Abstract
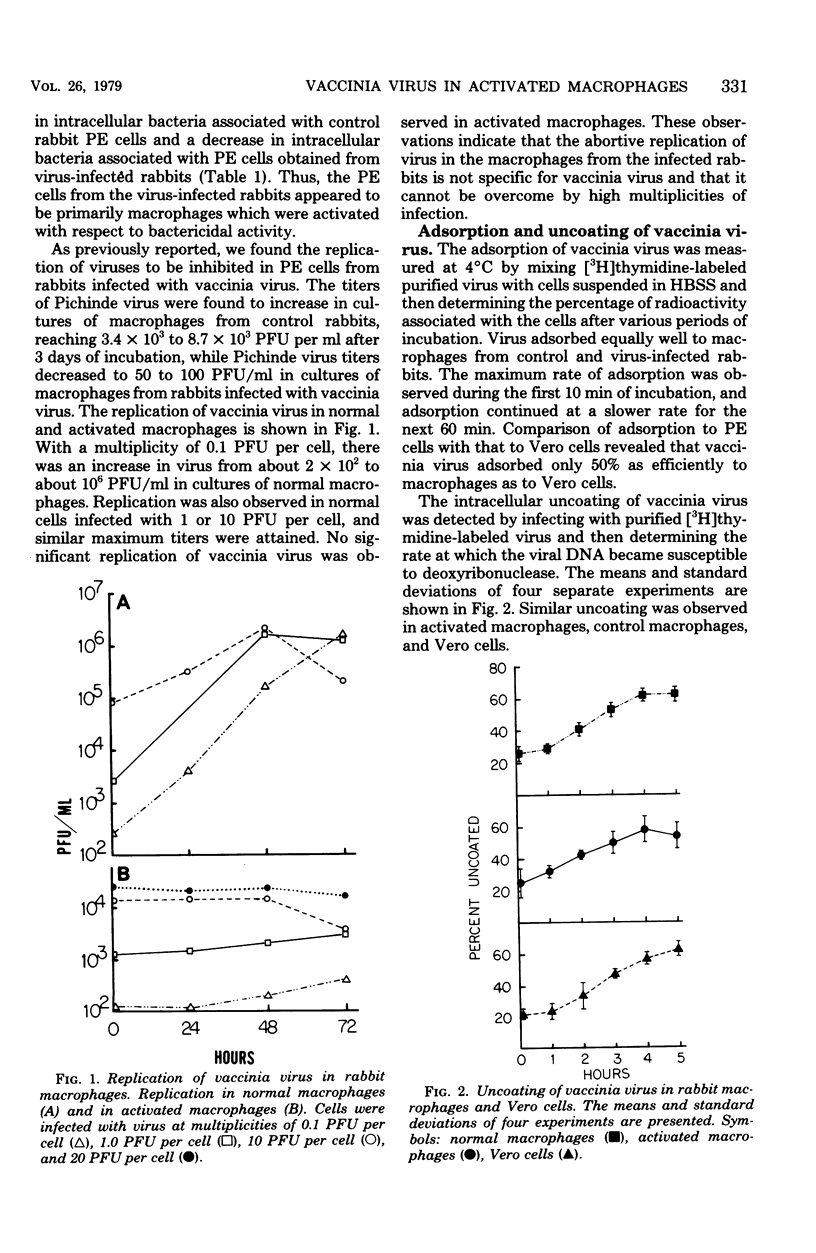
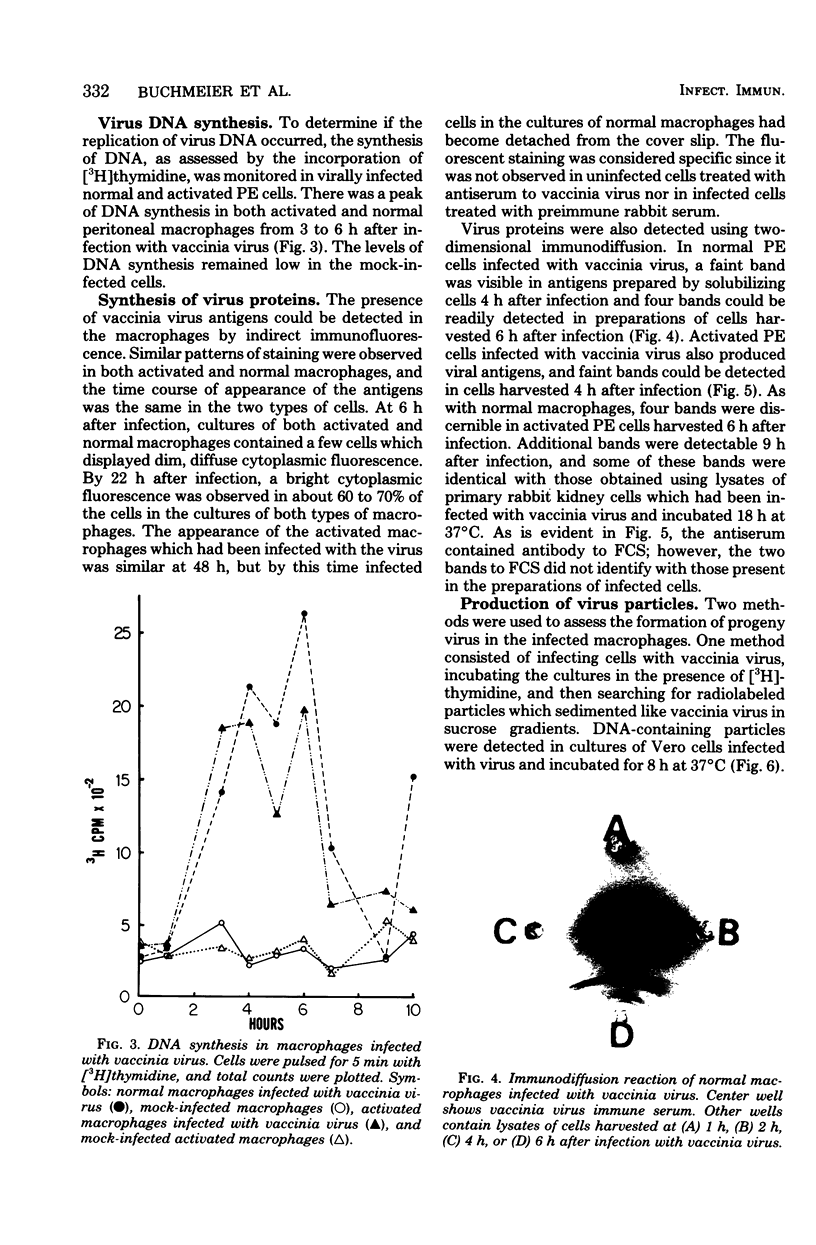
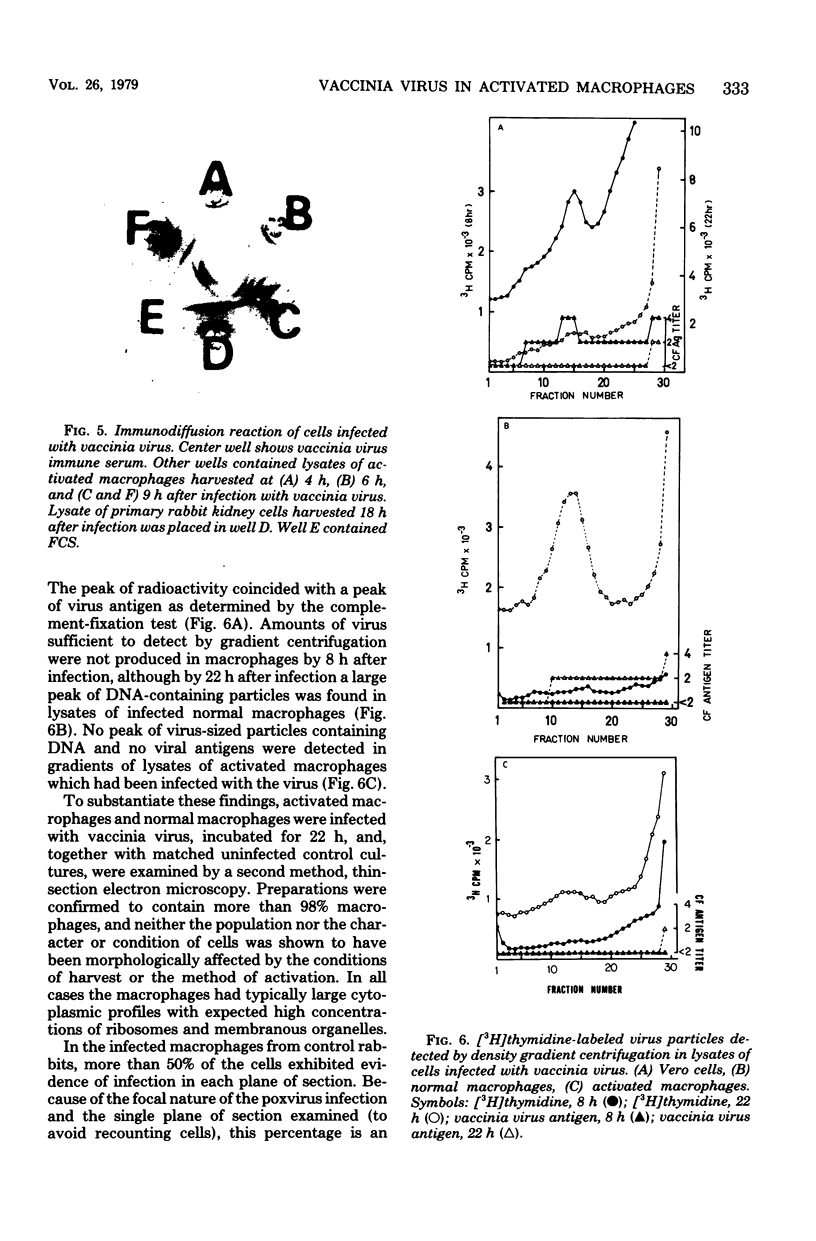
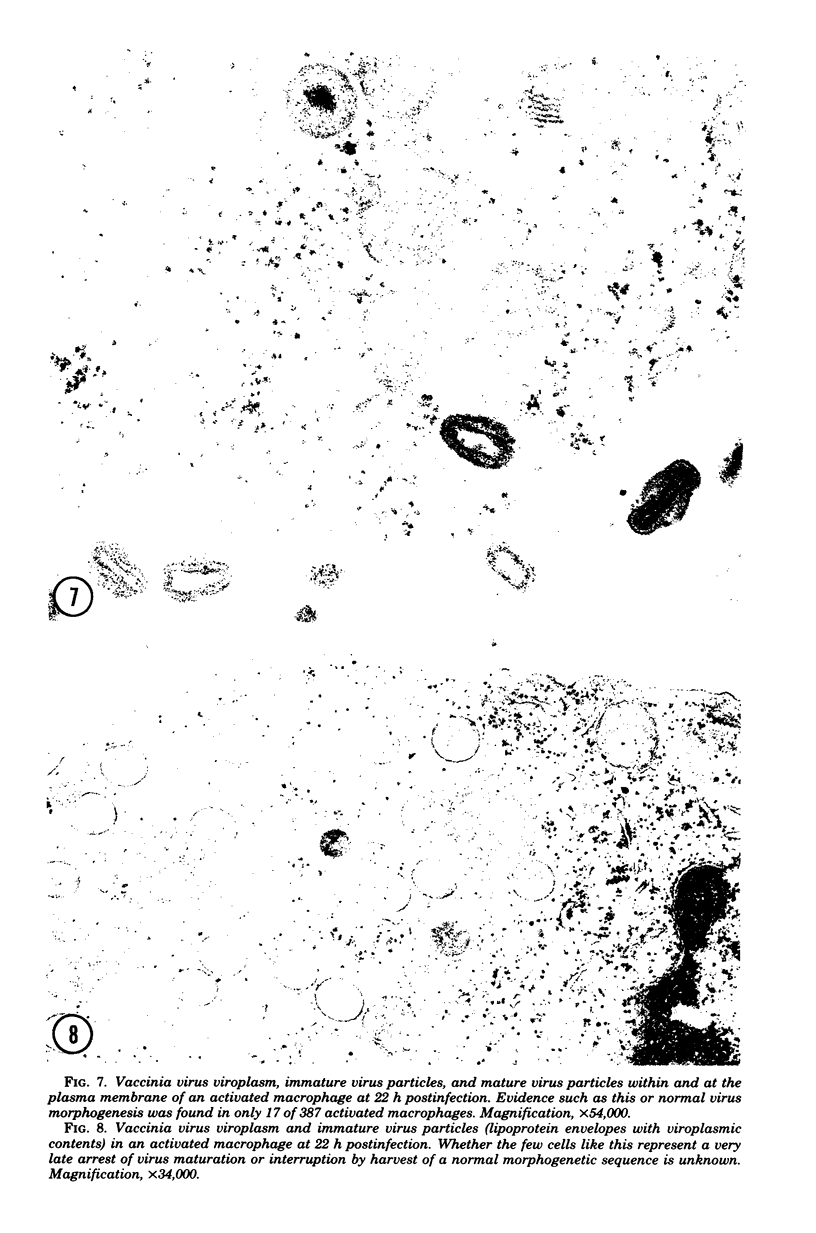
During the course of infection of rabbits with vaccinia virus, macrophages obtained from the peritoneal cavity develop bactericidal activity and the replication of vaccinia virus becomes restricted in these cells. The abortive replication of vaccinia virus in the activated macrophages was characterized in the present study. The virus adsorbed to and was uncoated equally well in macrophages from both normal and infected rabbits. A burst of deoxyribonucleic acid synthesis of comparable magnitude took place 3 to 6 h after infection in both normal and activated macrophages. Although the production of viral antigens, as detected by immunodiffusion and immunofluorescence, was the same in both types of cells, very few virus particles were formed in activated as compared with normal macrophages. We conclude that a block in a late step of the virus replication cycle occurred in the activated macrophages.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison A. C. Genetic factors in resistance against virus infections. Arch Gesamte Virusforsch. 1965;17(2):280–294. doi: 10.1007/BF01267912. [DOI] [PubMed] [Google Scholar]
- Allison A. C. On the role of mononuclear phagocytes in immunity against viruses. Prog Med Virol. 1974;18(0):15–31. [PubMed] [Google Scholar]
- Avila F. R., Schultz R. M., Tompkins W. A. Specific macrophage immunity to vaccinia virus: macrophage-virus interaction. Infect Immun. 1972 Jul;6(1):9–16. doi: 10.1128/iai.6.1.9-16.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bang F. B., Warwick A. MOUSE MACROPHAGES AS HOST CELLS FOR THE MOUSE HEPATITIS VIRUS AND THE GENETIC BASIS OF THEIR SUSCEPTIBILITY. Proc Natl Acad Sci U S A. 1960 Aug;46(8):1065–1075. doi: 10.1073/pnas.46.8.