Spinal cord injury (SCI) damages not only the gray matter neurons, but also the white matter axonal tracts that carry signals to and from the brain, resulting in permanent loss of function below injury. Neural stem cells (NSCs) have high therapeutic potential for reconstruction of the injured spinal cord since they can potentially form neuronal relays to bridge functional connectivity between separated spinal cord segments. This requires host axonal regeneration into and connectivity with donor neurons, and axonal growth and connectivity of donor neurons to host central nervous system (CNS) circuitry. In this mini-review, we will discuss key studies that explore novel neuronal relay formation by grafting NSCs in models of SCI, with emphasis on long-distance axonal growth and connectivity of NSCs grafted into injured spinal cord.
Traumatic spinal cord injury results in permanent loss of motor, sensory and autonomic function below injury. Unlike neurons of the peripheral nervous system, injured neurons of the CNS do not undergo spontaneous regeneration (Ramón y Cajal et al., 1991). One strategy to repair the injured spinal cord is transplantation of cells that can replace lost neural tissue in sites of SCI for potential functional recovery. This is especially true following severe SCI, in which there is little sparing of tissue and thus little chance to restore functional connectivity between severed spinal cord segments.
Neural stem cells, which can proliferate and differentiate into neurons and glia, have high therapeutic potential for reconstruction of the injured spinal cord. This includes host axonal regeneration and connection into permissive transplanted NSCs, and growth and connectivity of graft derived axons with host neurons (Jakeman and Reier, 1991; Bregman et al., 1993; Bamber et al., 1999). This reciprocal growth of host axons into the grafted neurons and from grafted neurons into host constitutes novel relay circuits that can bridge the injured spinal cord (Figure 1). In addition, transplanted NSCs may reduce secondary injury (Cummings et al., 2005) and have potential to re-myelinate spared, demyelinated axons (Keirstead et al., 2005).
Figure 1.
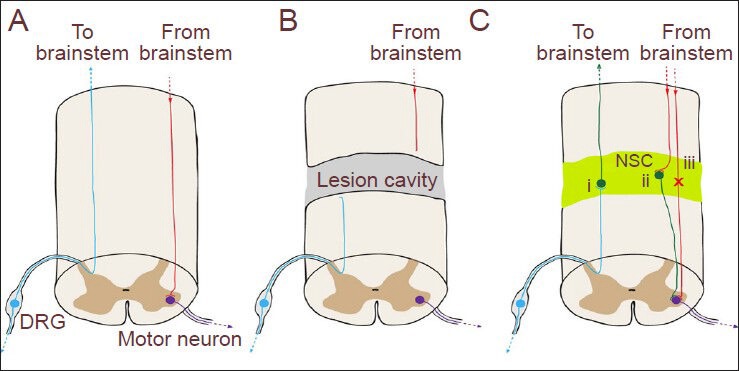
Functional neuronal relay formation by grafted neural stem cells (NSCs).
(A) Cartoon of the intact spinal cord depicting an ascending sensory axon (blue) originating in the dorsal root ganglion (DRG), and a descending motor axon (red) originating from brainstem and synapsing on a motor neuron (purple) in the spinal cord gray matter. (B) Following complete spinal cord transection, ascending and descending host axons are severed and undergo dieback. (C) NSC graft-derived neurons (green) send thousands of axonal projections over long distances to the brainstem and caudal spinal cord, forming functional connections with host spinal cord motor neurons and brainstem dorsal column nucleus neurons (Lu et al., 2012b). Regeneration of host axons into the NSC graft and innervation of grafted neurons with host generates novel functional neuronal relays to allow ascending (i) and descending (ii) neurotransmission across the graft, without the need for long-distance host axonal regeneration and reinnervation of postsynaptic targets (iii).
