Abstract
The microglia-mediated inflammatory reaction promotes neuronal damage under cerebral ischemia/hypoxia conditions. We therefore speculated that inhibition of hypoxia-induced microglial activation may alleviate neuronal damage. To test this hypothesis, we co-cultured ginsenoside Rb1, an active component of ginseng, and cortical neurons. Ginsenoside Rb1 protected neuronal morphology and structure in a single hypoxic culture system and in a hypoxic co-culture system with microglia, and reduced neuronal apoptosis and caspase-3 production. The protective effect was observable prior to placing in co-culture. Additionally, ginsenoside Rb1 inhibited levels of tumor necrosis factor-α in a co-culture system containing activated N9 microglial cells. Ginsenoside Rb1 also significantly decreased nitric oxide and superoxide production induced by N9 microglia. Our findings indicate that ginsenoside Rb1 attenuates damage to cerebral cortex neurons by downregulation of nitric oxide, superoxide, and tumor necrosis factor-α expression in hypoxia-activated microglia.
Keywords: nerve regeneration, traditional Chinese medicine, ischemia/hypoxia, microglia, neurons, apoptosis, ginsenoside Rb1, nerve inflammation factor, NSFC grant, neural regeneration
Introduction
Rat brain neurons are highly sensitive to ischemia; neuronal apoptosis occurs in the early stages of cerebral ischemia, with the reduction in neuronal number and neurotransmitter levels affecting learning and memory[1,2,3]. Preliminary studies from our research group demonstrated that hypoxia damages rat cerebral cortical neurons, and induces apoptosis[4,5], indicating that neuronal damage is an important pathological contributor to hypoxic-ischemic encephalopathy.
Neurons and glial cells comprise a unified organism in the central nervous system. Microglia account for 5–20% of total glial cells and play an important role in the regulation of neuronal function and in the maintenance of the microenvironment. Microglia are the first cells to be injured following a central nervous system insult. Cerebral ischemia induces excess activation of microglia, a complex reaction involving intracellular and extracellular ion imbalance, neurotransmitter disorders, and expression of a variety of soluble inflammatory cytokines[6,7,8,9,10,11]. These manifestations have a unique temporal and spatial pattern, accompanied by changes at the molecular level[6,7,8,9,10,11]. Early hypoxia can induce microglial proliferation and the activated microglia release free radicals, inflammatory cytokines, superoxide (O2−) and proinflammatory substances such as tumor necrosis factor-α (TNF-α) and inducible nitric oxide synthase. These factors promote neuronal injury and apoptosis, and aggravate ischemic stroke; in addition, hypoxia leads to changes in microglial mitochondrial structure and function[11,12,13,14,15,16].
Liu et al.[17,18,19,20,21] found that neurons in different brain regions exhibited different responses to hypoxia-ischemia, and microglial cell morphology varied significantly. We propose that the extent of microglial activation correlates with neuronal damage, and that activated microglia and apoptotic neurons are mainly localized to the peri-infarct zone. Indeed, similar responses are seen in cerebral ischemia-induced microglial activation that occurs before neuronal apoptosis[17,18,19,20,21]. The involvement of microglia in ischemic neuronal apoptosis can be observed through changes in expression time and distribution. Activated microglia-mediated inflammation promotes neuronal damage under cerebral hypoxic-ischemic conditions, so it is likely that inhibiting hypoxia-induced activation of microglia will alleviate neuronal damage.
Ginsenoside Rb1 has anti-aging, anti-oxidative and anti-apoptotic properties in neurons, and restores and promotes neuronal regeneration[22,23,24,25,26,27,28]. Preliminary studies from our research group showed that ginsenoside Rb1 alleviated apoptosis in rat hippocampal neurons and inhibited TNF-α, nitric oxide (NO) and O2− expression in activated microglia[29,30]. However, it remains unclear whether ginsenoside Rb1 reduces neuronal apoptosis by downregulating the activated microglia-mediated inflammatory response. In the present study, we observed the changes in cortical neuronal morphology, cell cycle, and caspase-3 production, as well as TNF-α, NO and O2− levels in different culture systems in the presence of ginsenoside Rb1, in an effort to explore the effect and mechanism of ginsenoside Rb1 on cerebral cortical neuronal damage mediated by activated microglia.
Results
Effect of ginsenoside Rb1 on the apoptosis of cortical neurons mediated by activated N9 microglia
Cell morphology
Under an inverted microscope, the cultured neurons in group A (normal neurons) and group B (neurons co-cultured in medium from microglia treated with ginsenoside Rb1) showed an even distribution and orderly arrangement, with rounded cell bodies, strong refraction, and unfolded cell processes linked into a network. The cultured neurons in groups C (neurons co-cultured in medium from microglia exposed to hypoxia) and E (neurons exposed to hypoxia and treated with ginsenoside Rb1) were distributed evenly but in a less orderly arrangement, and some cells exhibited deformation, large vacuoles, and distorted processes. In groups D (neurons exposed to hypoxia), G (pre-hypoxic neurons co-cultured in medium from microglia exposed to hypoxia and treated with ginsenoside Rb1) and H (pre-hypoxic neurons co-cultured in medium from microglia exposed to hypoxia and treated with ginsenoside-Rb1), the majority of cells were deformed and randomly arranged; projections were deformed and broken with some fragments or residues of apoptotic cells visible. In group F (pre-hypoxic neurons co-cultured in medium from microglia exposed to hypoxia), the number of cells was reduced, the residual cells were deformed, the processes were fractured or even absent, and some fragmented or residual apoptotic cells were visible. Ginsenoside Rb1 had no impact on the morphology of neurons in normal culture medium or in a co-culture system, but led to apparent changes in the morphology of neurons in single or co-culture hypoxic conditions. The extent of morphological changes was in the order group C < group D < group F, which suggests that ginsenoside Rb1 exerts a protective effect against hypoxia (Figure 1).
Figure 1.
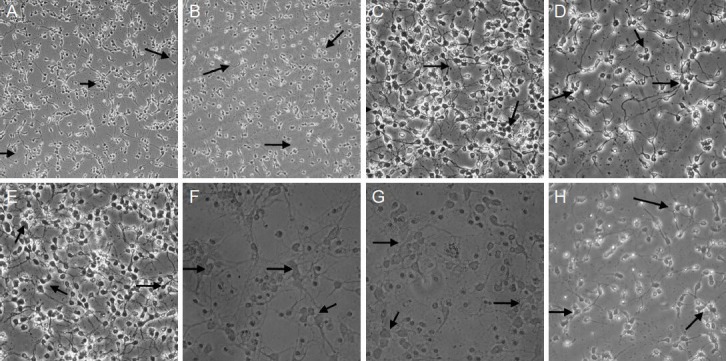
Effect of ginsenoside Rb1 on the morphology of cerebral cortical neurons after hypoxia (inverted microscope, scale bars: A, B: × 40; C–E, H: × 200; F, G: × 400).
