Abstract
Acupuncture at Baihui (GV20) and Dazhui (GV14) reduces neuronal loss and attenuates ultrastructural damage in cerebral ischemic rats. However, whether acupuncture can treat addiction and prevent readdiction through changes to brain cell ultrastructure remains unknown. In this study, cell apoptosis was observed in the hippocampus and frontal lobe of heroin readdicted rats by electron microscopy. Immunohistochemical staining displayed a reduction in Bcl-2 expression and an increase in Bax expression in the hippocampus and frontal lobe. After rats were given acupuncture at Baihui and Dazhui, the pathological damage in the hippocampus and frontal lobe was significantly reduced, Bcl-2 expression was upregulated and Bax expression was downregulated. Acupuncture exerted a similar effect with methadone, a commonly used drug for clinical treatment of drug addiction. Experimental findings suggest that acupuncture at Dazhui and Baihui can prevent brain cell apoptosis in heroin readdicted rats.
Keywords: nerve regeneration, traditional Chinese medicine, acupuncture, heroin readdiction, brain injury, ultrastructure, Bcl-2, Bax, apoptosis, neuroprotection, NSFC grant, neural regeneration
Introduction
Heroin addiction and readdiction can cause extensive damage to the brain, and result in irreversible damage, with longer drug abuse triggering severe brain damage[1,2,3,4,5,6]. Brain injury caused by heroin addiction and readdiction is highly involved in nerve cell apoptosis and nerve demyelination[7,8,9,10]. Apoptosis in the brain is a common symptom of heroin addicted rats and ultrastructural pathological changes are apparent in neurons of the midbrain ventral tegmental area and the nucleus accumbens, which plays an important role in the reward circuit. Therefore, apoptosis is a dominant mode of neuronal death caused by heroin addiction[7,8,9,10]. Accumulating evidence has shown ultrastructural changes in the cerebral cortex, hypothalamus, hippocampus, and pituitary gland of heroin addicted rats, rhesus monkeys and humans[11,12,13,14,15,16,17]. Ultrastructural changes such as degeneration, necrosis, and apoptosis of neurons, axons and dendrites are known to be found in the brain of heroin addicted rats, including the prefrontal cortex, nucleus accumbens, hippocampus, midbrain ventral tegmental area and hypothalamus[18,19,20,21,22].
Acupuncture has therapeutic effects on cerebral ischemia, dementia, epilepsy and other brain diseases, and also functions to repair the nervous system. Dazhui (GV14) and Baihui (GV20) are the preferred acupoints for treatment[23,24,25,26,27,28,29]. Acupuncture at Baihui and Dazhui improves oxygen atmosphere in the brain of cerebral ischemic rats, inhibits abnormal activation of the JAK2-STAT3 signal transduction pathways, and attenuates oxidative stress-induced brain injury[30,31,32]. In addition, acupuncture therapy can regulate the expression of apoptosis-related genes in the hippocampus, increase the number of nestin-positive cells, attenuate ultrastructural injury in ischemic neurons, endothelial cells, myelin and glial cells, improve cerebral cortex ultrastructural damage, and exert neuroprotective effects[33,34,35,36]. Acupuncture at Baihui and Dazhui contributes to the regulation of expression of various proteins, receptors and enzymes in the blood and hippocampus, modulates synaptic number and structure, promotes nerve regeneration and hippocampal long-term potentiation, accelerates synaptic transmission in hippocampal neurons, improves learning and memory ability of diabetic rats and dementia rats, and slows brain aging[37,38,39,40,41,42,43,44]. Finally, acupuncture at Baihui and Dazhui is responsible for inhibiting the reduced activity of mitochondrial complexes, protecting brain mitochondrial function, decreasing neuronal loss, and attenuating ultrastructural damage in Parkinson's disease mice[45,46].
Acupuncture therapy exerts a similar effect with other withdrawal therapies for the treatment of heroin toxic encephalopathy[47,48,49,50,51,52,53,54,55]. However, whether acupuncture improves central nervous system damage caused by heroin toxicity remains unknown. A review of relevant literatures revealed the influence of acupuncture on brain ultrastructure in heroin addicts[56,57,58]. However, to date, no studies have investigated if the role of acupuncture is mediated by changes to brain ultrastructure.
In this study, heroin readdiction was produced through repeated exposure and detoxification in rats, which more closely mimics the long-term or repeated drug addiction state in humans than other withdrawal models (one exposure and detoxification). We acupunctured heroin readdicted rats at Baihui and Dazhui, and then compared rats to methadone treated rats, a commonly used drug for the clinical treatment of drug addiction, in an effort to explore the neuroprotective effect of acupuncture on the brain of heroin readdicted rats at a cellular level.
Results
Quantitative analysis of experimental animals
Forty rats were randomly divided into four groups: normal control, model, acupuncture, and drug. Except for the normal control group, rats in the other three groups were intramuscularly injected with increasing doses of heroin into the hindlimb for 8 successive days, followed by a 5-day withdrawal. The injection and withdrawal were given three times to establish a heroin readdiction model. Rats in the acupuncture group were treated by acupuncture during the detoxification period, while those in the drug group were treated with methadone. No rats died during the experiment.
