphological effects of MWCNTs-EDA and MWCNTs-COOH at the concentrations listed above. Most PC12 cells in the control group were round, lucent, and well-attached. In contrast, with increasing concentration of MWCNTs, the number of adherent and viable cells gradually decreased, and the cells and MWCNTs gathered in clusters, suggesting an increase in the cytotoxicity of the MWCNTs. The cells treated with MWCNTs-EDA were more adherent and had higher refractivity compared with those treated with MWCNTs-COOH. Together, these results indicate that MWCNTs-EDA were less toxic to to PC12 cells than MWCNTs-COOH.
Figure 5.

Photographs of PC12 cells after incubation with MWCNTs-EDA for 48 hours (× 200).
Control group (A) contains no CNTs, only cells. From the inverted microscopy images, the numbers of adhered and viable cells decreased, and the refractivity (arrows) of PC12 cells falls, after culture with 50 μg/mL MWCNTs-COOH (B) and MWCNTs-EDA (C) for 48 hours. Furthermore, the changes in the MWCNTs-COOH group are greater than those in the MWCNTs-EDA group. MWCNTs: Multi-walled carbon nanotubes; EDA: eth-ylenediamine.
Transmission electron microscopy characterization of the MWCNTs-NGF complexes
The surface of MWCNTs-NGF complexes is rougher and thicker than nanotubes without amino functionalization (Figure 1).
Dispersity of MWCNTs-EDA and MWCNTs-NGF
The dispersity of MWCNTs after different treatments was studied (Figure 6) by dissolving 1 mg MWCNTs-COOH, 1 mg MWCNTs-EDA, 1 mg MWCNTs-COOH-NGF, and 1 mg MWCNTs-EDA-NGF complex each in 10 mL PBS and sonicating for 15 minutes. While MWCNTs-COOH formed apparent aggregates within 3 months, MWCNTs-EDA remained dispersed in PBS over the 3 months. Similarly, MWCNTs-COOH-NGF formed distinct aggregates in 3 months while MWCNTs-EDA-NGF displayed much better dispersity in PBS over the 3 months. These results suggest that the amino functionalization of CNTs enhanced their dispersibility in PBS.
Figure 6.
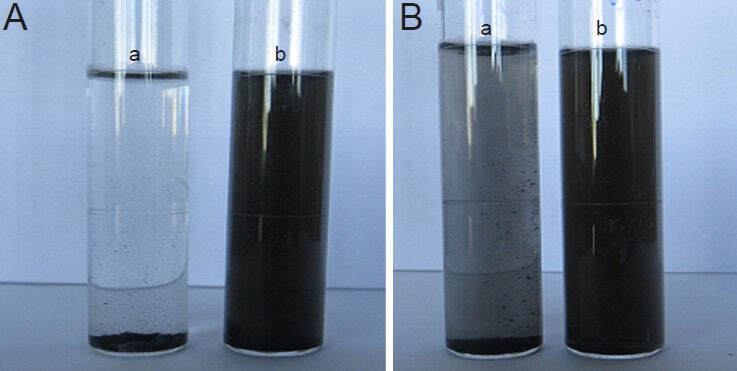
Dispersity of MWCNTs-EDA and MWCNT-COOH-NGF complexes in PBS after more than 3 months.
(A) MWCNT-COOH [a] and MWCNTs-EDA [b]. (B) MWCNT-COOH-NGF [a] and MWCNTs-EDA-NGF [b]. MWCNTs: Multi-walled carbon nanotubes; EDA: ethylenediamine; NGF: nerve growth factor.
Biological activity of MWCNTs-NGF complexes
Figure 7 illustrates the morphology of the PC12 cells cultured with MWCNTs-NGF complexes after 24 and 72 hours. Most of the cells in the negative control group were round, grew well, and showed no indicators of differentiation. In contrast, PC12 cells in the positive control group (NGF solution, 50 ng/mL) and incubated with MWCNTs-NGF complexes began to differentiate, extended protuberances, and stopped proliferating. The biological activity of cells incubated with MWCNTs-EDA-NGF was notably stronger than that of cells with MWCNTs-COOH-NGF. The MWCNTs-EDA-NGF complexes induced significant cell differentiation and cell protuberances were longer, more dense, and woven into meshes.