A (normal neurons) and B (neurons co-cultured in medium from microglia treated with ginsenoside Rb1): The majority of the cells showed even distribution, orderly arrangement, rounded cell bodies, strong refraction, and unfolded cell processes. C (neurons co-cultured in medium from microglia exposed to hypoxia) and E (neurons exposed to hypoxia and treated with ginsenoside Rb1): Cells were distributed uniformly, but a small number of the cells were deformed, with large vacuoles and distorted processes. D (neurons exposed to hypoxia), G (pre-hypoxic neurons co-cul-tured in medium from microglia exposed to hypoxia and treated with ginsenoside Rb1), and H (pre-hypoxic neurons co-cultured in medium from microglia exposed to hypoxia and treated with ginsenoside-Rb1): The majority of cells were scattered randomly and appeared deformed, with fractured processes; some cell debris was visible. F (pre-hypoxic neurons co-cultured in medium from microglia exposed to hypoxia): The number of cultured cells was significantly lower than in the control group, the residual cells appearing deformed, with fractured or absent processes, and a large amount of debris present from dead cells.
Cell cycle and apoptosis
Flow cytometry results showed that normal neurons were not apoptotic after being cultured with N9 microglia culture medium and ginsenoside Rb1. Ginsenoside Rb1 reduced the apoptosis peak of pre-hypoxic neurons, indicating that the compound has anti-apoptotic effects. The medium harvested after N9 microglial activation could induce the apoptosis of pre-hypoxic or normal neurons. After microglia were activated by hypoxia, the resulting culture medium contained active substances which aggravated neuronal apoptosis, while ginsenoside Rb1 attenuated neuronal apoptosis and exhibited a protective effect before co-culture. Activated microglia-mediated damage of cerebral cortex neurons in Sprague-Dawley rats also affected the neuronal cell cycle, and those neurons cultured in the Rb1-free medium presented no features of the cell cycle. This evidence suggests that ginsenoside Rb1 can enhance the stability of the neuronal cell cycle and alleviate hypoxic injury (Figure 2).
Figure 2.
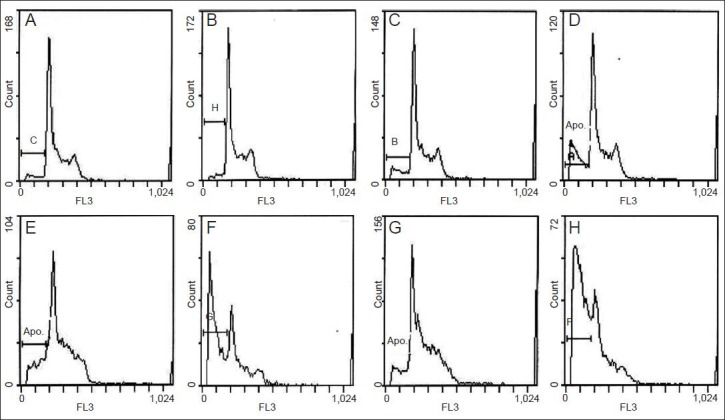
Effect of ginsenoside Rb1 on the cell cycle and apoptosis of cerebral cortical neurons (flow cytometry).
A, B (Group A: normal neurons; group B: neurons co-cultured in medium from microglia treated with ginsenoside Rb1). Essentially normal cell cycle: no apparent apoptotic peak, G2/M phase twice as high as G0–G1 phase, and S phase between G2/M and G0/G1. D, E (Group D: neurons ex-posed to hypoxia; group E: neurons exposed to hypoxia and treated with ginsenoside Rb1). After ginsenoside Rb1 was added to the culture system, the apoptotic peak was significantly reduced. C, F–H (Group C: neurons co-cultured in medium from microglia exposed to hypoxia; group F: pre-hypoxic neurons co-cultured in medium from microglia exposed to hypoxia; group G: pre-hypoxic neurons co-cultured in medium from mi-croglia exposed to hypoxia and treated with ginsenoside Rb1; Group H: pre-hypoxic neurons co-cultured in medium from microglia exposed to hypoxia and treated with ginsenoside-Rb1). An apoptotic peak was found in each group. In groups F–H, different levels of cell cycle distortion can be observed, the mildest in group G and most severe in group F, where no boundary between G2/M phase and G0–G1 phase was found, and no mul-tiple proportion existed. APO: Apoptotic peak.
Caspase-3 expression in neurons
Western blot analysis showed that ginsenoside Rb1 had no impact on caspase-3 expression in the neurons in a normal co-culture system (P > 0.05); hypoxia-activated N9 microglia medium upregulated caspase-3 expression in normally cultured neurons (P < 0.05), while ginsenoside Rb1 downregulated caspase-3 expression in hypoxia-induced neurons (P < 0.05). Hypoxia-activated N9 microglial medium elevated caspase-3 expression in neurons (P < 0.05), while ginsenoside Rb1 inhibited the effect of hypoxia on caspase-3 expression (P < 0.05; Figure 3).
Figure 3.
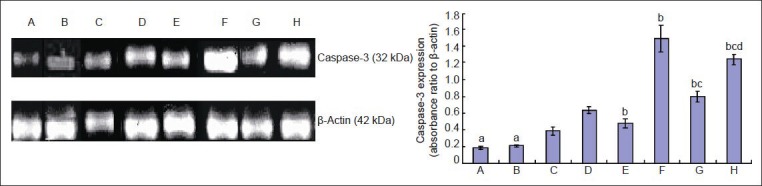
Effect of ginsenoside Rb1 on caspase-3 expression in hypoxic cerebral cortical neurons.
Data are expressed as mean ± SD. Comparisons between groups were tested by one-way analysis of variance, and pairwise comparisons were per-formed using the least significant difference test. aP < 0.05, vs. group C; bP < 0.05, vs. group D; cP < 0.05, vs. group F; dP < 0.05, vs. group G. Group A: Normal neurons. Group B: Neurons co-cultured in medium from microglia treated with ginsenoside Rb1. Group C: Neurons co-cultured in medium from microglia exposed to hypoxia. Group D: Neurons exposed to hypoxia. Group E: Neurons exposed to hypoxia and treated with ginse-noside Rb1. Group F: Pre-hypoxic neurons co-cultured in medium from microglia exposed to hypoxia. Group G: Pre-hypoxic neurons co-cultured in medium from microglia exposed to hypoxia and treated with ginsenoside Rb1. Group H: Pre-hypoxic neurons co-cultured in medium from microglia exposed to hypoxia and treated with ginsenoside-Rb1.