Acupuncture improved the morphology of nerve cells in the frontal cortex and hippocampus of heroin readdicted rats
Under transmission electron microscopy, we found clear nuclear membranes, normal structure of rough endoplasmic reticulum, ribosomes and cell nuclei, abundant rough endoplasmic reticulum and mitochondria, as well as mild expansion of rough endoplasmic reticulum in the hippocampus and frontal lobe of normal control rats.
In model rats, the frontal cortex and hippocampal neurons exhibited nuclear pyknosis, chromatin margination, rough endoplasmic reticulum expansion, mitochondria degeneration, widened perinuclear gaps, and apparent apoptosis. In the acupuncture group, frontal cortex and hippocampal neurons had clear nuclear membranes and abundant mitochondria and rough endoplasmic reticulum, rough endoplasmic reticulum structure was normal or mildly dilated, and some mitochondrial vacuolar degeneration was observed. Neuronal morphology in the frontal cortex and hippocampus was similar between the acupuncture and drug group (Figure 1).
Figure 1.
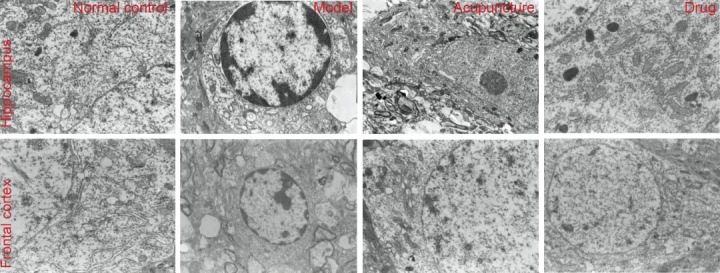
Effect of acupuncture on the morphology of nerve cells in the frontal cortex and hippocampus of heroin readdicted rats (transmission electron microscopy, × 10,000).
In the normal control group, the nuclear membrane in hippocampal nerve cells was clearly visible, there were abundant rough endoplasmic reticulum, ribosomes and mitochondria, mitochondrial cristae were clear; fewer ribosomes were observed in the frontal cortex, some mitochondria degenerated, and rough endoplasmic reticulum mildly expanded. In the model group, hippocampal neuronal cells were apoptotic, chromatin aggregated and rough endoplasmic reticulum expanded, partial mitochondrial expansion and vacuolar degeneration were observed, intermembrane space widened; the frontal lobe also exhibited nerve cell apoptosis, nuclear chromatin aggregation, and enlarged perinuclear gaps. In the acupuncture group, hippocampal nerve cells had clear nuclear membranes, expanded rough endoplasmic reticulum, intact mitochondria; frontal lobe nerve cells had clear nuclear membranes, partial mitochondrial degeneration, and abundant rough endoplasmic reticulum. In the drug group, hippocampal nerve cells had clear nuclear membranes, there were a large number of mitochondria with clear mitochondrial cristae, rough endoplasmic reticulum mildly dilated; frontal lobe nerve cells also presented clear nuclear membranes, the ribosomes and rough endoplasmic reticulum were abundant, and a few mitochondria appeared degenerated.
Acupuncture at Baihui and Dazhui enhanced Bcl-2 expression in frontal cortex and hippocampal nerve cells of heroin readdicted rats
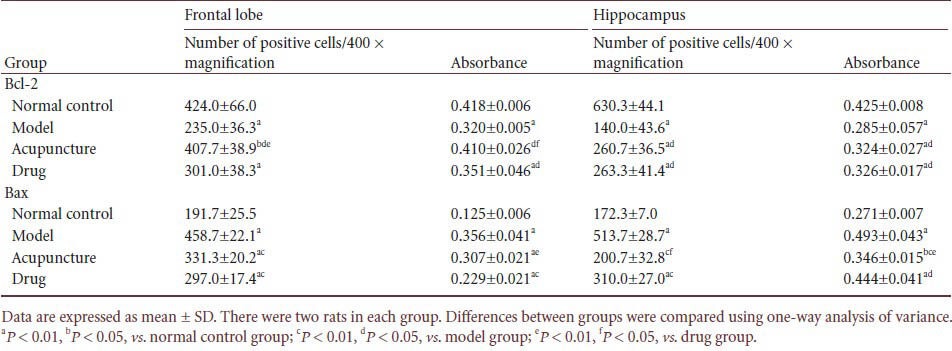
Immunohistochemical staining showed that the number of Bcl-2-positive cells and Bcl-2 expression in the frontal cortex and hippocampus of heroin readdicted rats significantly decreased compared with the normal control group (P < 0.01). In the acupuncture and drug groups, the number of Bcl-2 positive cells and Bcl-2 expression significantly increased compared with the model group (P < 0.05). In addition, the acupuncture group showed more Bcl-2-positive cells and higher Bcl-2 expression than the drug group (P < 0.01 or P < 0.05; Figure 2A, Table 1).
Figure 2.