Grafted NSCs as a permissive substrate for host axonal regeneration
One important objective for NSC transplantation is to promote regenera-tion of injured host axons, since differentiation of NSCs into neural tissue mimics a state of CNS development that can provide a permissive envi-ronment for host axonal growth and regeneration. In addition, NSCs are unique among other cell types since they can generate new neurons that can serve as targets for regenerating host axons. Indeed, early studies reported that host axons could penetrate fetal spinal cord grafts, including some long-tract systems, such as corticospinal, serotonergic, noradrenergic, local propriospinal axons, and sensory axons (Jakeman and Reier, 1991; Bregman et al., 1993; Bamber et al., 1999). However, the number of regenerated axons is modest and the distance into the graft is relatively short (within 0.5 mm). This is in contrast to robust regeneration or regrowth into and beyond fetal spinal cord grafts by host axons in the neonatal stage where CNS neurons still have great regenerative capacity (Bregman et al., 1993; Iwashita et al., 1994). Recent studies, including ours, confirmed regeneration of both ascending and descending long tract axons into NSC grafts (Bonner et al., 2011; Lu et al., 2012b). Furthermore, these regenerated host axons formed synaptic connections with graft-derived neurons.
To enhance the intrinsic growth capacity of injured adult CNS axons, additional therapeutic manipulations are needed (Seijffers and Benowitz, 2008). One strategy is to deliver neurotrophins to the injured spinal cord for promotion of host axonal regeneration into NSC grafts and potentially beyond (Bregman et al., 1997; Coumans et al., 2001; Lu et al., 2003). Neurotrophins delivered via protein infusion can increase the number and extent of supraspinal axons penetrating the fetal graft (Bregman et al., 1997; Coumans et al., 2001). We have successfully delivered neurotrophins into NSC grafts or beyond to promote host axonal regeneration using gene therapy for precise local delivery (Lu et al., 2003; Lu et al., 2012a). However, prolonged delivery of neurotrophins may modulate activities of host spinal cord neurons and affect overall behavioral outcomes (Lu et al., 2012a). An other strategy is to enhance intrinsic growth ability of injured adult neurons by genetic manipulation of growth associated genes, such as deletion of phosphatase and tensin homolog (PTEN) (Liu et al., 2010) or overexpression of Krüppel-like Factor 7 (Blackmore et al., 2012). The combination of these strategies with NSC grafts may greatly enhance host axonal regeneration and connectivity with graft-derived neurons.
Growth and connectivity of grafted NSCs
One of the hallmark features of a functional neuron is its connectivity to target cells by an axon and axonal terminals. Therefore, a successful NSC graft for SCI will display integration and connectivity of donor neurons to host CNS circuitry. Jakeman and Reier (1991) were among the first to perform extensive anatomical assessments of such projection patterns between graft and host tissue at chronic time points after grafting fetal spinal cord tissue. They demonstrated that graft derived axons extended up to 5 mm into host spinal cord tissue, though the majority of axons associated with the graft-host border as determined by anterograde and retrograde tracer labeling. Fischer's group (Lepore and Fischer, 2005) was the first to directly demonstrate axonal growth of grafted embryonic spinal cord cells into host spinal cord as far as 5 mm using the reporter gene alkaline phosphatase. However, grafts of cultured neuronal-restricted precursor (NRP) cells/glial-restricted precursor (GRP) cells into injured spinal cord failed to extend axons into host unless neurotrophins were delivered near the graft (Lepore and Fischer, 2005; Bonner et al., 2011). Graft-derived axon terminals formed synaptic connections with host dorsal column neurons where brain-derived neurotrophic factor (BDNF) was delivered (Bonner et al., 2011).
Besides NSCs from rodents, human NSCs derived from fetal CNS tissue have been reported to extend axons into host CNS tissue when transplanted into the injured spinal cord. Wictorin and Bjorklund (1992) grafted human fetal spinal cord cell suspension into adult rat spinal cord one segment above or below a partial lesion site, and observed human axonal extension into host white matter for up to 10 mm at 3–4 months post-graft using a human specific neurofilament marker. This finding along with a previous study of human axonal growth in adult lesioned rat brain (Wictorin et al., 1990) is the first report of long-distance axonal growth in the inhibitory environment of adult CNS tissue. Although this study shows long-distance growth of human NSC projections in the injured adult spinal cord, the human cells were implanted into normal cord parenchyma one segment (3–4 mm) above or below the lesion (Wictorin and Bjorklund, 1992). Whether human NSCs implanted into sites of SCI can survive or differentiate into neurons that extend their axons into host spinal cord to establish functional connections is unknown.