Effect of ginsenoside Rb1 on the expression of inflammatory cytokines (TNF-α, NO, O2−) secreted from hypoxia-activated N9 microglia
Using an ELISA assay, we detected that TNF-α expression was very poorly expressed in the culture systems without N9 microglial cells (groups A, D, E), and there was no significant difference among these three groups (P > 0.05). This evidence indicates that ginsenoside Rb1 has no impact on TNF-α expression in normally cultured cortical neurons. Compared with normal neurons cultured in hypoxia-activated N9 microglia culture medium (group C), low levels of TNF-α expression were found in normal neurons cultured in normal N9 microglial activation medium (group B) (P < 0.05). Compared with hypoxic neurons cultured in hypoxia-activated N9 microglial medium (group F), TNF-α expression was reduced after ginsenoside Rb1 was added to the culture medium in groups G and H (P < 0.05). Furthermore, group G was lower than group H (P < 0.05), suggesting that ginsenoside Rb1 suppressed the production of TNF-α in the co-culture system, and in particular in hypoxia-activated N9 microglia (Figure 4A).
Figure 4.
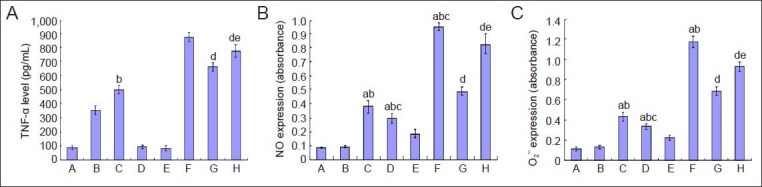
Effect of ginsenoside Rb1 on the expression of inflammatory mediators (TNF-α, NO, O2−) secreted from hypoxia-activated N9 microglia.
Data are expressed as mean ± SD. Comparisons between groups were tested by one-way analysis of variance, and pairwise comparisons were per-formed using the least significant difference test. aP < 0.05, vs. group A; bP < 0.05, vs. group B; cP < 0.05, vs. group C; dP < 0.05, vs. group F; eP < 0.05, vs. group G. Group A: Normal neurons. Group B: Neurons co-cultured in medium from microglia treated with ginsenoside Rb1. Group C: Neurons co-cultured in medium from microglia exposed to hypoxia. Group D: Neurons exposed to hypoxia. Group E: Neurons exposed to hypoxia and treat-ed with ginsenoside Rb1. Group F: Pre-hypoxic neurons co-cultured in medium from microglia exposed to hypoxia. Group G: Pre-hypoxic neurons co-cultured in medium from microglia exposed to hypoxia and treated with ginsenoside Rb1. Group H: Pre-hypoxic neurons co-cultured in medi-um from microglia exposed to hypoxia and treated with ginsenoside-Rb1. TNF-α: Tumor necrosis factor-α; NO: nitric oxide; O2−: superoxide.
Production of NO and O2− was determined using Griess reagent and the water-soluble tetrazolium assay, respectively. The results showed that a hypoxic environment in any of the culture systems significantly increased NO or O2− production in cells, and microglial cells were more sensitive than neurons (P < 0.05). Ginsenoside Rb1 inhibited NO or O2− production in the hypoxia-induced cells, mainly N9 microglial cells (Figure 4B, C).
Discussion
After cerebral ischemia or brain damage, microglia move rapidly from a resting state to a state of activation. These cells begin to proliferate, migrate and differentiate, release cytotoxins and inflammatory mediators, secrete cytokines, and upregulate immune expression. A series of previous studies showed that activated microglia in vitro released a large number of free radicals and inflammatory cytokines, which activated or aggravated neuronal damage; conversely, neuronal damage activated microglial cells, thus leading to a pathological cascade and aggravating the damage induced by ischemia[31,32,33,34,35,36,37,38,39].
Previous preliminary studies from our research group showed that: (1) Hypoxia can induce microglial activation, increasing the expression of secreted neurotoxic cytokines; hypoxia also causes neuronal damage or apoptosis, and increases caspase-3 production; microglial activation is consistent with neuronal apoptosis in time distribution and extent, suggesting that stress-induced microglial activation is closely linked with neuronal apoptosis. (2) After 12 hours of hypoxia, microglial medium alone can inhibit the growth and proliferation of normally cultured cortical neurons in Sprague-Dawley rats, and trigger neuronal apoptosis, while the co-culture with hypoxia medium aggravates the decline of neuronal viability and apoptosis. (3) In a co-culture system containing hypoxic microglia, the production of NO, O2−, and TNF-α was higher than that in normal microglial single- or co-culture, and normal hypoxic neuronal culture systems. This evidence indicates that the high level of neurotoxins produced by microglia is an important factor in hypoxic neuronal damage[5,9,10]. From these preliminary results, we hypothesized that neuronal damage can be alleviated by regulating hypoxia-activated microglia. Therefore, in the present study, we added ginsenoside Rb1 to microglia and cortical neurons in a co-culture system in an effort to explore the role of, and mechanism underlying, cerebral cortical neuronal injury mediated by hypoxia-activated microglia.
Ginsenoside Rb1 is a class of ginseng monomer[22,23,29,30]. Previous studies have found that ginsenoside Rb1/Rg1 can alleviate impairments in learning and memory, and regulate the cholinergic system in a variety of experimental animal models. In addition, ginsenoside Rb1/Rg1 exerts a protective effect on dopamine neurons by reducing the stress response induced by 1-methyl-4-phenyl 2,3,6-tetrahydropyridine/1-methyl-4-phenyl pyridine, and also alleviates tau hyperphosphorylation through regulation of the activities of glycogen synthase kinase 3β and protein phosphatase 2A[40,41,42,43,44,45,46,47,48]. The present study demonstrates the protective effect of ginsenoside Rb1 on the damage to cerebral cortical neurons in rats mediated by hypoxia-activated microglia. The main findings are summarized as follows: (1) Ginsenoside Rb1 protects against neuronal morphology damage and structure in single- and co-culture hypoxic systems. (2) Ginsenoside Rb1 alleviates neuronal apoptosis in alone hypoxia system and hypoxia co-culture system, and its protective effect appears before the neurons are placed in co-culture. (3) Ginsenoside Rb1 enhances the stability of the neuronal cell cycle and alleviates hypoxic injury. (4) Ginsenoside Rb1 reduces caspase-3 production of neurons in single- and co-culture hypoxic systems, and its inhibition effect appears before the co-culture. (5) Ginsenoside Rb1 inhibits the production of TNF-α, NO, and O2− in a hypoxia-activated N9 microglial co-culture system. Together, these results indicate that by downregulating the expression of TNF-α, NO, O2− and other inflammatory mediators in hypoxia-activated microglia, ginsenoside Rb1 regulates inflammation and attenuates neuronal injury.