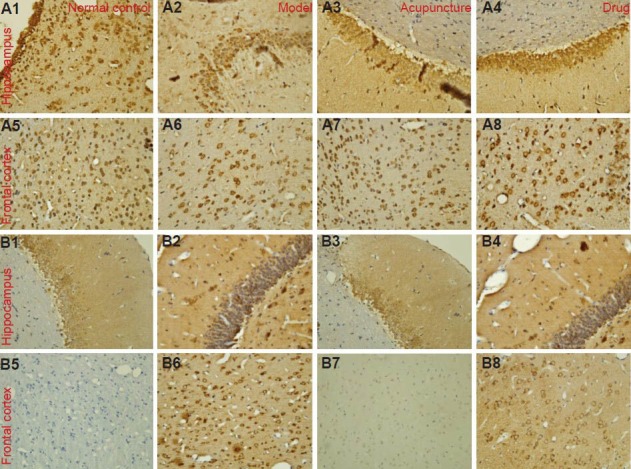
Effect of acupuncture at Baihui and Dazhui on Bcl-2 (A1–A8) and Bax (B1–B8) expression in the frontal lobe and hippocampus of heroin readdicted rats (immunohistochemical staining, × 400).
Bcl-2 expression in the hippocampus and frontal lobe of model rats was decreased, while Bax epxression was increased compared with the normal control group. Both acupuncture and drug therapy increased Bcl-2 expression but decreased Bax expression in the frontal cortex and hippocampus of heroin readdicted rats. Bcl-2- and Bax-positive expression appeared brown.
Table 1.
Effect of acupuncture at Baihui and Dazhui on Bcl-2- and Bax-positive cells in the frontal lobe and hippocampus of heroin readdicted rats
Acupuncture at Baihui and Dazhui reduced Bax expression in frontal lobe and hippocampal nerve cells of heroin readdicted rats
Immunohistochemical staining showed that the number of Bax-positive cells and Bax expression in the frontal cortex and hippocampus of heroin readdicted rats significantly increased compared with the normal control group (P < 0.01). In the acupuncture and drug groups, the number of Bax-positive cells and Bax expression significantly decreased compared with the model group (P < 0.05). In addition, the acupuncture group showed more Bax-positive cells and higher Bax expression than the drug group (P < 0.01 or P < 0.05; Figure 2B, Table 1).
Discussion
In this study, we observed ultrastructural damage in the hippocampus and prefrontal cortex of heroin readdicted rats. Once the stability of the cerebral environment is destroyed, it inevitably affects the basic function and hinders information transmission between the brain and the periphery[59,60]. After the nerve nuclear group in the limbic system is partially destroyed, neuroendocrine immune function may change[59,60], resulting in withdrawal symptoms.
Our findings showed that ultrastructure pathological damage to brain nerve cells of heroin readdicted rats was markedly attenuated after acupuncture. This evidence indicates that acupuncture can reverse heroin readdiction-caused brain injury to some extent. Furthermore acupuncture has the potential to maintain mitochondria and rough endoplasmic reticulum function, promote the transport of proteins synthesized by membrane-bound ribosomes, and help to synthesize ATP, which are conducive to providing energy for a variety of physiological activities of cells and restoring the function of nerve nuclear groups in the hippocampus and prefrontal cortex[37,38,39,40,41,42,43,44,45,46]. Based on the aforementioned evidence, we speculate that acupuncture may potentially restore learning and memory disorders, cognitive disorders, and mood disorders caused by heroin, and prevent readdiction.
In this study, heroin readdiction caused the downregulation of Bcl-2 expression in the frontal lobe and hippocampus, while increased proapoptotic Bax expression levels. Acupuncture at Baihui and Dazhui increased Bcl-2 expression in the frontal lobe and hippocampus of heroin readdicted rats, while decreased Bax expression levels. Excess Bax produces Bax-Bax homodimers and induces apoptosis; the decline of Bax expression levels leads to the generation of heterodimers with Bcl-2, thus inhibiting apoptosis[61,62,63,64]. Electron microscopy results showed that acupuncture can prevent brain cell apoptosis in heroin readdicted rats, which is most likely mediated by altering cell ultrastructure, regulating the expression of the apoptosis-related genes Bcl-2 and Bax, and changing Bcl-2/Bax ratio. However apoptosis is a complex physiological process involving many factors and mechanisms.
We also compared the therapeutic effect of two therapies (acupuncture vs. methadone) in the treatment of heroin readdiction. The results showed consistent variations in brain cell ultrastructure and apoptosis-related genes (Bcl-2 and Bax) in the two groups. Acupuncture was more effective than methadone on local lesions.
Overall, our study has provided a partial mechanism for the neuroprotective effect of acupuncture on brain cell death in heroin readdicted rats, and provided insights on the possibility of using acupuncture for the treatment of heroin readdiction. However, further studies are needed to understand how acupuncture prevents apoptosis of brain cells in heroin readdicted rats.
Materials and Methods
Design
A randomized controlled animal experiment.
Time and setting
Experiments were performed from May to June in 2010 at Research Institute of Acupuncture and Meridian, Anhui University of Traditional Chinese Medicine, Anhui Key Laboratory of Foundation and Technology of Acupuncture and Moxibustion (Cultivating Base), China.