Figure 7.
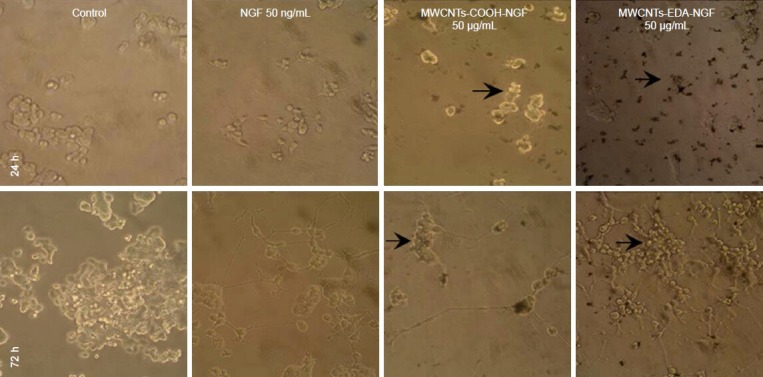
Optical micrographs of PC12 cells treated with different MWCNTs-NGF samples (× 200).
The MWCNTs-EDA-NGF complexes significantly induce cell differentiation and the growing protuberances are long, dense, and woven into mesh-es. The density of these cells is clearly higher than that of cells cultured with MWCNTs-COOH-NGF complexes. MWCNTs: Multi-walled carbon nanotubes; EDA: ethylenediamine; NGF: nerve growth factor; h: hours.
The density of these cells was clearly higher than those cultured with MWCNTs-COOH-NGF complexes, which suggests indirectly that the cytotoxicity of MWCNTs-COOH-NGF was higher than that of MWCNTs-EDA-NGF.
The 90% F12 with 10% horse serum media was chosen for the biological activity study because dorsal root ganglia cultured in it for 72 hours proliferated were firmly adherent, and showed no dendritic protuberances. When dorsal root ganglia were cultured in 90%F12 with fetal bovine serum, dendritic nerve fibers grew outwards, which would affect the results of the biological activity tests. Cells cultured in only F12 proliferated slowly and were not firmly adherent (Figure 8).
Figure 8.
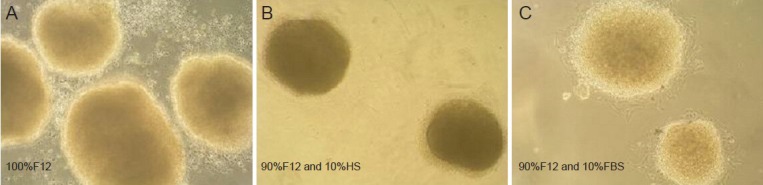
Optical micrographs of dorsal root ganglia (DRG) incubated in different solutions (× 100).
(A) DRG cultured with only F12 show poor growth and are not firmly adherent. (B) The 90% F12 and 10% horse serum were chosen for the bio-logical activity testing because DRG cultured in it for 72 hours proliferated and were firmly adherent without developing dendritic protuberances. (C) Dendritic nerve fibers extending out of cells cultured with media containing 90% F12 and 10% fetal bovine serum.
Figure 9 shows images of dorsal root ganglions that were co-cultured with MWCNTs-NGF for 24 and 72 hours. Dorsal root ganglion morphology in the negative control group was not changed, while a large number of slender radial nerve fibers grew outwards when dorsal root ganglia in the positive control group were cultured with 50 ng/mL NGF solution for 72 hours. On the contrary, the morphology of dorsal root ganglia that were co-cultured with MWCNTs-NGF complexes at different concentrations of 1640 solution (25, 50, 100, 200, 400, and 500 μg/mL) for 72 hours was altered remarkably. MWCNTs-COOH-NGF were concentrated around the dorsal root ganglion, where a small number of short and thick nerve fibers had grown. In contrast, there was no accumulation of CNTs around the dorsal root ganglia exposed to MWCNTs-EDA-NGF, where a large number of long and thin nerve fibers had grown. Based on these results, it appears that MWCNTs-EDA-NGF complexes promoted the growth of dorsal root ganglion neurons and demonstrated less toxicity to dorsal root ganglia than the MWCNTs-COOH-NGF complexes.