In summary, the present study is the first to demonstrate that ginsenoside Rb1 protects rat cerebral cortical neurons against hypoxia-activated microglia-mediated injury, and that the associated mechanism is mediated by the downregulation of NO, O2−, and TNF-α in hypoxia-activated microglia. The present results suggest that ginsenoside Rb1 is a promising candidate for clinical use in the prevention of neuronal degeneration following cerebral ischemia.
Materials and Methods
Design
A comparative cytological observation.
Time and setting
Experiments were performed in July 2012 at the Neurobiology Research Center of Fujian Medical University, China.
Materials
Cells
N9 mouse microglial cell lines were provided by the U.S. National Cancer Institute.
Animals
Twenty pregnant, clean-grade Sprague-Dawley rats at 19 days of gestation were purchased from the Experimental Animal Center of Fujian Medical University, China (license No. SCXK (Min) 2012-0001).
Drugs
Ginsenoside Rb1 is one of the main active ingredients of ginseng, and also the glycoside derived from protopanaxadiol. It is extracted from the stem, basal part of stem, root, and alabastrum root of Panax ginseng C.A. Meyer. The chemical formula is C54H92O23 (Figure 5)[49] and its molecular weight is 1,109.29. Ginsenoside Rb1 was obtained at > 98% purity from the Department of Organic Chemistry, Jilin University, China. According to previous reports of ginsenoside Rb1 in nerve cells[22,23,29,30], we selected 100 μg/mL as the drug concentration and 6 hours as the administration time.
Figure 5.
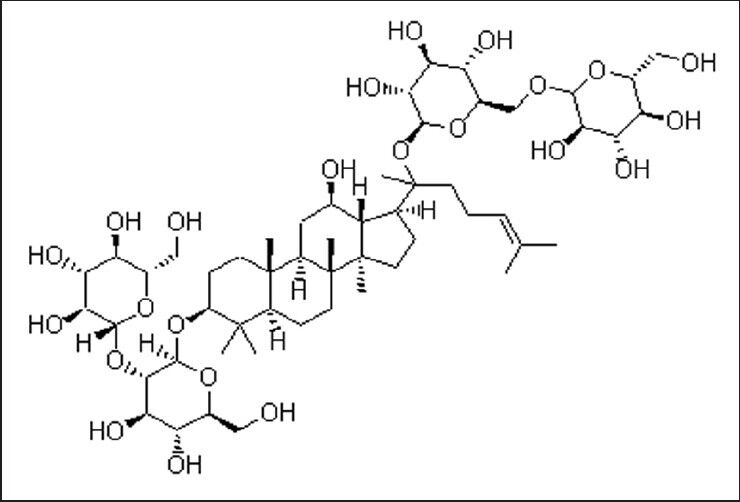
Chemical structure of ginsenoside Rb1.
Methods
Culture of microglia
N9 microglial cell lines cryopreserved in liquid nitrogen were regarded as passage 2 cells, which were then seeded at 4 × 105 /mL in cell culture flasks at 37°C and 5% CO2. After 3 days, the adherent cells covered 50–60% of the bottom of the culture flask and were trypsinized using 0.02% EDTA. Then cells were passaged at 1:3 and cultured in another flask, and cultured cells at passages 3–8 were selected for experiments. The culture medium was composed of 89% high-glucose Dulbecco's modified Eagle medium (DMEM) + 10% fetal bovine serum + 1% penicillin-streptomycin.
Immunofluorescence identification of microglia
Mononuclear cell membrane adhesion molecule III complement receptor CD11b served as a specific microglial marker[50,51,52]. After the cells were stabilized for 24 hours, they were stained and identified. The culture medium was discarded and fixed with 4% paraformaldehyde at room temperature for 30 minutes, then treated with 0.3% Triton X-100 for 20 minutes, and incubated with 3% H2O2 at room temperature for 10 minutes to eliminate endogenous peroxidase activity. Between the steps, cells were rinsed with 0.01 mol/L PBS (pH 7.4). The washed cells were then incubated with blocking solution (PBS containing 5% bovine serum albumin + 0.1% Triton X-100) for 30 minutes at room temperature to block nonspecific background staining. Subsequently, the blocking solution was discarded and the cells were incubated with rabbit anti-CD11b polyclonal antibody (1:1,000; Chemicon, Santa Cruz, CA, USA) at 37°C for 1 hour and then overnight at 4°C. After washing three times in PBS, 5 minutes each time, the cells were further incubated with rhodamine-labeled goat anti-rabbit IgG (1:200; Chemicon) at 37°C for an additional 1 hour. After a final PBS wash, the cells were mounted using phosphoglycerol and observed under a fluorescent microscope (Leica, Solms, Germany). The negative control was incubated with 0.01 mol/L PBS instead of primary antibody. Thirty fields of view were photographed at 200 × magnification and randomly selected for counting the percentage of positive cells under a phase contrast microscope (Nikon, Tokyo, Japan). Images were analyzed using an image analysis system (Bio-Rad, Hercules, CA, USA). Images containing 95% microglia were included in the study.
Establishment of a hypoxic microglial culture system
To establish the hypoxic culture system, the microglial cells were resuspended and the cell density was adjusted accordingly. The hypoxic microglial culture system was established at 37°C in 94% N2, 1% O2 and 5% CO2, followed by 12 hours hypoxia. Hypoxia-induced activation of microglia was defined based on microglial function (proliferation activity, expression of neurotoxic factors such as TNF-α, NO, O2−, and mitochondrial structure)[9,10,53]. The resulting medium provided conditioned culture medium (50% of microglia culture fluid + 50% of neuronal culture fluid) for the subsequent establishment of co-culture medium.