Materials
Animals
Forty Wistar rats of clean grade, aged 4 months, half male and half female, weighing 200–220 g, were provided by the Experimental Center of Nanjing Medical University, China, under the permission number of SCXK (Su) 2008-0004. Prior to experimentation, rats were given a normal diet for 7 days to adapt to vivarium conditions, under a natural lighting cycle, at 25°C and 58–60% humidity. All experimental procedures were conducted in accordance with the Guidance Suggestions for the Care and Use of Laboratory Animals, issued by the Ministry of Science and Technology of China[65].
Drugs
Heroin powder (purity 90%; chemical name: diacetylmorphine; Figure 3A), was provided by the Narcotics Control Commission of Anhui Province, China. Methadone hydrochloride is chemically described as 6-(dimethylamino)-4,4-diphenyl-3-heptanone hydrochloride (Figure 3B), and was provided by Tianjin Central Pharmaceutical Co., Ltd., Tianjin, China. The approval No. was (97) X-313 (2), lot No. was 090919, and the specification was 10 mg/10 mL.
Figure 3.
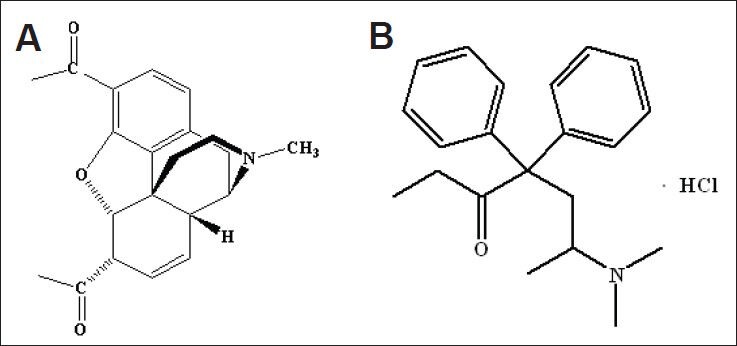
Chemical structure of heroin (A) and methadone hydrochloride (B).
Methods
Establishment of heroin readdiction model
Rats were given an intramuscular injection of heroin into the hindlimb for 8 successive days. The injection dose was initially 0.8 mg/kg on day 1 and gradually increased to 3.6 mg/kg on day 8, with a 0.4 mg/kg increment per day. Heroin was dissolved in saline and 0.5 mL was given to each rat per day[21]. At days 4–8, each rat received two injections of heroin, as the exposure (addiction) process. After 8 days of injection, the intramuscular injection of heroin was withdrawn for 5 days (detoxification). The exposure and detoxification procedures were repeated three times to establish heroin readdiction models[66,67]. In the normal control group, rats were given intramuscular saline 0.5 mL per day.
Acupuncture intervention
Each rat was treated with acupuncture during the detoxification period. According to the Rat Brain in Stereotaxic Coordinates[68], we selected Baihui (parietal bone) and Dazhui (between the seventh cervical vertebrae and the first thoracic vertebrae, in the middle of the back). Rats were fixed in a special holder and remained awake. A 25-mm-long stainless steel needle (Suzhou Medical Supplies Factory, Suzhou, Jiangsu Province, China) was flatly inserted into the Baihui and Dazhui points, to a depth of 12 mm, then the needle was retained for 30 minutes. The acupuncture therapy was given at 8:00 daily for 5 successive days.
Methadone intervention
Each rat was treated with methadone at decreasing doses for 5 days during the detoxification period. The daily dose of methadone was 0.4, 0.3, 0.2, 0.1, 0 mg per day.
Immunohistochemical detection of Bcl-2 and Bax expression in the frontal cortex and hippocampus
Two rats in each group were anesthetized with 10% (v/v) chloral hydrate via peritoneal injection. The chest was opened, and a small incision was made on the right atrial appendage. The catheter was then engaged via the left ventricle into the aorta and the rat was rapidly perfused with 250 mL saline until the effluent from the right atrial appendage became clear, followed by a slow infusion of 4% (w/v) paraformaldehyde for 30 minutes[69]. The frontal cortex and hippocampus were harvested and fixed in 4% (w/v) paraformaldehyde for 1 week. Subsequently, paraffin sections were dewaxed and placed in 3% (v/v) H2O2 methanol solution at room temperature for 10–20 minutes. After three washes with distilled water, endogenous peroxidase was blocked. The sections were immersed in 0.01 mol/L citrate buffer (pH 6.0) and heated to boiling on an electric heater twice with an interval of 5 minutes. After cooling to room temperature, sections were rinsed using 0.1 mol/L PBS (pH 7.3) twice and incubated with normal goat blocking solution (1:20) at room temperature for 20 minutes to block non-specific binding sites.