Giovanini and colleagues (Giovanini et al., 1997) characterized human fetal spinal cord transplantation into sites of adult rat SCI under different lesion and grafting conditions. Although they demonstrated extensive human neurite growth into host, these neurites grew only into host gray matter for a short distance, but not into host white matter. The short distance growth of human axons into host may relate to poor survival and integration of human fetal spinal cord cells into lesion center of host since no immunosuppression was described in the methodology of this xenograft study (Giovanini et al., 1997).
Recently, our group focused on characterization of axonal growth and connectivity of grafted NSCs in models of severe SCI. We developed new methodology to improve NSC tracking, survival, and differentiation/maturation in the injured adult spinal cord (Lu et al., 2012b). We began with isolation of NSCs/progenitor cells from a stable transgenic Fischer 344 rat line expressing a green fuorescent protein (GFP) reporter gene to unequivocally label grafted cells in order to follow their axonal growth and connectivity. The Fischer 344 rats are inbred and isogenic, thereby avoiding immunological rejection when cells are grafted between rats of the same strain. Next, we used committed NSCs and progenitor cells from freshly dissociated embryonic day 14 (E14) spinal cord tissue to avoid glial differentiation or lack of axonal growth from cultured rat NSCs (Cao et al., 2001; Lepore and Fischer, 2005). In addition, we embedded isolated NSCs/progenitor cells into a fibrin matrix containing growth factor cocktails to retain these cells and support their survival and differentiation in severe lesioned spinal cord. Finally, we delayed grafting of NSCs/neural progenitor cells for two weeks after SCI, a clinically relevant time point, to avoid peak inflammation for further enhancement of their survival (Lu et al., 2012b).
We observed that grafted NSCs/progenitors survived well, completely flled the upper thoracic complete transection site and differentiated into both neurons and glia (Lu et al., 2012b). Most remarkably, the grafted neurons extended their axons in remarkable densities in both rostral and caudal directions (29,000 axons emerging from the graft in the caudal direction) and over very long distances (7–9 spinal cord segments and over 20 mm) in the host spinal cord. Such numbers and distance of growth of graft-derived axons are unprecedented to the best of our knowledge. Furthermore, many grafted axons were myelinated and exhibited extensive synapse formation with host neurons (Lu et al., 2012b). Similarly, E14 brainstem derived NSCs grafted into T3 transection site also extended large number of axons into host for long distances, including graft-derived catecholaminergic and serotonergic neurons and their innervations of caudal sympathetic preganglionic neurons (Hou et al., 2013). These results indicate that, despite the initial non-growth-supporting environment of the lesioned adult CNS, our protocol transforms the lesion site into one that is highly permissive for axonal growth from graft-derived neurons within the lesion site.
Because of the therapeutic implications for human cell transplantation, we tested whether cultured human fetal spinal cord derived NSCs grafted into severe injured rodent spinal cord exhibit similar axonal growth and connectivity as rodent NSCs. We chose an FDA approved human cell line (566RSC cells) that is approved for clinical trials for amyotrophic lateral sclerosis (ALS) and is well characterized both in vitro and in vivo for differentiation (Yan et al., 2007; Guo et al., 2010), and grafted these cells into sites of T3 complete transection in adult immunodeficient athymic rats (Lu et al., 2012b). Again, we observed long-distance growth and connectivity of GFP-labeled human axons from the graft even at the short survival time of 7 weeks. In a follow-up study, the same human line was grafted into a compression injury site of adult rats under immunosuppressive conditions (van Gorp et al., 2013). Numerous human neurites extended from the graft into the host in both rostral and caudal directions, especially along the lateral white matter. In addition, human neurites expressed the pre-synaptic marker synaptophysin, suggesting connection with host neurons. These findings indicate that human NSCs have similar intrinsic growth capacity to specify suffcient information for extensive, long-distance axonal growth even in an inhibitory adult CNS environment.