Harvesting, purification and culture of rat cerebral cortical neurons
Rats at 19 days of gestation were anesthetized, the uterus was dissected out, and fetal brain was harvested, ground, filtered, resuspended, and centrifuged. After the supernatant was discarded, the cells were triturated and inoculated in high-glucose DMEM containing 10% fetal bovine serum, 1% L-glutamine, 1% MEM vitamins, 0.1 IU/L penicillin G, 0.1 IU/L streptomycin, 2 g/L NaHCO3) at 37°C and 5% CO2. The cells were purified and cultured in Neurobasal-A Medium (Life Technologies, Gaithersburg, MD, USA) containing 2% B-27 additive, 1% fetal bovine serum, 1% cytosine arabinoside, 0.2% L-glutamine, 0.1% kynurenic acid stock solution, 0.1% fluorodeoxyuridine stock solution, 0.175% β-mercaptoethanol stock solution, 50 U/mL penicillin G, and 50 U/mL streptomycin. On day 7, cells were collected for identification[54,55].
Identification of cortical neurons using immunofluorescence staining
The neuron-specific microtubule-associated protein 2 was used to identify neurons[56,57]. Briefly, after the culture medium was discarded, the cells were fixed in cold 4% paraformaldehyde for 30 minutes, treated with 0.3% Triton X-100 for 20 minutes, and then incubated with 30 μL of 0.3% H2O2/methanol solution at room temperature for 10 minutes, 30 μL of normal goat serum at room temperature for 10 minutes, 50 μL of mouse anti-microtubule-associated protein 2 polyclonal antibody (1:1,000; Chemicon) at 4°C overnight, and FITC-labeled sheep anti-mouse IgG (1:100; Chemicon) at 37°C for 1 hour. Between the incubation steps, cells were rinsed with PBS. Finally, the slices were mounted with phosphoglycerol[54,55,56] and observed with fluorescence microscopy. The negative control was incubated with 0.01 mol/L PBS instead of primary antibody. Thirty fields of view at 200 × magnification were randomly selected for counting the percentage of positive cells under a phase contrast microscope (Nikon). The images were then analyzed using an image analysis system (Bio-Rad) and those containing 90% microglia were included in the study.
Establishment of cortical neuron hypoxia culture system
To establish culture system, cortical neurons were resuspended according to the requirements of different experimental methods, and the cell density was adjusted. The hypoxic culture system was established at 37°C in 94% N2, 1% O2 and 5% CO2, followed by 12 hours of hypoxia. The hypoxia-induced activation of cortical neurons was defined based on biological function (proliferation activity, expression of neurotoxic factors such as TNF-α, NO, O2−, and mitochondrial structure)[9,10,53]. This also provided the conditioned culture medium (50% of microglia culture fluid + 50% of neuronal culture fluid) for the subsequent establishment of co-culture medium.
Establishment of microglial and cortical neuronal co-culture system
Rat cortical neurons were primarily cultured in conditioned medium in which the microglia were previously cultured, to establish the co-culture system. The co-culture (or co-hypoxia) time was 12 hours.
Cell grouping
Group A: Normal neurons. Group B: Normal neurons co-cultured in medium from microglia treated with ginsenoside Rb1; normal N9 microglia were treated with ginsenoside Rb1 for 6 hours, then 3 mL culture medium was harvested and co-cultured with normal neurons for 12 hours. Group C: Normal neurons co-cultured in medium from microglia exposed to hypoxia; after microglia were subjected to hypoxia for 12 hours, 3 mL culture medium was harvested and co-cultured with normal neurons for 12 hours. Group D: Hypoxia-exposed neurons; neurons were subjected to hypoxia for 12 hours. Group E: Hypoxia-exposed neurons treated with ginsenoside Rb1; neurons were subjected to hypoxia for 12 hours and then treated with ginsenoside Rb1 for 6 hours. Group F: Pre-hypoxic neurons co-cultured in medium from microglia exposed to hypoxia; after microglia were subjected to hypoxia for 12 hours, 3 mL culture medium was harvested and co-cultured with pre-hypoxic neurons for an additional 12 hours. Group G: Pre-hypoxic neurons co-cultured in medium from microglia exposed to hypoxia and treated with ginsenoside Rb1; after neurons were subjected to hypoxia for 12 hours and ginsenoside Rb1 was applied for 6 hours, 3 mL culture medium was harvested and co-cultured with pre-hypoxic neurons for an additional 12 hours. Group H: Pre-hypoxic neurons co-cultured in medium from microglia exposed to hypoxia and treated with ginsenoside-Rb1; after microglia were exposed to hypoxia for 12 hours, 3 mL culture medium was harvested and co-cultured with pre-hypoxic neurons for an additional 12 hours, followed by application of ginsenoside Rb1 for 6 hours. Cells in each group were cultured in 6-well plates (Corning, Michigan, USA) with 3 × 106 cells per well.
The growth and distribution of neurons were observed under an inverted microscope. The single cell suspension in each group was prepared, centrifuged, washed, fixed, filtrated, and stained with propidium iodide (Beyotime Institute of Biotechnology, Shanghai, China). The proliferation cycle and apoptosis of neurons were determined using a flow cytometer (BD Company, Franklin Lakes, NJ, USA). Neuronal caspase-3 expression was determined with western blot analysis. Briefly, the protein sample was extracted from the lysed cells, loaded onto the gel, electrophoresed, and proteins transferred onto a polyvinylidene fluoride membrane, which was then rinsed and blocked to prevent nonspecific binding. The membrane was incubated with mouse anti-rat caspase-3 monoclonal antibody (1:500; Beyotime Institute of Biotechnology) at 4°C overnight, and then with goat anti-mouse IgG (1:100; Beyotime Institute of Biotechnology) at 37°C for 90 minutes. Electrochemiluminescence was used to visualize the proteins with absorbance at 405 nm detected using a UV spectrophotometer (Beckman Company, Kraemer Boulevard Brea, CA, USA). The experiment was repeated four times. The ratio of caspase-3 to β-actin absorbance was used to represent caspase-3 expression level.
Detection of the production of neurotoxic factors in culture medium
The expression of TNF-α in culture medium was detected using an ELISA kit (R & D Systems Inc., Minneapolis, MN, USA) according to the manufacturer's instructions. Absorbance at 450 nm was determined using a UV spectrophotometer (Beckman) and the concentration of samples (pg/mL) was calculated from a standard curve.
The expression of NO in the culture medium was detected with a Griess reagent assay. Cells were lysed in nitrogen-free lysate, centrifuged, and the supernatant was discarded. NO standards (1–100 mmol/L; Beyotime Institute of Biotechnology) and samples were added to the culture plate (50 mL/well), to which Griess reagents I and II were added. The absorbance at 540 nm was measured with a UV spectrophotometer.