Figure 9.
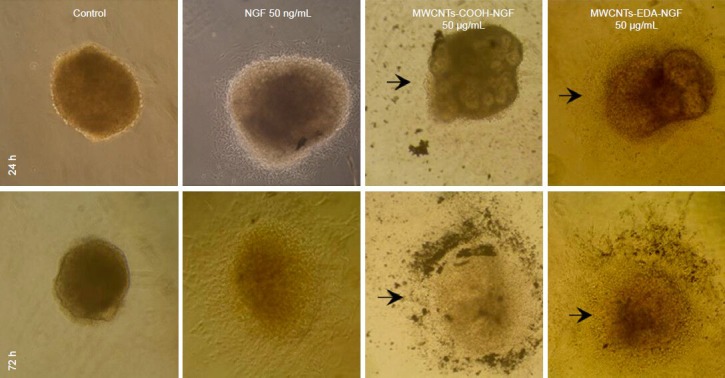
Optical micrographs of dorsal root ganglia (DRG) incubated with different MWCNTs-NGF samples (× 100).
The MWCNTs-COOH-NGF accumulate near the DRG and a small number of short and thick nerve fibers are seen growing (arrows). In contrast, there is no CNTs accumulation near the DRG and a large number of long and thin nerve fibers are found growing from the ganglion of MWCNTs- EDA-NGF samples (arrows). MWCNTs: Multi-walled carbon nanotubes; EDA: ethylenediamine; NGF: nerve growth factor. h: hours.
Discussion
The PC12 cells were fully and firmly adherent, difficult to cluster, and proliferated in the 1640 medium containing 10% horse serum and 5% fetal bovine serum that was recommended in a study by Zheng et al. The MTT assay showed that the cells treated with MWCNTs-EDA displayed significantly higher viability than those treated with MWCNTs-COOH. Cell morphology and state of adherence, which directly affect survival, were enhanced more after exposure to MWCNTs-EDA than to MWCNTs-COOH. Other groups have reported that amino-functionalized MWCNTs modified with EDA exhibited high cell viability[44], largely due to the promotion of nerve cell growth. We found no indicators of differentiation of the PC12 cells after co-culture with amino-functionalized MWCNTs for 48 hours. This result is not consistent with the findings of Yen et al.[44], which may be due to the short co-culture duration of PC12 cells with amino-functionalized MWCNTs. Dispersibility testing showed that MWCNTs-EDA had good dispersion stability before and after modification with nerve growth factor, suggesting that the functionalization with amino groups improved the CNT solubility.
Our results confirmed that MWCNTs-NGF complexes prepared by non-covalent grafting have similar biological effects. The biological activity tests showed that both MWCNTs-COOH and MWCNTs-EDA non-covalently grafted with NGF can promote nerve cell differentiation. Because ELISA methods can not accurately measure the content of complexed nerve growth factor, it is difficult to quantitatively explain its enhancement of nerve cell differentiation, and we will try to determine the amount of nerve growth factor in the complexes in future experiments. MWCNTs-EDA-NGF complexes, in comparison with MWCNTs-COOH-NGF complexes, showed better cell adherence, higher cell density, clear cell differentiation, and a denser nerve fiber web.