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanden R. V. Mechanisms of recovery from a generalized viral infection: mousepox. 3. Regression infectious foci. J Exp Med. 1971 May 1;133(5):1090–1104. doi: 10.1084/jem.133.5.1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanden R. V. Mechanisms of recovery from a generalized viral infection: mousepox. II. Passive transfer of recovery mechanisms with immune lymphoid cells. J Exp Med. 1971 May 1;133(5):1074–1089. doi: 10.1084/jem.133.5.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanden R. V., Mims C. A. Macrophage activation in mice infected with ectromelia or lymphocytic choriomeningitis viruses. Aust J Exp Biol Med Sci. 1973 Jun;51(3):393–398. doi: 10.1038/icb.1973.35. [DOI] [PubMed] [Google Scholar]
- Bodo G., Scheirer W., Suh M., Schultze B., Horak I., Jungwirth C. Protein synthesis in pox-infected cells treated with interferon. Virology. 1972 Oct;50(1):140–147. doi: 10.1016/0042-6822(72)90354-6. [DOI] [PubMed] [Google Scholar]
- CAIRNS J. The initiation of vaccinia infection. Virology. 1960 Jul;11:603–623. doi: 10.1016/0042-6822(60)90103-3. [DOI] [PubMed] [Google Scholar]
- Cohen G. H., Wilcox W. C. Soluble antigens of vaccinia-infected mammalian cells. I. Separation of virus-induced soluble antigens into two classes on the basis of physical characteristics. J Bacteriol. 1966 Sep;92(3):676–686. doi: 10.1128/jb.92.3.676-686.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DALES S. The uptake and development of vaccinia virus in strain L cells followed with labeled viral deoxyribonucleic acid. J Cell Biol. 1963 Jul;18:51–72. doi: 10.1083/jcb.18.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels C. A., Kleinerman E. S., Snyderman R. Abortive and productive infections of human mononuclear phagocytes by type I herpes simplex virus. Am J Pathol. 1978 Apr;91(1):119–136. [PMC free article] [PubMed] [Google Scholar]