The expression of O2− in the culture medium was detected using the water-soluble tetrazolium-1 reduction assay. Briefly, when the cells reached 85% confluence in the 96-well culture plate, the culture medium was discarded, the cells were washed with Dulbecco's PBS and incubated with 200 μL/well detection solution (Beyotime Institute of Biotechnology) at 37°C for 5 minutes. There were six parallel wells in each group. The absorbance value at 450 nm was measured with a UV spectrophotometer.
Statistical analysis
Data are expressed as mean ± SD and analyzed using SPSS version 13.0 (SPSS, Chicago, IL, USA). The difference between groups was compared using one-way analysis of variance and further pairwise comparisons were performed using the least significant difference test. P < 0.05 was considered statistically significant.
Acknowledgments:
We would like to thank Professor Chen XC from Institute of Gerontology, Fujian Union Hospital; Professor Zhu L from Department of Immunology, Fujian Medical University; Professor Lin JY and Zhao R from Molecular Biology Research Center, Fujian Medical University; Professor Zhao XZ and Zhang G from Department of Human Anatomy, Histology and Embryology, Fujian Medical University, China for providing technical support. We also appreciate Wang JM, M.D. from the National Cancer Institute of USA for providing N9 cell line.
Footnotes
Funding: This study was supported by the National Natural Science Foundation of China, No. 81041054; China Postdoctoral Science Foundation funded project (General Program), No. 2013M542193.
Conflicts of interest: None declared.
Peer review: This study aims to explore the role and mechanism associated with ginsenoside Rb1 on the damage of rat cerebral cortical neurons mediated by hypoxia-induced activation of microglia through immunochemistry and molecular biology detections. The present study found the neuroprotective effect of ginsenoside Rb1, which provide experimental evidence for clinical application.
Copyedited by Murphy JS, Guo GQ, Cai WJ, Yu J, Yang Y, Li CH, Song LP, Zhao M
References
- [1].Zhang C, An J, Strickland DK, et al. The low-density lipoprotein receptor-related protein 1 mediates tissue-type plasminogen activator-induced microglial activation in the ischemic brain. Am J Pathol. 2009;174(2):586–594. doi: 10.2353/ajpath.2009.080661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Wang W, Wang J, Xu Y, et al. Expressions of Smac/Diablo and caspase-9 in the cytoplasm of cerebral cortex of neonatal rats with hypoxic-ischemic brain damage and interference effects of shenfu injection on them. Zhonghua Shenjing Yixue Zazhi. 2011;10(3):228–231. [Google Scholar]
- [3].Li W, Suo AQ, Zhang JW, et al. Effects of β-amyloid-induced microglial inflammatory supernatant on neuronal apoptosis. Zhonghua Yi Xue Za Zhi. 2011;91(3):203–206. [PubMed] [Google Scholar]
- [4].Guo LL, Guan ZZ, Huang Y, et al. The neurotoxicity of β-amyloid peptide toward rat brain is associated with enhanced oxidative stress, inflammation and apoptosis, all of which can be attenuated by scutellarin. Exp Toxicol Pathol. 2013;65(5):579–584. doi: 10.1016/j.etp.2012.05.003. [DOI] [PubMed] [Google Scholar]
- [5].Ke LN, Wang W, Lin L, et al. Effects of hypoxia on biological function of the neurons in the hippocampus of the rat. Shenjing Jiepou Xue Zazhi. 2009;25(2):135–140. [Google Scholar]
- [6].Wang HB, Guo WT, Liu HL, et al. Inhibition of inflammatory mediator release from microglia can treat ischemic/hypoxic brain injury. Neural Regen Res. 2013;8(13):1157–1168. doi: 10.3969/j.issn.1673-5374.2013.13.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Sawada M, Sawada H, Nagatsu T. Effects of aging on neuroprotective and neurotoxic properties of microglia in neurodegenerative diseases. Neurodegener Dis. 2008;5(3-4):254–256. doi: 10.1159/000113717. [DOI] [PubMed] [Google Scholar]
- [8].Gao JH, Yan ZF, Zhang W. Microglia NOX2 is the potential target of neural immune inflammatory dopaminergic neurons in progressive degeneration. Zhonghua Shenjing Ke Zazhi. 2011;44(6):426–429. [Google Scholar]
- [9].Ke LN, Wang W, Zhang G. Biological function of cultured N9 cell in different stages during anoxia. Jiepou Xue Zazhi. 2009;32(2):195–199. [Google Scholar]
- [10].Ke LN, Zhao XZ, Xu JW, et al. Action of activated microglia in hippocampal neurons of rat damage induced by hypoxia. Jiepou Xuebao. 2009;40(5):737–742. [Google Scholar]
- [11].Chern CM, Liou KT, Wang YH, et al. Andrographolide inhibits PI3K/AKT-dependent NOX2 and iNOS expression protecting mice against hypoxia/ischemia-induced oxidative brain injury. Planta Med. 2011;77(15):1669–1679. doi: 10.1055/s-0030-1271019. [DOI] [PubMed] [Google Scholar]
- [12].Chen Y, Zhou J, Li J, et al. Electroacupuncture pretreatment prevents cognitive impairment induced by limb ischemia-reperfusion via inhibition of microglial activation and attenuation of oxidative stress in rats. Brain Res. 2012;1432:36–45. doi: 10.1016/j.brainres.2011.11.002. [DOI] [PubMed] [Google Scholar]
- [13].Choi DY, Lee YJ, Hong JT, et al. Antioxidant properties of natural polyphenols and their therapeutic potentials for Alzheimer's disease. Brain Res Bull. 2012;87(2-3):144–153. doi: 10.1016/j.brainresbull.2011.11.014. [DOI] [PubMed] [Google Scholar]
- [14].Das Sarma J. Microglia-mediated neuroinflammation is an amplifier of virus-induced neuropathology. J Neurovirol. doi: 10.1007/s13365-013-0188-4. in press. [DOI] [PubMed] [Google Scholar]