Isolation and cultivation of the dorsal root ganglion is the first problem encountered when investigating the biological activity of these complexes. Embryos 8–9 days old were the most suitable material for isolation of dorsal root ganglia. Dorsal root ganglia exhibited different states after being cultured with various media formulations. The results from this study showed that 90% F12 with 10% horse serum can be used for studying biological activity because dorsal root ganglia cultured in this formulation for 72 hours exhibited proliferation, were firmly adherent, and had no dendritic processes. The dorsal root ganglia cultured with 10% fetal bovine serum and 90% F12 were stretched out with branched nerve fibers, which could influence the biological activity tests. The dorsal root ganglia that were cultured with medium containing only F12 were not firmly adherent and proliferated poorly. The biological activity tests of the complexes cultured with dorsal root ganglia showed that both kinds of MWCNTs-NGF complexes promoted the growth of nerve fibers from the dorsal root ganglia, but to different degrees. The MWCNTs-EDA-NGF complexes induced a denser radial nerve fiber net compared with the MWCNTs-COOH-NGF complexes.
Taken together, these data suggest that MWCNTs-EDA had higher dispersibility and lower cytotoxicity than carboxylated CNTs. Non-covalently grafted MWCNTs-NGF complexes maintained biological activity. Furthermore, MWCNTs-EDA-NGF complex promoted PC12 cell differentiation and dorsal root ganglion nerve growth more than MWCNTs-COOH-NGF complexes.
Materials and Methods
Design
An in vitro cytology experiment.
Time and setting
This study was performed at the Key Laboratory of the Ministry of Education of Xinjiang Uygur Autonomous Region, School of Pharmacy, Shihezi University, China between September 2011 and January 2013.
Materials
Pathogen-free 9-day-old chick embryos were provided by the Laboratory Animal Center, Xinjiang Medical University, China with the license No. SCXK 2003-001 and included in this study. The obtained chick embryos were incubated in a thermotank at 38°C and 75% humidity.
PC12 cells were purchased from The Cell Bank of Type Culture Collection of Chinese Academy of Sciences, China and then cultured in RPMI 1640 culture medium (Gibco, Carlsbad, CA, USA) containing 10% horse serum (Gibco), 5% fetal bovine serum (Gibco), and 100 U penicillin/streptomycin (Gibco) in a 5% CO2 incubator at 37°C.
Methods
Preparation of MWCNTs-EDA
Dried MWCNTs-COOH (1.0 g) and excess neat SOCl2(100 mL) were first sonicated for 30 minutes and then stirred with a magnetic stirrer at room temperature for 48 hours. The suspension was filtered through a 0.45-μm microporous membrane. The excess SOCl2 was washed at least 5 times with tetrahydrofuran and evaporated under vacuum at room temperature for 20 minutes. The residue was then reacted with 100 mL of EDA at room temperature for 10 hours with magnetic stirring. Next, the suspension was filtered through a 0.45 μm microporous membrane and washed at least 5 times with tetrahydrofuran, and then evaporated under vacuum at room temperature for 20 minutes. Finally, the blank solid was dialyzed (MWCO 14 kDa) in pure water for 72 hours and dried at room temperature under vacuum to yield MWCNTs-EDA. A JEM-1230 transmission electron microscope (JEOL, Tokyo, Japan) was used to observe the morphology of MWCNTs-EDA, an STA409PC type differential TGA scanning calorimeter (NETZSCH Scientific Instrument Trading Co., Ltd., Shanghai, China) was used to measure the TG curve of MWCNTs-EDA, and the IR spectra of MWCNTs-EDA was detected by an FTIR-8400S Fourier transform infrared spectrometer (Shimadzu, Kyoto, Japan) to characterize the chemical structure of MWCNTs-EDA. MWCNTs-COOH was used as a control.