- Eustatia J. M., Maase E., van Helden P., van der Veen J. Viral replication in mouse macrophages. Arch Gesamte Virusforsch. 1972;39(4):376–380. doi: 10.1007/BF01241017. [DOI] [PubMed] [Google Scholar]
- GLASGOW L. A. LEUKOCYTES AND INTERFERON IN THE HOST RESPONSE TO VIRAL INFECTIONS. I. MOUSE LEUKOCYTES AND LEUKOCYTE-PRODUCED INTERFERON IN VACCINIA VIRUS INFECTION IN VITRO. J Exp Med. 1965 Jun 1;121:1001–1018. doi: 10.1084/jem.121.6.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOODMAN G. T., KOPROWSKI H. Study of the mechanism of innate resistance to virus infection. J Cell Comp Physiol. 1962 Jun;59:333–373. doi: 10.1002/jcp.1030590313. [DOI] [PubMed] [Google Scholar]
- Glasgow L. A., Fischbach J., Bryant S. M., Kern E. R. Immunomodulation of host resistance to experimental viral infections in mice: effects of Corynebacterium acnes, Corynebacterium parvum, and Bacille calmette-guérin. J Infect Dis. 1977 May;135(5):763–770. doi: 10.1093/infdis/135.5.763. [DOI] [PubMed] [Google Scholar]
- Glasgow L. A. Leukocytes and interferon in the host response to viral infections. II. Enhanced interferon response of leukocytes from immune animals. J Bacteriol. 1966 Jun;91(6):2185–2191. doi: 10.1128/jb.91.6.2185-2191.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer B., Delfs D., McElree H. Electron microscope study of the interaction of vaccinia virus with macrophages from immunized and nonimmunized rabbits. Infect Immun. 1974 Feb;9(2):452–459. doi: 10.1128/iai.9.2.452-459.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch M. S., Zisman B., Allison A. C. Macrophages and age-dependent resistance to Herpes simplex virus in mice. J Immunol. 1970 May;104(5):1160–1165. [PubMed] [Google Scholar]