- [15].Wang SX, Guo H, Hu LM, et al. Caffeic acid ester fraction from Erigeron breviscapus inhibits microglial activation and provides neuroprotection. Chin J Integr Med. 2012;18(6):437–444. doi: 10.1007/s11655-012-1114-y. [DOI] [PubMed] [Google Scholar]
- [16].Franco EC, Cardoso MM, Gouvêia A, et al. Modulation of microglial activation enhances neuroprotection and functional recovery derived from bone marrow mononuclear cell transplantation after cortical ischemia. Neurosci Res. 2012;73(2):122–132. doi: 10.1016/j.neures.2012.03.006. [DOI] [PubMed] [Google Scholar]
- [17].Lee CH, Yoo KY, Choi JH, et al. Neuronal damage is much delayed and microgliosis is more severe in the aged hippocampus induced by transient cerebral ischemia compared to the adult hippocampus. J Neurol Sci. 2010;294(1-2):1–6. doi: 10.1016/j.jns.2010.04.014. [DOI] [PubMed] [Google Scholar]
- [18].Nimmervoll B, White R, Yang JW, et al. LPS-induced microglial secretion of TNFα increases activity-dependent neuronal apoptosis in the neonatal cerebral cortex. Cereb Cortex. 2013;23(7):1742–1755. doi: 10.1093/cercor/bhs156. [DOI] [PubMed] [Google Scholar]
- [19].Cutando L, Busquets-Garcia A, Puighermanal E, et al. Microglial activation underlies cerebellar deficits produced by repeated cannabis exposure. J Clin Invest. 2013;123(7):2816–2831. doi: 10.1172/JCI67569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Zarruk JG, Fernández-López D, García-Yébenes I, et al. Cannabinoid type 2 receptor activation downregulates stroke-induced classic and alternative brain macrophage/microglial activation concomitant to neuroprotection. Stroke. 2012;43(1):211–219. doi: 10.1161/STROKEAHA.111.631044. [DOI] [PubMed] [Google Scholar]
- [21].Lv M, Liu Y, Zhang J, et al. Roles of inflammation response in microglia cell through Toll-like receptors 2/interleukin-23/interleukin-17 pathway in cerebral ischemia/reperfusion injury. Neuroscience. 2011;176:162–172. doi: 10.1016/j.neuroscience.2010.11.066. [DOI] [PubMed] [Google Scholar]
- [22].Zhu J, Jiang Y, Wu L, et al. Suppression of local inflammation contributes to the neuroprotective effect of ginsenoside Rb1 in rats with cerebral ischemia. Neuroscience. 2012;202:342–351. doi: 10.1016/j.neuroscience.2011.11.070. [DOI] [PubMed] [Google Scholar]
- [23].Hu JF, Song XY, Chu SF, et al. Inhibitory effect of ginsenoside Rg1 on lipopolysaccharide-induced microglial activation in mice. Brain Res. 2011;1374:8–14. doi: 10.1016/j.brainres.2010.11.069. [DOI] [PubMed] [Google Scholar]
- [24].Joh EH, Lee IA, Jung IH, et al. Ginsenoside Rb1 and its metabolite compound K inhibit IRAK-1 activation--the key step of inflammation. Biochem Pharmacol. 2011;82(3):278–286. doi: 10.1016/j.bcp.2011.05.003. [DOI] [PubMed] [Google Scholar]
- [25].Ortega FJ, Gimeno-Bayon J, Espinosa-Parrilla JF, et al. ATP-dependent potassium channel blockade strengthens microglial neuroprotection after hypoxia-ischemia in rats. Exp Neurol. 2012;235(1):282–296. doi: 10.1016/j.expneurol.2012.02.010. [DOI] [PubMed] [Google Scholar]
- [26].Kong HL, Li ZQ, Zhao YJ, et al. Ginsenoside Rb1 protects cardiomyocytes against CoCl2-induced apoptosis in neonatal rats by inhibiting mitochondria permeability transition pore opening. Acta Pharmacol Sin. 2010;31(6):687–695. doi: 10.1038/aps.2010.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Gao XQ, Yang CX, Chen GJ, et al. Ginsenoside Rb1 regulates the expressions of brain-derived neurotrophic factor and caspase-3 and induces neurogenesis in rats with experimental cerebral ischemia. J Ethnopharmacol. 2010;132(2):393–399. doi: 10.1016/j.jep.2010.07.033. [DOI] [PubMed] [Google Scholar]
- [28].Ye R, Yang Q, Kong X, et al. Ginsenoside Rd attenuates early oxidative damage and sequential inflammatory response after transient focal ischemia in rats. Neurochem Int. 2011;58(3):391–398. doi: 10.1016/j.neuint.2010.12.015. [DOI] [PubMed] [Google Scholar]
- [29].Ke LN, Wang W, Xu JW, et al. Effect of ginsenoside Rb1 on N9 cell activation induced by oxygen deficit. Jiepou Xuebao. 2009;40(4):533–538. [Google Scholar]
- [30].Ke LN, Wang W, Zhao XZ, et al. Effect of ginsenoside Rb1 on hypoxia-induced damage of hippocampal neurons in SD rats. Shanxi Yike Daxue Xuebao. 2009;40(8):688–692. [Google Scholar]
- [31].Wang JY, Shum AY, Chao CC, et al. Production of macrophage inflammatory protein-2 following hypoxia/reoxygenation in glial cells. Glia. 2000;32(2):155–164. [PubMed] [Google Scholar]
- [32].Fan X, Heijnen CJ, van der Kooij MA, et al. The role and regulation of hypoxia-inducible factor-1alpha expression in brain development and neonatal hypoxic-ischemic brain injury. Brain Res Rev. 2009;62(1):99–108. doi: 10.1016/j.brainresrev.2009.09.006. [DOI] [PubMed] [Google Scholar]
- [33].Schaible EV, Steinsträßer A, Jahn-Eimermacher A, et al. Single administration of tripeptide α-MSH(11-13) attenuates brain damage by reduced inflammation and apoptosis after experimental traumatic brain injury in mice. PLoS One. 2013;8(8):e71056. doi: 10.1371/journal.pone.0071056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Chen Y, Zhou J, Li J, et al. Electroacupuncture pretreatment prevents cognitive impairment induced by limb ischemia-reperfusion via inhibition of microglial activation and attenuation of oxidative stress in rats. Brain Res. 2012;1432:36–45. doi: 10.1016/j.brainres.2011.11.002. [DOI] [PubMed] [Google Scholar]