Cytotoxicity of MWCNTs-EDA
PC12 cells in the logarithmic growth phase were seeded in 96-well plates at a density of 1 × 104 per well and incubated at 37°C in a 5% CO2 incubator for 24 hours. Concentrations of MWCNTs-COOH and MWCNTs-EDA in PBS (3.125, 6.25, 12.5, 25, 50, 100, and 200 μg/mL) were added to the 96-well plates with 100 μL in each well, and eight parallel wells were designated for each concentration. Furthermore, a blank experimental group (carbon nanotubes, no PC12 cells), a control group (PC12 cells, no carbon nanotubes) and a blank control group (no PC12 cells, no carbon nanotubes) were assessed. The culture conditions were the same for the experimental and control groups. PC12 cells were incubated at 37°C in a 5% CO2 incubator for 48 hours. A 20-μL volume of MTT solution (Sigma, St. Louis, MO, USA) was added to each well and they were cultured for another 4 hours. The culture medium in each well was carefully aspirated and the reaction was stopped by adding 150 μL dimethyl sulfoxide. After low speed oscillation for 10 minutes to fully dissolve the formed crystals, the absorbance value at 490 nm was measured with a Thermo 3001 multifunctional ELISA reader (Thermo, Franklin, MA, USA).
Cell viability was calculated as: cell viability = (Aexperimental group–Ablank experimental group)/(Acontrol group–Ablank control group) × 100%.
Each experiment was repeated three times. The morphology of PC12 cells was observed with an IX71 fluorescence inverted microscope (Olympus, Tokyo, Japan).
Preparation of MWCNTs-NGF complexes
A 2-mg mass of MWCNTs-EDA was fully mixed with 9 mL PBS and then 1 mL of PBS containing rat nerve growth factor (Sigma, 30 μg/mL) was added. The resultant mixture was stirred for 24 hours at room temperature and then centrifuged at 10,000 r/min for 10 minutes in centrifuge with a radius of 86 mm. After removing the supernatant, the product was centrifuged again after washing with PBS. Next, the product was dispersed in 10 mL PBS to create the solution of MWCNTs-NGF complex. The micromorphology of the MWCNTs-NGF complex was observed using an H600 transmission electron microscope (Hitachi, Tokyo, Japan).
Dispersity of the MWCNTs-NGF complex
MWCNTs-EDA (1 mg) and MWCNTs-NGF (1 mg) complexes were separately added to 10 mL PBS, and the suspensions were then sonicated for 15 minutes. The dispersed state of the complexes was observed for a certain period of time to study the dispersibility of the CNTs and the complexes, using MWCNTs-COOH and MWCNTs-COOH-NGF complex as controls.
Isolation and cultivation of chick embryo dorsal root ganglia
Chick embryo dorsal root ganglia were isolated using an Olympus SZX7 dissecting microscope (Olympus, Tokyo, Japan), and were grown in a 37°C, 5% CO2 incubator. Several chick embryo dorsal root ganglia were cultured with three different media formulations and were separately investigated after 72 hours. The medium ratios were: 100% F12 (Hyclone, Logan, UT, USA); 90%F12 and 10% fetal bovine serum (Gibco); and 90% F12 and 10% horse serum (Gibco). The medium containing 90% F12 and 10% horse serum (Gibco) was selected for general use based on the results.
Biological activity of the MWCNTs-NGF complex
Biological activity testing was performed to determine the effects of the MWCNTs-NGF complex on PC12 cells and chick embryo dorsal root ganglia. The MWCNTs-COOH and NGF complex formed by non-covalent grafting was taken as a control. Nerve growth factor at 50 ng/mL was used as a positive control group, and serum-free RPMI 1640 culture medium was used as a negative control group. The two types of MWCNTs-NGF complex samples were separately dissolved in RPMI 1640 culture medium to produce different solution concentrations: 25, 50, 100, 200, and 400 μg/mL with 500 μL per well. PC12 cells were seeded into 24-well plates at a density of 2 × 104 per well, and chick embryo dorsal root ganglia were seeded into the 24-well plates at a density of 8 ganglia per well. PC12 cells were observed with an IX71 fluorescence inverted microscope (Olympus) after incubation at 37°C in a 5% CO2 incubator for 24 or 72 hours.
Statistical analysis
All data are expressed as mean ± SD and were analyzed using SPSS 13.0 software (SPSS, Chicago, IL, USA). One-way analysis of variance was used to compare differences am-ong groups. A level of P < 0.05 was considered statistically significant.
Footnotes
Funding: This study was supported by the National Natural Science Foundation of China, No. 81160395.
Conflicts of interest: None declared.