- JOHNSON R. T. THE PATHOGENESIS OF HERPES VIRUS ENCEPHALITIS. II. A CELLULAR BASIS FOR THE DEVELOPMENT OF RESISTANCE WITH AGE. J Exp Med. 1964 Sep 1;120:359–374. doi: 10.1084/jem.120.3.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOKLIK W. K., BECKER Y. THE REPLICATION AND COATING OF VACCINIA DNA. J Mol Biol. 1964 Dec;10:452–474. doi: 10.1016/s0022-2836(64)80066-8. [DOI] [PubMed] [Google Scholar]
- JOKLIK W. K. THE INTRACELLULAR UNCOATING OF POXVIRUS DNA. I. THE FATE OF RADIOACTIVELY-LABELED RABBITPOX VIRUS. J Mol Biol. 1964 Feb;8:263–276. doi: 10.1016/s0022-2836(64)80136-4. [DOI] [PubMed] [Google Scholar]
- JOKLIK W. K. The preparation and characteristics of highly purified radioactively labelled poxvirus. Biochim Biophys Acta. 1962 Aug 20;61:290–301. doi: 10.1016/0926-6550(62)90091-9. [DOI] [PubMed] [Google Scholar]
- Joklik W. K., Merigan T. C. Concerning the mechanism of action of interferon. Proc Natl Acad Sci U S A. 1966 Aug;56(2):558–565. doi: 10.1073/pnas.56.2.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joklik W. K. The poxviruses. Annu Rev Microbiol. 1968;22:359–390. doi: 10.1146/annurev.mi.22.100168.002043. [DOI] [PubMed] [Google Scholar]
- Kates J. R., McAuslan B. R. Relationship between protein synthesis and viral deoxyribonucleic acid synthesis. J Virol. 1967 Feb;1(1):110–114. doi: 10.1128/jvi.1.1.110-114.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz E., Moss B. Vaccinia virus structural polypeptide derived from a high-molecular-weight precursor: formation and integration into virus particles. J Virol. 1970 Dec;6(6):717–726. doi: 10.1128/jvi.6.6.717-726.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koszinowski U., Kruse F., Thomssen R. Interactions between vaccinia virus and sensitized macrophages in vitro. Arch Virol. 1975;48(4):335–345. doi: 10.1007/BF01317432. [DOI] [PubMed] [Google Scholar]