- [35].Neumann J, Sauerzweig S, Rönicke R, et al. Microglia cells protect neurons by direct engulfment of invading neutrophil granulocytes: a new mechanism of CNS immune privilege. J Neurosci. 2008;28(23):5965–5975. doi: 10.1523/JNEUROSCI.0060-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Nam KN, Park YM, Jung HJ, et al. Anti-inflammatory effects of crocin and crocetin in rat brain microglial cells. Eur J Pharmacol. 2010;648(1-3):110–116. doi: 10.1016/j.ejphar.2010.09.003. [DOI] [PubMed] [Google Scholar]
- [37].Zhou J, Huang WQ, Li C, et al. Intestinal ischemia/reperfusion enhances microglial activation and induces cerebral injury and memory dysfunction in rats. Crit Care Med. 2012;40(8):2438–2448. doi: 10.1097/CCM.0b013e3182546855. [DOI] [PubMed] [Google Scholar]
- [38].Guo LL, Guan ZZ, Huang Y, et al. The neurotoxicity of β-amyloid peptide toward rat brain is associated with enhanced oxidative stress, inflammation and apoptosis, all of which can be attenuated by scutellarin. Exp Toxicol Pathol. 2013;65(5):579–584. doi: 10.1016/j.etp.2012.05.003. [DOI] [PubMed] [Google Scholar]
- [39].Wang JP, Yang ZT, Liu C, et al. Correlation of microglia activation number and content of GDNF and TNF-α in ischemic mouse brain. Zhongguo Laonian Xue Zazhi. 2012;32(11):2334–2335. [Google Scholar]
- [40].Jiang Z, Wang Y, Zhang X, et al. Preventive and therapeutic effects of ginsenoside Rb1 for neural injury during cerebral infarction in rats. Am J Chin Med. 2013;41(2):341–352. doi: 10.1142/S0192415X13500250. [DOI] [PubMed] [Google Scholar]
- [41].Wang J, Qiao L, Li S, et al. Protective effect of ginsenoside Rb1 against lung injury induced by intestinal ischemia-reperfusion in rats. Molecules. 2013;18(1):1214–1226. doi: 10.3390/molecules18011214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Sun Q, Meng QT, Jiang Y, et al. Ginsenoside Rb1 attenuates intestinal ischemia reperfusion induced renal injury by activating Nrf2/ARE pathway. Molecules. 2012;17(6):7195–7205. doi: 10.3390/molecules17067195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Liang YY, Wang B, Qian DM, et al. Inhibitory effects of Ginsenoside Rb1 on apoptosis caused by HSV-1 in human glioma cells. Virol Sin. 2012;27(1):19–25. doi: 10.1007/s12250-012-3220-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Lu T, Jiang Y, Zhou Z, et al. Intranasal ginsenoside Rb1 targets the brain and ameliorates cerebral ischemia/reperfusion injury in rats. Biol Pharm Bull. 2011;34(8):1319–1324. doi: 10.1248/bpb.34.1319. [DOI] [PubMed] [Google Scholar]
- [45].Li Y, Tang J, Khatibi NH, et al. Treatment with ginsenoside rb1, a component of panax ginseng, provides neuroprotection in rats subjected to subarachnoid hemorrhage-induced brain injury. Acta Neurochir Suppl. 2011;110(Pt 2):75–79. doi: 10.1007/978-3-7091-0356-2_14. [DOI] [PubMed] [Google Scholar]
- [46].Wu Y, Xia ZY, Dou J, et al. Protective effect of ginsenoside Rb1 against myocardial ischemia/reperfusion injury in streptozotocin- induced diabetic rats. Mol Biol Rep. 2011;38(7):4327–4335. doi: 10.1007/s11033-010-0558-4. [DOI] [PubMed] [Google Scholar]
- [47].Park JS, Park EM, Kim DH, et al. Anti-inflammatory mechanism of ginseng saponins in activated microglia. J Neuroimmunol. 2009;209(1-2):40–49. doi: 10.1016/j.jneuroim.2009.01.020. [DOI] [PubMed] [Google Scholar]
- [48].Lu HF, Chen ZY. Investigation on the neuroprotective effect of ginsenoside Rb1 on glutamate induced hippocampal neurons injury. Zhonghua Linchuang Yishi Zazhi: Dianzi Ban. 2013;7(8):3467–3470. [Google Scholar]
- [49].2005 ed. Beijing: Chemical Industry Press; 2005. China Pharmacopoeia Committee. Pharmacopoeia of the People's Republic of China. [Google Scholar]
- [50].Webster CM, Hokari M, McManus A, et al. Microglial P2Y12 deficiency/inhibition protects against brain ischemia. PLoS One. 2013;8(8):e70927. doi: 10.1371/journal.pone.0070927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Jin R, Yu S, Song Z, et al. Phosphoinositide 3-kinase-gamma expression is upregulated in brain microglia and contributes to ischemia-induced microglial activation in acute experimental stroke. Biochem Biophys Res Commun. 2010;399(3):458–464. doi: 10.1016/j.bbrc.2010.07.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Lee CH, Moon SM, Yoo KY, et al. Long-term changes in neuronal degeneration and microglial activation in the hippocampal CA1 region after experimental transient cerebral ischemic damage. Brain Res. 2010;1342:138–149. doi: 10.1016/j.brainres.2010.04.046. [DOI] [PubMed] [Google Scholar]
- [53].Nam KN, Park YM, Jung HJ, et al. Anti-inflammatory effects of crocin and crocetin in rat brain microglial cells. Eur J Pharmacol. 2010;648(1-3):110–116. doi: 10.1016/j.ejphar.2010.09.003. [DOI] [PubMed] [Google Scholar]
- [54].Li YK, Chen XC, Zhu YG, et al. Ginsenoside Rb1 attenuates okadaic acid-induced Tau protein hyperphosphorylation in rat hippocampal neurons. Sheng Li Xue Bao. 2005;57(2):154–160. [PubMed] [Google Scholar]
- [55].Chen XC, Zhou YC, Chen Y, et al. Ginsenoside Rg1 reduces MPTP-induced substantia nigra neuron loss by suppressing oxidative stress. Acta Pharmacol Sin. 2005;26(1):56–62. doi: 10.1111/j.1745-7254.2005.00019.x. [DOI] [PubMed] [Google Scholar]
- [56].Jiang PF, Zhu T, Gao JD, et al. The effect of maternal infection on cognitive development and hippocampus neuronal apoptosis, proliferation and differentiation in the neonatal rats. Neuroscience. 2013;246:422–434. doi: 10.1016/j.neuroscience.2013.04.021. [DOI] [PubMed] [Google Scholar]
- [57].Lambertsen KL, Clausen BH, Babcock AA, et al. Microglia protect neurons against ischemia by synthesis of tumor necrosis factor. J Neurosci. 2009;29(5):1319–1330. doi: 10.1523/JNEUROSCI.5505-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]