The generation of embryonic stem (ES) cells and induced pluripotent stem (IPS) cells opens new doors for SCI repair since these pluripotent stem cells are able to proliferate indefinitely and can differentiate into various types of cells, including neurons and glia, therefore providing endless resource for cell transplantation. In addition, IPS cells are adult derived and can be transplanted autologously to avoid both ethical and immunorejection concerns. Several studies reported grafting of either ES cell-derived NSCs (Kimura et al., 2005; Erceg et al., 2010; Rossi et al., 2010; Cui et al., 2011; Niapour et al., 2012) or IPS cell-derived NSCs (Tsuji et al., 2010; Nori et al., 2011; Fujimoto et al., 2012) into injured rodent spinal cord. These studies, however, did not explore the growth and connectivity of grafted neurons with host. Our group first showed extensive outgrowth of human axons over long distance into host from human ES cells (HUES7) derived NSC graft placed in C5 hemisection site for 3 months. Human ES cell graft-derived axons reached the brainstem in the rostral direction and the lumbar spinal cord in caudal direction, with axonal growth spanning over 17 spinal segments and more than 50 mm (Lu et al., 2012b). Graft of another human ES cell line (H9) derived NSCs into C5 hemisection site for 3 months exhibited similar extensive axonal growth (Figure 2, unpublished result). Notably, human axons grew in highly organized, rostro-caudal linear trajectories in host white matter (Figure 2A). Throughout the course of their white matter projections, human axons sent their collateral branches into host gray matter for innervation of target neurons (Figure 2B). Immunohistochemistry demonstrates that human axons expressing the pre-synaptic marker synaptophysin surrounded host gray matter neurons, including choline acetyltransferase-expressing host spinal motor neurons, indicating synaptic connections with host neurons (Lu et al., 2012b). Similarly, grafted primitive neural stem cells (pNSCs) derived from human ES cells (HUES9) into sites of SCI also extended long axonal projections into host spinal cord, including human serotonergic axons from the graft (Zhao et al., 2013). Recently, we also transplanted human IPS cells derived NSCs into C5 hemisection site of adult immunodeficient rats for 3 months and found, once again, extensive axonal outgrowth in a preliminary study (Lu et al., unpublished results). Indeed, human axons traversed virtually the entire rostral-caudal extent of the rat CNS. The robust growth of human IPS cell-derived NSCs may relate to the integration of four genes that induce stemness of adult somatic cells (Takahashi and Yamanaka, 2006). These fndings indicate that NSCs derived from human ES or IPS cells have similar properties of axonal growth and connectivity as CNS derived NSCs.
Figure 2.
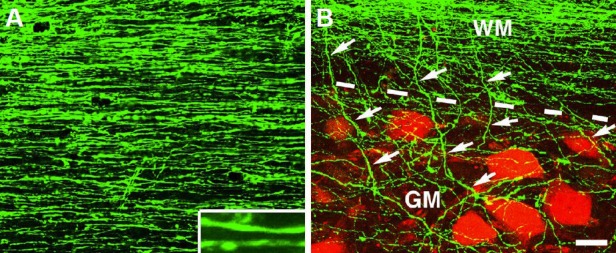
Growth and innervation of human axons after spinal cord injury in rats.
(A) Growth of green fluorescent protein (GFP) expressing human axons (H9 derived) in the host white matter 3 mm caudal to a neural stem cell (NSC) graft placed in C5 hemisection site for 3 months. Inset shows individual axons at higher magnification in a horizontal section. (B) Innervation of human axons (green) from host white matter (WM) into gray matter (GM) is indicated by arrows. Host neurons are labeled with NeuN (red). Dashed lines indicate white matter and gray matter interface. Scale bar: 32 μm.