- LaColla P., Weissbach A. Vaccinia virus infection of HeLa cells. I. Synthesis of vaccinia DNA in host cell nuclei. J Virol. 1975 Feb;15(2):305–315. doi: 10.1128/jvi.15.2.305-315.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine S., Magee W. E., Hamilton R. D., Miller O. V. Effect of interferon on early enzyme and viral DNA synthesis in vaccinia virus infections. Virology. 1967 May;32(1):33–40. doi: 10.1016/0042-6822(67)90249-8. [DOI] [PubMed] [Google Scholar]
- Lopez C., Dudas G. Replication of herpes simplex virus type 1 in macrophages from resistant and susceptible mice. Infect Immun. 1979 Feb;23(2):432–437. doi: 10.1128/iai.23.2.432-437.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MIMS C. A. ASPECTS OF THE PATHOGENESIS OF VIRUS DISEASES. Bacteriol Rev. 1964 Mar;28:30–71. doi: 10.1128/br.28.1.30-71.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magee W. E., Levine S., Miller O. V., Hamilton R. D. Inhibition by interferon of the uncoating of vaccinia virus. Virology. 1968 Aug;35(4):505–511. doi: 10.1016/0042-6822(68)90280-8. [DOI] [PubMed] [Google Scholar]
- McLaren C., Cheng H., Spicer D. L., Tompkins W. A. Lymphocyte and macrophage responses after vaccinia virus infections. Infect Immun. 1976 Oct;14(4):1014–1021. doi: 10.1128/iai.14.4.1014-1021.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metz D. H., Esteban M. Interferon inhibits viral protein synthesis in L cells infected with vaccinia virus. Nature. 1972 Aug 18;238(5364):385–388. doi: 10.1038/238385a0. [DOI] [PubMed] [Google Scholar]
- Mogensen S. C. Genetics of macrophage-controlled resistance to hepatitis induced by herpes simplex virus type 2 in mice. Infect Immun. 1977 Aug;17(2):268–273. doi: 10.1128/iai.17.2.268-273.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morahan P. S., Glasgow L. A., Crane J. L., Jr, Kern E. R. Comparison of antiviral and antitumor activity of activated macrophages. Cell Immunol. 1977 Feb;28(2):404–415. doi: 10.1016/0008-8749(77)90122-8. [DOI] [PubMed] [Google Scholar]