After excess liquid was removed, sections were incubated with rabbit anti-rat Bcl-2 monoclonal antibody (1:100; Boster Biological Engineering Co., Ltd., Wuhan, Hubei Province, China) and rabbit anti-rat Bax monoclonal antibody (1:100; Boster Biological Engineering Co., Ltd.) in a wet box at 4°C overnight. The negative control was treated with PBS instead of primary antibody. Sections were then incubated with biotinylated goat anti-rabbit IgG and SABC at 37°C for 30 minutes. Between each step, sections were rinsed using 0.1 mol/L PBS (pH 7.3) twice for 5 minutes each. Subsequently, sections were developed with 3,3′-diaminobenzidine for 5–20 minutes, washed with distilled water and counterstained with hematoxylin for 10 seconds, followed by dehydration, and mounting. Using the DP801 morphological microscopic image analysis system (Jetta Technology Development Co., Ltd., Nanjing, Jiangsu Province, China), five random sections of each rat were examined at five different visual fields (400 × magnification). Positively stained cells showing brown particles were counted in each field and averaged to obtain the mean absorbance values.
Electron microscopy observation of prefrontal cortex and hippocampal ultrastructure
After the experiment was complete, rats were sacrificed by cervical dislocation, and prefrontal cortex and hippocampal tissue were cut according to the Rat Brain Stereotaxic Coordinates[69] on ice. The specimens were then cut into 1-mm3 blocks and fixed in 1% (v/v) osmium tetroxide fixative, then serially sliced into thin slices at 70 nm thickness for transmission electron microscopy (JEOL, Tokyo, Japan) observation.
Statistical analysis
Data were analyzed using SPSS 11.0 software (SPSS, Chicago, IL, USA) and results were expressed as mean ± SD. Differences among groups were compared using one-way analysis of variance. A P < 0.05 value was considered statistically significant.
Acknowledgments:
We would like to thank all staffs from Research Institute of Acupuncture and Meridian, Anhui University of Traditional Chinese Medicine; Scientific Research Center, Anhui University of Traditional Chinese Medicine; Department of Pathology, Anhui Provincial Hospital, China for great support and help.
Footnotes
Funding: This study was financially sponsored by the Foundation for Excellent Young Talents in Universities of Anhui Province in China, No. 2010SQRL105; the National Natural Science Foundation of China, No. 81173325.
Conflicts of interest: None declared.
Copyedited by Diwakarla S, Song XG, Bai J, Yu J, Yang Y, Li CH, Song LP, Liu WJ, Zhao M
References
- [1].Gekht AB, Polunina AG, Briun EA, et al. Neurological disturbances in heroin addicts in acute withdrawal and early post-abstinence periods. Zh Nevrol Psikhiatr Im S S Korsakova. 2003;103(2):9–15. [PubMed] [Google Scholar]
- [2].Ryan A, Molloy FM, Farrell MA, et al. Fatal toxic leukoencephalopathy: clinical, radiological, and necropsy findings in two patients. J Neurol Neurosurg Psychiatry. 2005;76(7):1014–1016. doi: 10.1136/jnnp.2004.047134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Offiah C, Hall E. Heroin-induced leukoencephalopathy: characterization using MRI, diffusion-weighted imaging, and MR spectroscopy. Clin Radiol. 2008;63(2):146–152. doi: 10.1016/j.crad.2007.07.021. [DOI] [PubMed] [Google Scholar]
- [4].Gardini S, Venneri A. Reduced grey matter in the posterior insula as a structural vulnerability or diathesis to addiction. Brain Res Bull. 2012;87(2-3):205–211. doi: 10.1016/j.brainresbull.2011.11.021. [DOI] [PubMed] [Google Scholar]
- [5].Zhang XL, Zhang Y, Qiu SJ. Magnetic resonance imaging findings in comparison with histopathology of heroin-associated encephalopathy. Nan Fang Yi Ke Da Xue Xue Bao. 2007;27(2):121–125. [PubMed] [Google Scholar]
- [6].Jiang SA, Wang XY, Hao W. Controlled study of cerebral gray matter concentration in patients with heroin dependence. Zhongguo Yaowu Yilaixing Zazhi. 2006;15(5):373–375. [Google Scholar]
- [7].Khurdayan VK, Buch S, El-Hage N, et al. Preferential vulnerability of astroglia and glial precursors to combined opioid and HIV-1 Tat exposure in vitro. Eur J Neurosci. 2004;19(12):3171–3182. doi: 10.1111/j.0953-816X.2004.03461.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Wei XL, Ye J, Zhou Y. Observation of ultrastructural changs in the brain of heroin-addicted rat under electronic microscope. Guangxi Yike Daxue Xuebao. 2003;20(6):835–837. [Google Scholar]