Functional neuronal relay formation
The reciprocal growth and connection of grafted neurons into host and host neurons into graft could result in potential functional neuronal relay formation. Early studies partially documented the ingrowth of host axons into graft and outgrowth of grafted neurons into host, supporting this hypothesis (Jakeman and Reier, 1991; Bregman et al., 1993; Bregman et al., 1997; Bamber et al., 1999). Recent studies confirmed functional connectivity between grafted neurons and host target neurons by injection of the transneuronal tracer pseudorabies virus and electrophysiological recordings (White et al., 2010; Lee et al., 2014). The formation of a functional neuronal relay by grafted neurons was first documented by Bonner et al. (2011) who demonstrated that host ascending sensory axons formed active synapses with graft neurons at the dorsal column injury site with the signal propagating by graft derived axons to the dorsal column nuclei using stimulus-evoked c-Fos expression and electrophysiological recording. Our group first documented partial restoration of descending neuronal transmission signals crossing complete transected spinal cord that received NSC graft using electrophysiological recording (Lu et al., 2012b). In addition, we observed locomotor behavioral improvement in NSC transplanted subjects compared to controls (Lu et al., 2012b). Re-transection above the graft abolished this recovery, indicating novel relay circuit formation by grafted neurons that connected host neurons above and below the lesion. Other studies have reported various degrees of functional improvement with NSC graft, probably partially due to this novel neuronal relay formation (Coumans et al., 2001; Cummings et al., 2005; Lowry et al., 2008; Erceg et al., 2010; Rossi et al., 2010; Nori et al., 2011; Fujimoto et al., 2012; Hou et al., 2013). Together, these findings demonstrate growth and connectivity between NSC graft and host tissue, lending strong support to the concept that donor neurons can serve as a novel functional relay between intact host neurons separated by a spinal cord lesion.
Future perspectives and challenges
Despite these recent advances, there remain several challenges facing future research toward therapeutic repair for SCI. Our recent work reveals the incredible capacity for intrinsic growth of transplanted NSCs within the injured adult CNS (Lu et al., 2012b); however, fine-tuning graft-derived projections by rehabilitation training and promoting their growth toward specifc targets by axon guiding molecules and neurotrophins will require further investigation. Additional future studies are also needed to characterize graft-derived neuronal and glial populations, in order to understand and shape the nature of host-graft relay circuitry. An ongoing goal remains to identify new methods to enhance regeneration of host axons into NSC grafts, especially for functionally important pathways like the corticospinal tract, which is particularly refractory to regeneration. The potential for formation of novel neuronal relay circuits by NSC transplantation opens up new possibilities for combinatorial therapies for the treatment of SCI.
Footnotes
Conflicts of interest: None declared.
Funding: This work was funded by grants from the Veterans Administration, the Canadian Spinal Research Organization and the California Institute for Regenerative Medicine.