- Morahan P. S., Kern E. R., Glasgow L. A. Immunomodulator-induced resistance against herpes simplex virus. Proc Soc Exp Biol Med. 1977 Apr;154(4):615–620. doi: 10.3181/00379727-154-39730. [DOI] [PubMed] [Google Scholar]
- North R. J. The concept of the activated macrophage. J Immunol. 1978 Sep;121(3):806–809. [PMC free article] [PubMed] [Google Scholar]
- Pathak P. N., Rao G. V., Tompkins W. A. In vitro cellular immunity to unrelated pathogens in chickens infected with fowlpox virus. Infect Immun. 1974 Jul;10(1):34–41. doi: 10.1128/iai.10.1.34-41.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROBERTS J. A. GROWTH OF VIRULENT AND ATTENUATED ECTROMELIA VIRUS IN CULTURED MACROPHAGES FROM NORMAL AND ECTROMELIAIMMUNE MICE. J Immunol. 1964 Jun;92:837–842. [PubMed] [Google Scholar]
- RONDLE C. J., DUMBELL K. R. Antigens of cowpox virus. J Hyg (Lond) 1962 Mar;60:41–49. doi: 10.1017/s0022172400039292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz R. M., Woan M. C., Tompkins W. A. Macrophage immunity to vaccina virus: factors affecting macrophage immunity in vitro. J Reticuloendothel Soc. 1974 Jul;16(1):37–47. [PubMed] [Google Scholar]
- Selgrade M. K., Osborn J. E. Role of macrophages in resistance to murine cytomegalovirus. Infect Immun. 1974 Dec;10(6):1383–1390. doi: 10.1128/iai.10.6.1383-1390.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sethi K. K., Brandis H. In vitro acquisition of resistance against herpes simplex virus by permissive murine macrophages. Arch Virol. 1979;59(3):157–172. doi: 10.1007/BF01317412. [DOI] [PubMed] [Google Scholar]