- [9].Wei XL, Ye J, Zhou Y. Observation of the ultrastructural changes of neuronal apoptosis in the brain of heroin-addicted rats. Guangxi Yike Daxue Xuebao. 2004;21(1):31–33. [Google Scholar]
- [10].Wei XL, Ye J, Liang Y, et al. Ultrastructural observation on the two kinds of death of neuron in the brain of heroin addicted-rats. Dianzi Xianwei Xuebao. 2004;23(4):318. [Google Scholar]
- [11].Lim KO, Wozniak JR, Mueller BA, et al. Brain macrostructural and microstructural abnormalities in cocaine dependence. Drug Alcohol Depend. 2008;92(1-3):164–172. doi: 10.1016/j.drugalcdep.2007.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Jang DP, Namkoong K, Kim JJ, et al. The relationship between brain morphometry and neuropsychological performance in alcohol dependence. Neurosci Lett. 2007;428(1):21–26. doi: 10.1016/j.neulet.2007.09.047. [DOI] [PubMed] [Google Scholar]
- [13].Chanraud S, Martelli C, Delain F, et al. Brain morphometry and cognitive performance in detoxified alcohol-dependents with preserved psychosocial functioning. Neuropsychopharmacology. 2007;32(2):429–438. doi: 10.1038/sj.npp.1301219. [DOI] [PubMed] [Google Scholar]
- [14].Miklossy J, Doudet DD, Schwab C, et al. Role of ICAM-1 in persisting inflammation in Parkinson disease and MPTP monkeys. Exp Neurol. 2006;197(2):275–283. doi: 10.1016/j.expneurol.2005.10.034. [DOI] [PubMed] [Google Scholar]
- [15].Walhovd KB, Moe V, Slinning K, et al. Volumetric cerebral characteristics of children exposed to opiates and other substances in utero. Neuroimage. 2007;36(4):1331–1344. doi: 10.1016/j.neuroimage.2007.03.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Weber RJ, Gomez-Flores R, Smith JE, et al. Neuronal adaptations, neuroendocrine and immune correlates of heroin self-administration. Brain Behav Immun. 2009;23(7):993–1002. doi: 10.1016/j.bbi.2009.05.057. [DOI] [PubMed] [Google Scholar]
- [17].Upadhyay J, Maleki N, Potter J, et al. Alterations in brain structure and functional connectivity in prescription opioid-dependent patients. Brain. 2010;133(Pt 7):2098–2114. doi: 10.1093/brain/awq138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Zhou Y, Ye J, Xian XL, et al. Research on neuron ultrastructure in the vta-nac and da neurotransmitter's change in the brain of heroin addicted and readdicted rats. Guangxi Yike Daxue Xuebao. 2005;22(2):185–188. [Google Scholar]
- [19].Qin W, Pan GS, Chen YS, et al. The study of ultrastructural changes of cerebral regions related to heroin dependent rats. Zunyi Yixueyuan Xuebao. 2007;30(1):8–10. [Google Scholar]
- [20].He GZ, Wei XL, Li JL. Study on the mechanism of brain lesions in heroin-addicted rats. Guangxi Yike Daxue Xuebao. 2007;24(6):835–837. [Google Scholar]
- [21].Zhang RJ, Cai XH, Song XG. Effect of neuronal ultrastructures in the brains of heroin re-addicted rats. Zhongguo Yaowu Yilaixing Zazhi. 2008;17(6):424–426. [Google Scholar]
- [22].Pan L, Pan GS. The effect of electroacupuncture on apoptosis of hippocampal neurocyte in heroin-addict rats. Baotou Yixueyuan Xuebao. 2008;24(4):331–334. [Google Scholar]
- [23].Wang DY, Huang XH, Wang Y, et al. Dynamic study of different acupuncture opportunity on acute cerebral rat neural function recovery. Zhongyiyao Xuebao. 2008;36(3):30–32. [Google Scholar]
- [24].Yi W, Xu NG, Wang GB, et al. Effect of electroacupuncture on nerve growth factor expression in ischemic cortex of rats with focal cerebral ischemia. Guangzhou Zhongyiyao Daxue Xuebao. 2006;23(1):35–38. [Google Scholar]
- [25].Wen XN, Huang YG, Wang JC, et al. Effect of electroacupuncture on hippocampal neurogenesis of rats with experimental epilepsy. Zhonghua Shenjing Yixue Zazhi. 2006;5(3):240–243. [Google Scholar]
- [26].Jin JM. Rules of acupoints selection analysis and main points of prescription evaluation of acupuncture treatment on vascular dementia. Zhongguo Zhongyiyao Keji. 2011;18(5):371–372. [Google Scholar]
- [27].Liu L, Liu LG, Lv M, et al. Clinical observation on infantile cerebral palsy treated with quick meridian needling therapy plus scalp acupuncture. Zhongguo Zhenjiu. 2010;30(10):826–829. [PubMed] [Google Scholar]