Copyedited by McCarty W, Norman C, Shi XY, Zhang W, Wang J, Li CH, Song LP, Zhao M
References
- [1].Zhang HW, Zhu BK, Xu YY. Proton conductivity and methanol permeability of composite membranes based on sulfonated poly (phthalazinone ether ketone) doped with phosphotungstic acid. Gongneng Cailiao. 2006;37(10):1590–1592. [Google Scholar]
- [2].Foldvar M, Bagonluri M. Carbon nanotubcs as functional excipients for nanomedicines: II. Drug delivery and biocompatibility issues. Nanomedicine. 2008;4(3):183–200. doi: 10.1016/j.nano.2008.04.003. [DOI] [PubMed] [Google Scholar]
- [3].Hamon MA, Chen J, Hu H, et al. Dissolution of single-walled carbon nanotubes. Adv Mater. 1999;11(10):834–840. [Google Scholar]
- [4].Magrez A, Kasas S, Salicio V, et al. Cellular toxicity of carbon-based nanomaterials. Nano Lett. 2006;6(6):1121–1125. doi: 10.1021/nl060162e. [DOI] [PubMed] [Google Scholar]
- [5].Dasgupta K, Joshi JB, Banerjee S. Fluidized bed synthesis of carbon nanotubes. Chem Eng J. 2011;171(3):841–869. [Google Scholar]
- [6].Cao W, Song XM, Wang B, et al. Research progress in carbon nanotube. Cailiao Daobao. 2007;21:77–82. [Google Scholar]
- [7].Huang DC, Huang DH. Carbon nanotubes and their applications. Wuli Xue Jinzhan. 2004;24(3):274–288. [Google Scholar]
- [8].Ajayan PM. Nanotubes from carbon. Chem Rev. 1999;99(7):1787–1800. doi: 10.1021/cr970102g. [DOI] [PubMed] [Google Scholar]
- [9].Xiao Q, Wang PH, Si ZC. Covalent functionalization of carbon nanotubes. Huaxue Jinzhan. 2007;19(1):101–106. [Google Scholar]
- [10].Dyke CA, Tour JM. Overcoming the insolubility of carbon nanotubes through high degrees of sidewall functionalization. Chemistry. 2004;10(4):812–817. doi: 10.1002/chem.200305534. [DOI] [PubMed] [Google Scholar]
- [11].Bianco A. Carbon nanotubes for the delivery of therapeutic molecules. Expert Opin Drug Deliv. 2004;1(1):57–65. doi: 10.1517/17425247.1.1.57. [DOI] [PubMed] [Google Scholar]
- [12].Sun YP, Huang WJ, Lin Y, et al. Soluble dendron-functionalized carbon nanotubes: preparation, characterization, and properties. Chem Mater. 2001;13(9):2864–2869. [Google Scholar]
- [13].Lin Y, Zhou B, Shiral Fernando KA, et al. Polymeric carbon nanocomposites from carbon nanotubes functionalized with matrix polymer. Macromolecules. 2003;36(19):7199–7204. [Google Scholar]
- [14].Huang WJ, Lin Y, Taylor S, et al. Sonication-assisted functionalization and solubilization of carbon nanotubes. Nano Lett. 2002;2(3):231–234. [Google Scholar]
- [15].Fernando KAS, Lin Y, Sun YP, et al. High aqueous solubility of functionalized single-walled carbon nanotubes. Langmuir. 2004;20(11):4777–4778. doi: 10.1021/la036217z. [DOI] [PubMed] [Google Scholar]
- [16].Liu Y, Wu DC, Zhang WD, et al. Polyethylenimine-grafted multiwalled carbon nanotubes for secure noncovalent immobilization and efficient delivery of DNA. Angewandte Chemie. 2005;117(30):4860–4863. doi: 10.1002/anie.200500042. [DOI] [PubMed] [Google Scholar]
- [17].Sun Y, Wilson SR, Schuster DI. High dissolution and strong light emission of carbon nanotubes in aromatic amine solvents. J Am Chem Soc. 2001;123(22):5348–5349. doi: 10.1021/ja0041730. [DOI] [PubMed] [Google Scholar]