- Stevens J. G., Cook M. L. Restriction of herpes simplex virus by macrophages. An analysis of the cell-virus interaction. J Exp Med. 1971 Jan 1;133(1):19–38. doi: 10.1084/jem.133.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tompkins W. A., Rama Rao G. V., Woan M. C. Immune and non-immune macrophage resistance to the fibroma-myxoma virus complex. J Reticuloendothel Soc. 1975 Jul;18(1):23–33. [PubMed] [Google Scholar]
- Tompkins W. A., RamaRao G. V. Defective macrophage immunity in newborn rabbits with fibroma virus-induced tumors. J Reticuloendothel Soc. 1978 Feb;23(2):161–166. [PubMed] [Google Scholar]
- Tompkins W. A., Zarling J. M., Rawls W. E. In vitro assessment of cellular immunity to vaccinia virus: contribution of lymphocytes and macrophages. Infect Immun. 1970 Dec;2(6):783–790. doi: 10.1128/iai.2.6.783-790.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner G. S., Ballard R. Interaction of mouse peritoneal macrophages with fixed rabies virus in vivo and in vitro. J Gen Virol. 1976 Feb;30(2):223–231. doi: 10.1099/0022-1317-30-2-223. [DOI] [PubMed] [Google Scholar]
- Ueda S., Nozima T. Delayed hypersensitivity in vaccinia-infected mice. II. Resistance of peritoneal macrophages against vaccinia infection. Acta Virol. 1973 Jan;17(1):41–49. [PubMed] [Google Scholar]
- Virelizier J. L., Allison A. C., de Maeyer E. Production by mixed lymphocyte cultures of a type II interferon able to protect macrophages against virus infection. Infect Immun. 1977 Aug;17(2):282–285. doi: 10.1128/iai.17.2.282-285.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zisman B., Hirsch M. S., Allison A. C. Selective effects of anti-macrophage serum, silica and anti-lymphocyte serum on pathogenesis of herpes virus infection of young adult mice. J Immunol. 1970 May;104(5):1155–1159. [PubMed] [Google Scholar]
- Zisman B., Wheelock E. F., Allison A. C. Role of macrophages and antibody in resistance of mice against yellow fever virus. J Immunol. 1971 Jul;107(1):236–243. [PubMed] [Google Scholar]