- [28].Wan SY, Tan F, Wu HK, et al. Study of electro-acupuncture intervention in neonatal cells and blood vessels in rats with hypertensive cerebral ischemia reperfusion injury. Zhonghua Zhongyiyao Zazhi. 2011;26(2):350–353. [Google Scholar]
- [29].Chen CT, Tian HM, Zhang H, et al. Effects of acupuncture on neurologic impairment and infarct size after local cerebral ischemia in rats. Hunan Zhongyiyao Daxue Xuebao. 2011;31(7):63–66. [Google Scholar]
- [30].Li R, Li ZR, Shen MH, et al. Study on the influence of electroacupuncture on serum GSH and GSH-Px in cerebral ischemia rats. Shanghai Zhenjiu Zazhi. 2006;25(9):40–41. [Google Scholar]
- [31].Cheng J, Li ZR, Zhu Y, et al. Effects of electroacupuncture on expression of calmodulin in the hippocampus of rats with cerebral ischemia-reperfusion injury. Zhongguo Zhenjiu. 2011;31(11):1015–1019. [PubMed] [Google Scholar]
- [32].Liu R, Xu NG, Yi W, et al. Effect of electro-acupuncture on the JAK-STAT signal transduction pathway in rats with the focal cerebral ischemia. Shenjing Jiepou Xue Zazhi. 2011;27(6):617–622. [Google Scholar]
- [33].Wan SY, Tan F, Wu HK, et al. Study of electro-acupuncture and ultrastructure of thrombin sensitive protein-1mRNA in hypertensive cerebral ischemia rats. Shizhen Guoyi Guoyao. 2011;22(2):488–490. [Google Scholar]
- [34].Yu DQ, Pei HT, Zhang PH, et al. Effects of electroacupuncture on the expression of nestin in endogenous neural stem cell in hippocampus in rats with focal cerebral ischemia-reperfusion. Zhongguo Zhenjiu. 2010;30(11):929–932. [PubMed] [Google Scholar]
- [35].Shen MH, Li ZR, Xiang XR, et al. Effect of electroacupuncture on cerebral cortex ultrastructure in rats with cerebral ischemia-reperfusion injury. Zhenci Yanjiu. 2009;34(3):167–170. [PubMed] [Google Scholar]
- [36].Chen F, Yan ZK, Yang B. Effects of acupoint-injection of compound angelica-root injectio on cerebral bcl-2 and bax immunoactivity and hemorheology in rats with cerebral ischemia-reperfusion injury. Zhenci Yanjiu. 2011;36(2):85–89. [PubMed] [Google Scholar]
- [37].Yang CZ, Li MQ, Wang Y, et al. The effect of acupuncture on behavior and contents of AchE of rats with vascular dementia. Zhenjiu Linchuang Zazhi. 2011;27(7):71–73. [Google Scholar]
- [38].Zhu XD, Jiang XC, Mao X. Effect of acupuncture on β-AP and Tau protein expression in mice brain tissue with Alzheimer disease duplicated by D-galactose. Zhongyi Xuebao. 2011;26(8):954–955. [Google Scholar]
- [39].Wang YL, Jin Z, Sun ZR. Experimental study of acupuncture on SOD, MDA in the brain and liver tissue of aging model rat induced by D-gal. Zhongyiyao Xinxi. 2011;28(4):102–104. [Google Scholar]
- [40].Zhang XD, Liu Y, Wang JC, et al. The influence of electroacupuncture therapy on the expression of hippocampus NOS, CaMK in rats’ models of Alzheimer's disease. Zhenjiu Linchuang Zazhi. 2011;27(6):70–74. [Google Scholar]
- [41].Jin ZG, He YF, Jing XH. Effect of acupuncture on the expression of CREB in hippocampus and amygdala in diabetic rats. Zhongguo Zhongyi Jichu Yixue Zazhi. 2011;17(1):102–105. [Google Scholar]
- [42].Wang W, Tang W, Sun ZR. Effect of electroacupuncture on memory and synaptic plasticity of hippocampal neurons in rats with senile dementia learning. Zhongguo Shiyong Shenjing Jibing Zazhi. 2010;13(18):29–31. [Google Scholar]
- [43].Li M, Xu GH, Wanlai SQ. Effect of electroacupuncture on long-term potentiation,calmodulin and calmodulin protein kinase ‡U mRNA expression in vascular dementia rats. Guangzhou Zhongyiyao Daxue Xuebao. 2010;27(4):337–340. [Google Scholar]
- [44].Yao HB, Jiang GH, Gong XW. Effect of electropuncture on expressions of c-fos, Caspase-3 and AD rats’ learning and memory ability. Qiqihar Yixueyuan Xuebao. 2009;30(18):2225–2226. [Google Scholar]
- [45].Sun HM, He X, Wang YY, et al. Influence of acupuncture in Baihui (GV20) and Dazhui (GV14) on activity of brain mitochondrial complex in mice with Parkinson's disease. Beijing Zhongyiyao Daxue Xuebao. 2011;34(4):250–253. 262. [Google Scholar]
- [46].Sun HM, Wu HX, Xu H, et al. Protective effect of acupuncture in acupoints of Governor Vessel on dopaminergic neurons protection and influence on ultrastructure in mice with Parkinson's disease. Beijing Zhongyiyao Daxue Xuebao. 2010;33(4):257–261. [Google Scholar]
- [47].Gao XY, Ma QL. Analysis on relationship between Du meridian and brain. Shijie Zhongyiyao. 2007;2(3):134–137. [Google Scholar]