References
- [1].Bamber NI, Li H, Aebischer P, Xu XM. Fetal spinal cord tissue in mini-guidance channels promotes longitudinal axonal growth after grafting into hemisected adult rat spinal cords. Neural Plast. 1999;6:103–121. doi: 10.1155/NP.1999.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Blackmore MG, Wang Z, Lerch JK, Motti D, Zhang YP, Shields CB, Lee JK, Goldberg JL, Lemmon VP, Bixby JL. Kruppel-like Factor 7 engineered for transcriptional activation promotes axon regeneration in the adult corticospinal tract. Proc Natl Acad Sci U S A. 2012;109:7517–7522. doi: 10.1073/pnas.1120684109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Bonner JF, Connors TM, Silverman WF, Kowalski DP, Lemay MA, Fischer I. Grafted neural progenitors integrate and restore synaptic connectivity across the injured spinal cord. J Neurosci. 2011;31:4675–4686. doi: 10.1523/JNEUROSCI.4130-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Bregman BS, McAtee M, Dai HN, Kuhn PL. Neurotrophic factors increase axo-nal growth after spinal cord injury and transplantation in the adult rat. Exp Neurol. 1997;148:475–494. doi: 10.1006/exnr.1997.6705. [DOI] [PubMed] [Google Scholar]
- [5].Bregman BS, Kunkel-Bagden E, Reier PJ, Dai HN, McAtee M, Gao D. Recovery of function after spinal cord injury: mechanisms underlying transplant-mediated recovery of function differ after spinal cord injury in newborn and adult rats. Exp Neurol. 1993;123:3–16. doi: 10.1006/exnr.1993.1136. [DOI] [PubMed] [Google Scholar]
- [6].Cao QL, Zhang YP, Howard RM, Walters WM, Tsoulfas P, Whittemore SR. Plu-ripotent stem cells engrafted into the normal or lesioned adult rat spinal cord are restricted to a glial lineage. Exp Neurol. 2001;167:48–58. doi: 10.1006/exnr.2000.7536. [DOI] [PubMed] [Google Scholar]
- [7].Coumans JV, Lin TT, Dai HN, MacArthur L, McAtee M, Nash C, Bregman BS. Axonal regeneration and functional recovery after complete spinal cord transec-tion in rats by delayed treatment with transplants and neurotrophins. J Neurosci. 2001;21:9334–9344. doi: 10.1523/JNEUROSCI.21-23-09334.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Cui YF, Xu JC, Hargus G, Jakovcevski I, Schachner M, Bernreuther C. Embry-onic stem cell-derived L1 overexpressing neural aggregates enhance recovery after spinal cord injury in mice. PloS One. 2011;6:e17126. doi: 10.1371/journal.pone.0017126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Cummings BJ, Uchida N, Tamaki SJ, Salazar DL, Hooshmand M, Summers R, Gage FH, Anderson AJ. Proc Natl Acad Sci U S A. Proc Natl Acad Sci U S A. 2005;102:14069–14074. doi: 10.1073/pnas.0507063102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Erceg S, Ronaghi M, Oria M, Rosello MG, Arago MA, Lopez MG, Radojevic I, More-no-Manzano V, Rodriguez-Jimenez FJ, Bhattacharya SS, Cordoba J, Stojkovic M. Transplanted oligodendrocytes and motoneuron progenitors generated from human embryonic stem cells promote locomotor recovery after spinal cord transection. Stem Cells. 2010;28:1541–1549. doi: 10.1002/stem.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Fujimoto Y, Abematsu M, Falk A, Tsujimura K, Sanosaka T, Juliandi B, Semi K, Namihira M, Komiya S, Smith A, Nakashima K. Treatment of a mouse model of spinal cord injury by transplantation of human induced pluripotent stem cell-derived long-term self-renewing neuroepithelial-like stem cells. Stem Cells. 2012;30:1163–1173. doi: 10.1002/stem.1083. [DOI] [PubMed] [Google Scholar]
- [12].Giovanini MA, Reier PJ, Eskin TA, Wirth E, Anderson DK. Characteristics of human fetal spinal cord grafts in the adult rat spinal cord: infuences of lesion and grafting conditions. Exp Neurol. 1997;148:523–543. doi: 10.1006/exnr.1997.6703. [DOI] [PubMed] [Google Scholar]