- [18].Zhang Y, Bai YH, Yan B. Functionalized carbon nanotubes for potential medicinal applications. Drug Discov Today. 2010;15(11-12):428–435. doi: 10.1016/j.drudis.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Feazell RP, Nakayama-Ratchford N, Dai H, et al. Soluble Ringle-walled carbon nanotubes as longboat delivery systems for platinum (IV) anticancer drug design. J Am Chem Soc. 2007;129(27):8438–8439. doi: 10.1021/ja073231f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Hamon MA, Chen J, Hu H, et al. Dissolution of single-walled carbon nanotubes. Adv Mater. 1999;11(10):834–840. [Google Scholar]
- [21].Chen J, Rao AM, Lyuksyutov S, et al. Dissolution of full-length single-walled carbon nanotubes. J Phys Chem B. 2001;105(13):2525–2528. [Google Scholar]
- [22].Xu HY, Meng J, Kong H. What are carbon nanotubes’ roles in anti-tumor therapies (invited)? Sci China Chem. 2010;53(11):2250–2256. [Google Scholar]
- [23].Coccini T, Roda E, Sarigiannis DA, et al. Effects of watersoluble functionalized multi-walled carbon nanotubes examined by different cytotoxicity methods in human astrocyte D384 and lung A549 cells. Toxicology. 2010;269(1):41–53. doi: 10.1016/j.tox.2010.01.005. [DOI] [PubMed] [Google Scholar]
- [24].Jiang L, Li LL, He H, et al. Preparation methods of amino-functionalized carbon nanotubes and their application in pharmaceutical field. Yaoxue Jinzhan. 2012;36(9):400–405. [Google Scholar]
- [25].Li JX, Zeng L. Study of nerve growth factor and progress of peripheral nerve injuries. Guangxi Yixue. 2007;29(1):60–62. [Google Scholar]
- [26].Macdonald DS, Weerapura M, Beazely MA, et al. Modulation of NMDA receptors by pituitary adenylate cyclase activating peptide in CA1 neurons requires G alpha q, protein kinase C, and activation of Src. J Neurosci. 2005;25(49):11374–11384. doi: 10.1523/JNEUROSCI.3871-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Giordano G, Sanchez-Perez AM, Burgal M, et al. Chronic exposure to ammonia induces isoform-selective alterations in the intracellular distribution and NMDA receptor mediated translocation of protein kinase C in cerebellar neurons in culture. J Neurochem. 2005;92(1):143–157. doi: 10.1111/j.1471-4159.2004.02852.x. [DOI] [PubMed] [Google Scholar]
- [28].Lallemend F, Hadjab S, Hans G, et al. Activation of protein kinase CbetaI constitutes a new neurotrophic pathway for deafferented spiral ganglion neurons. J Cell Sci. 2005;118(Pt 19):4511–4525. doi: 10.1242/jcs.02572. [DOI] [PubMed] [Google Scholar]
- [29].Zhang H, Ding J, Tian W, et al. Ganglioside GM1 binding the N-terminus of amyloid precursor protein. Neurobiol Aging. 2009;30(8):1245–1253. doi: 10.1016/j.neurobiolaging.2007.11.013. [DOI] [PubMed] [Google Scholar]
- [30].Huang F, Wang J, Chen A. Effects of co-grafts mesenchymal stem cells and nerve growth factor suspension in the repair of spinal cord injury. J Huazhong Univ Sci Technolog Med Sci. 2006;26(2):206–210. doi: 10.1007/BF02895817. [DOI] [PubMed] [Google Scholar]
- [31].Madduri S, Feldman K, Tervoort T, et al. Collagen nerve conduits releasing the neurotrophic factors GDNF and NGF. J Control Release. 2010;143(2):168–174. doi: 10.1016/j.jconrel.2009.12.017. [DOI] [PubMed] [Google Scholar]