- [48].Wang ZT, Yuan YQ, Wang J, et al. Clinical curative effect of acupuncture combined drug therapy on heroin dependence. Zhongguo Zhenjiu. 1999;19(11):657–658. [Google Scholar]
- [49].Song XG, Zhang H, Wang ZH, et al. Clinical observation on acupuncture combined with methadone for improving heroin withdrawal syndrome. Zhongguo Zhenjiu. 2002;22(12):795–797. [Google Scholar]
- [50].Wu JM, Wei DY, Luo YF, et al. Clinic reaseach on heroin deaddiction effects of acupuncture and its potentiality of preventing relapse. Zhongxiyi Jiehe Xuebao. 2003;1(4):268–272. doi: 10.3736/jcim20030412. [DOI] [PubMed] [Google Scholar]
- [51].Wen TQ, Yang ZJ, Lei XL, et al. Clinical application of acupuncture for treatment of heroin withdrawal syndrome. Zhongguo Zhenjiu. 2005;25(7):449–453. [PubMed] [Google Scholar]
- [52].Song XG, Xu GL, Tang ZL, et al. Auricular acupuncture on heroin addicts: a 70 cases clinical observation. Zhongguo Zhongyiyao Keji. 2006;13(3):189–190. [Google Scholar]
- [53].Rong J, Liu ZY, Asihae Clinical study on heroin withdrawal syndrome treated by scalp acupuncture in addicts. Zhongguo Yaowu Lanyong Fangzhi Zazhi. 2006;12(4):205–208. [Google Scholar]
- [54].Song XG, Hu L, Wang J, et al. Clinical observation on the effects of acupuncture combined with psychological desensitization therapy for sleep disorders and anxiety symptom of heroin-addicts. Zhongguo Yaowu Lanyong Fangzhi Zazhi. 2010;19(4):269–272. [Google Scholar]
- [55].Shi FZ, Wei KC, Xu DB, et al. Acupuncture and moxibustion in alleviating sleep disorders and anxiety in heroin addicts: a reports of 35 cases. Anhui Zhongyi Xueyuan Xuebao. 2011;30(2):37–39. [Google Scholar]
- [56].Zeng B, Wang JY, Zhang YL, et al. Expression of LC3B in rat hippocampal CA1 neurons after global cerebral ischemia-reperfusion. Zhongguo Linchuang Jiepou Xue Zazhi. 2012;30(6):667–669. [Google Scholar]
- [57].Zeng R. Research of the related factors between location of carebral lesion and PSD. Huazhong Yixue Zazhi. 2008;32(2):100–102. [Google Scholar]
- [58].Liu HY, Yao ZJ, Lu Q, et al. The study on the relationship of cognitive function and the frontal gray matter concentration in female major depression. Zhongguo Shenjing Jingshen Jibing Zazhi. 2009;35(8):463–466. [Google Scholar]
- [59].Zhang ZJ, Zou YB, Tang MS. Endoplasmic reticulum stress and nervous system diseases. Huanan Guifang Yixue Zazhi. 2011;25(6):548–551. [Google Scholar]
- [60].Zhao XQ, Wang XR, Zhang J. Mitochondria related substances and cell apoptosis. Hebei Beifang Xueyuan Xuebao: Yixue Ban. 2009;6(6):80–82. [Google Scholar]
- [61].Antonsson B, Conti F, Ciavatta A, et al. Inhibition of Bax channel-forming activity by Bcl-2. Science. 1997;277(5324):370–372. doi: 10.1126/science.277.5324.370. [DOI] [PubMed] [Google Scholar]
- [62].Pan G, O’Rourke K, Dixit VM. Caspase-9, Bcl-XL, and Apaf-1 form a ternary complex. J Biol Chem. 1998;273(10):5841–5845. doi: 10.1074/jbc.273.10.5841. [DOI] [PubMed] [Google Scholar]
- [63].Chang SF, Sun YY, Yang LY, et al. Bcl-2 gene family expression in the brain of rat offspring after gestational and lactational dioxin exposure. Ann N Y Acad Sci. 2005;1042:471–480. doi: 10.1196/annals.1338.040. [DOI] [PubMed] [Google Scholar]
- [64].Tsukahara S, Yamamoto S, Tin-Tin-Win-Shwe, et al. Inhalation of low-level formaldehyde increases the Bcl-2/Bax expression ratio in the hippocampus of immunologically sensitized mice. Neuroimmunomodulation. 2006;13(2):63–68. doi: 10.1159/000094829. [DOI] [PubMed] [Google Scholar]
- [65].The Ministry of Science and Technology of the People's Republic of China. Guidance Suggestions for the Care and Use of Laboratory Animals. 2006-09-30 [Google Scholar]
- [66].Ye J, Meng ZQ, Wei SR, et al. A study on establishment of animal models of heroin dependence relapse. Guangxi Yike Daxue Xuebao. 2003;20(6):832–835. [Google Scholar]
- [67].Wei XL, Ye J, Zheng Y. Establishment of the model of heroin readdicted rats. Guangxi Yixue. 2004;26(6):783–785. [Google Scholar]
- [68].Hua XB. Study of Rat Brain in Stereotaxic Coordinates. Shiyan Dongwu yu Dongwu Shiyan. 1991;1:1–5. [Google Scholar]
- [69].Zhuge QC. 3rd ed. Beijing: People's Medical Publishing House; 2005. The Rat Brain in Stereotaxic Coordinates. [Google Scholar]