- [13].Guo X, Johe K, Molnar P, Davis H, Hickman J. Characterization of a human fetal spinal cord stem cell line, NSI-566RSC, and its induction to functional moto-neurons. J Tissue Eng Regen Med. 2010;4:181–193. doi: 10.1002/term.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Hou S, Tom VJ, Graham L, Lu P, Blesch A. Partial restoration of cardiovascular function by embryonic neural stem cell grafts after complete spinal cord transec-tion. J Neurosci. 2013;33:17138–17149. doi: 10.1523/JNEUROSCI.2851-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Iwashita Y, Kawaguchi S, Murata M. Restoration of function by replacement of spinal cord segments in the rat. Nature. 1994;367:167–170. doi: 10.1038/367167a0. [DOI] [PubMed] [Google Scholar]
- [16].Jakeman LB, Reier PJ. Axonal projections between fetal spinal cord transplants and the adult rat spinal cord: a neuroanatomical tracing study of local interactions. J Comp Neurol. 1991;307:311–334. doi: 10.1002/cne.903070211. [DOI] [PubMed] [Google Scholar]
- [17].Keirstead HS, Nistor G, Bernal G, Totoiu M, Cloutier F, Sharp K, Steward O. Hu-man embryonic stem cell-derived oligodendrocyte progenitor cell transplants remye-linate and restore locomotion after spinal cord injury. J Neurosci. 2005;25:4694–4705. doi: 10.1523/JNEUROSCI.0311-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Kimura H, Yoshikawa M, Matsuda R, Toriumi H, Nishimura F, Hirabayashi H, Na-kase H, Kawaguchi S, Ishizaka S, Sakaki T. Transplantation of embryonic stem cell-derived neural stem cells for spinal cord injury in adult mice. Neurol Res. 2005;27:812–819. doi: 10.1179/016164105X63629. [DOI] [PubMed] [Google Scholar]
- [19].Lee KZ, Lane MA, Dougherty BJ, Mercier LM, Sandhu MS, Sanchez JC, Reier PJ, Fuller DD. Intraspinal transplantation and modulation of donor neuron electro-physiological activity. Exp Neurol. 2014;251:47–57. doi: 10.1016/j.expneurol.2013.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Lepore AC, Fischer I. Lineage-restricted neural precursors survive, migrate, and differentiate following transplantation into the injured adult spinal cord. Exp Neu-rol. 2005;194:230–242. doi: 10.1016/j.expneurol.2005.02.020. [DOI] [PubMed] [Google Scholar]
- [21].Liu K, Lu Y, Lee JK, Samara R, Willenberg R, Sears-Kraxberger I, Tedeschi A, Park KK, Jin D, Cai B, Xu B, Connolly L, Steward O, Zheng B, He Z. PTEN deletion enhances the regenerative ability of adult corticospinal neurons. Nat Neurosci. 2010;13:1075–1081. doi: 10.1038/nn.2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Lowry N, Goderie SK, Adamo M, Lederman P, Charniga C, Gill J, Silver J, Temple S. Multipotent embryonic spinal cord stem cells expanded by endothelial fac-tors and Shh/RA promote functional recovery after spinal cord injury. Exp Neurol. 2008;209:510–522. doi: 10.1016/j.expneurol.2007.09.031. [DOI] [PubMed] [Google Scholar]
- [23].Lu P, Jones LL, Snyder EY, Tuszynski MH. Neural stem cells constitutively secrete neurotrophic factors and promote extensive host axonal growth after spinal cord injury. Exp Neurol. 2003;181:115–129. doi: 10.1016/s0014-4886(03)00037-2. [DOI] [PubMed] [Google Scholar]
- [24].Lu P, Blesch A, Graham L, Wang Y, Samara R, Banos K, Haringer V, Havton L, Weishaupt N, Bennett D, Fouad K, Tuszynski MH. Motor axonal regenera-tion after partial and complete spinal cord transection. J Neurosci. 2012a;32:8208–8218. doi: 10.1523/JNEUROSCI.0308-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Lu P, Wang Y, Graham L, McHale K, Gao M, Wu D, Brock J, Blesch A, Rosenzweig ES, Havton LA, Zheng B, Conner JM, Marsala M, Tuszynski MH. Long-distance growth and connectivity of neural stem cells after severe spinal cord injury. Cell. 2012b;150:1264–1273. doi: 10.1016/j.cell.2012.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Niapour A, Karamali F, Nemati S, Taghipour Z, Mardani M, Nasr-Esfahani MH, Baha-rvand H. Cotransplantation of human embryonic stem cell-derived neural progenitors and schwann cells in a rat spinal cord contusion injury model elicits a distinct neurogenesis and functional recovery. Cell Transplant. 2012;21:827–843. doi: 10.3727/096368911X593163. [DOI] [PubMed] [Google Scholar]