- [32].Son SJ, Bai X, Nan AJ, et al. Template synthesis of multifunctional nanotubes for controlled release. J Control Release. 2006;114(2):143–152. doi: 10.1016/j.jconrel.2006.06.004. [DOI] [PubMed] [Google Scholar]
- [33].Kam NW, O’Connell M, Wisdom JA, et al. Carbon nanotubes as multifunctional biological transporters and near-infrared agents for selective cancer cell destruction. Proc Natl Acad Sci U S A. 2005;102(33):11600–11605. doi: 10.1073/pnas.0502680102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Pantarotto D, Singh R, McCarthy D, et al. Functionalized carbon nanotubes for plasmid DNA gene delivery. Angew Chem Int Edn Engl. 2004;43(39):5242–5246. doi: 10.1002/anie.200460437. [DOI] [PubMed] [Google Scholar]
- [35].Liu Y, Wu DC, Zhang WD, et al. Polyethylenimine-grafted multiwalled carbon nanotubes for secure noncovalent immobilization and efficient delivery of DNA. Angew Chem Int Ed Engl. 2005;44(30):4782–4785. doi: 10.1002/anie.200500042. [DOI] [PubMed] [Google Scholar]
- [36].Moon HK, Chang CI, Lee DK, et al. Effect of nucleases on the cellular internalization of fluorescent labeled DNA-functionalized single-walled carbon nanotubes. Nano Res. 2008;1(4):351–360. [Google Scholar]
- [37].Kam NW, Liu Z, Dai H. Functionalization of carbon nanotubes via cleavable disulfide bonds for efficient intracellular delivery of siRNA and potent gene silencing. J Am Chem Soc. 2005;127(36):12492–12493. doi: 10.1021/ja053962k. [DOI] [PubMed] [Google Scholar]
- [38].Valenti LE, Fiorito PA, Garcia CD, et al. The adsorption-desorption process of bovine serum albumin on carbon nanotubes. J Colloid Interface Sci. 2007;307(2):349–356. doi: 10.1016/j.jcis.2006.11.046. [DOI] [PubMed] [Google Scholar]
- [39].Kam NW, Dai H. Carbon nanotubes as intracellular protein transporters: generality and biological functionality. J Am Chem Soc. 2005;127(16):6021–6026. doi: 10.1021/ja050062v. [DOI] [PubMed] [Google Scholar]
- [40].Feazell RP, Nakayama-Ratchford N, Dai H, et al. Soluble Ringle- walled carbon nanotubes as longboat delivery systems for platinum (IV) anticancer drug design. J Am Chem Soc. 2007;129(27):8438–8439. doi: 10.1021/ja073231f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Xu HY, Meng J, Kong H. What are carbon nanotubes’ roles in anti-tumor therapies. Sci China Chem. 2010;53(11):2250–2256. [Google Scholar]
- [42].Kam NW, Jessop TC, Wender PA, et al. Nanotube molecular transporters internalization of carbon nanotube-protein conjugates into mammalian cells. J Am Chem Soc. 2004;126(22):6850–6851. doi: 10.1021/ja0486059. [DOI] [PubMed] [Google Scholar]
- [43].Murugesan S, Myers K, Subramanian V. Aminofunctionalized and acid treated multi-walled carbon nanotubes as supports for electrochemical oxidation of formic acid. Appl Catal B Environ. 2011;103(3-4):266–274. [Google Scholar]
- [44].Yen SJ, Hsu WL, Chen YC, et al. The enhancement of neural growth by amino-functionalization on carbon nanotubes as a neural electrode. Biosens Bioelectron. 2011;26(10):4124–4132. doi: 10.1016/j.bios.2011.04.003. [DOI] [PubMed] [Google Scholar]
- [45].Li J, Wang YB, Qiu JD, et al. Biocomposites of covalently linked glucose oxidase on carbon nanotubes for glucose biosensor. Anal Bioanal Chem. 2005;383:918–922. doi: 10.1007/s00216-005-0106-6. [DOI] [PubMed] [Google Scholar]