- [27].Nori S, Okada Y, Yasuda A, Tsuji O, Takahashi Y, Kobayashi Y, Fujiyoshi K, Koike M, Uchiyama Y, Ikeda E, Toyama Y, Yamanaka S, Nakamura M, Okano H. Grafted human-induced pluripotent stem-cell-derived neurospheres promote motor functional recovery after spinal cord injury in mice. Proc Natl Acad Sci U S A. 2011;108:16825–16830. doi: 10.1073/pnas.1108077108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Ramón y Cajal S, DeFelipe J, Jones EG. New York: Oxford University Press; 1991. Cajal's degeneration and regeneration of the nervous system. [Google Scholar]
- [29].Rossi SL, Nistor G, Wyatt T, Yin HZ, Poole AJ, Weiss JH, Gardener MJ, Dijkstra S, Fischer DF, Keirstead HS. Histological and functional benefit following transplantation of motor neuron progenitors to the injured rat spinal cord. PloS One. 2010;5:e11852. doi: 10.1371/journal.pone.0011852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Seijffers R, Benowitz LI. Intrinsic determinants of axon regeneration. In: Kordower JH, Tuszynski MH, editors. CNS regeneration: Basic science and clinical advances. 2nd Edition. Elsevier; 2008. pp. 1–39. [Google Scholar]
- [31].Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fbroblast cultures by defned factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- [32].Tsuji O, Miura K, Okada Y, Fujiyoshi K, Mukaino M, Nagoshi N, Kitamura K, Kumagai G, Nishino M, Tomisato S, Higashi H, Nagai T, Katoh H, Kohda K, Matsuzaki Y, Yuzaki M, Ikeda E, Toyama Y, Nakamura M, Yamanaka S, et al. (2010) Therapeutic potential of appropriately evaluated safe-induced pluripotent stem cells for spinal cord injury. Proc Natl Acad Sci U S A. 107:12704–12709. doi: 10.1073/pnas.0910106107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].van Gorp S, Leerink M, Kakinohana O, Platoshyn O, Santucci C, Galik J, Joosten EA, Hruska-Plochan M, Goldberg D, Marsala S, Johe K, Ciacci JD, Marsala M. Amelioration of motor/sensory dysfunction and spasticity in a rat model of acute lumbar spinal cord injury by human neural stem cell transplantation. Stem Cell Res Ther. 2013;4:57. doi: 10.1186/scrt209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].White TE, Lane MA, Sandhu MS, O’Steen BE, Fuller DD, Reier PJ. Neuronal progenitor transplantation and respiratory outcomes following upper cervical spinal cord injury in adult rats. Exp Neurol. 2010;225:231–236. doi: 10.1016/j.expneurol.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Wictorin K, Bjorklund A. Axon outgrowth from grafts of human embryonic spinal cord in the lesioned adult rat spinal cord. Neuroreport. 1992;3:1045–1048. doi: 10.1097/00001756-199212000-00003. [DOI] [PubMed] [Google Scholar]
- [36].Wictorin K, Brundin P, Gustavii B, Lindvall O, Bjorklund A. Reformation of long axon pathways in adult rat central nervous system by human forebrain neuroblasts. Nature. 1990;347:556–558. doi: 10.1038/347556a0. [DOI] [PubMed] [Google Scholar]
- [37].Yan J, Xu L, Welsh AM, Hatfeld G, Hazel T, Johe K, Koliatsos VE. Extensive neuronal differentiation of human neural stem cell grafts in adult rat spinal cord. PLoS Med. 2007;4:e39. doi: 10.1371/journal.pmed.0040039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Zhao J, Sun W, Cho HM, Ouyang H, Li W, Lin Y, Do J, Zhang L, Ding S, Liu Y, Lu P, Zhang K. Integration and long distance axonal regeneration in the central nervous system from transplanted primitive neural stem cells. J Biol Chem. 2013;288:164–168. doi: 10.1074/jbc.M112.433607. [DOI] [PMC free article] [PubMed] [Google Scholar]


