Abstract
Functional magnetic resonance imaging studies have shown that the insular cortex has a significant role in pain identification and information integration, while the default mode network is associated with cognitive and memory-related aspects of pain perception. However, changes in the functional connectivity between the default mode network and insula during pain remain unclear. This study used 3.0 T functional magnetic resonance imaging scans in 12 healthy subjects aged 24.8 ± 3.3 years to compare the differences in the functional activity and connectivity of the insula and default mode network between the baseline and pain condition induced by intramuscular injection of hypertonic saline. Compared with the baseline, the insula was more functionally connected with the medial prefrontal and lateral temporal cortices, whereas there was lower connectivity with the posterior cingulate cortex, precuneus and inferior parietal lobule in the pain condition. In addition, compared with baseline, the anterior cingulate cortex exhibited greater connectivity with the posterior insula, but lower connectivity with the anterior insula, during the pain condition. These data indicate that experimental low back pain led to dysfunction in the connectivity between the insula and default mode network resulting from an impairment of the regions of the brain related to cognition and emotion, suggesting the importance of the interaction between these regions in pain processing.
Keywords: nerve regeneration, low back pain, resting-state, functional connectivity, functional magnetic resonance imaging, default mode network, insula, hypertonic saline, cognitive, emotion, visual analogue scale, the Science and Technology Foundation of Guangdong Province of China, neural regeneration
Introduction
Pain is commonly viewed as a sensation with multiple components, which are shaped by a combination of cultural, psychological and biological factors[1,2]. Low back pain of longer duration and increased severity causes cognitive impairments beyond the feeling of pain, which include depression, anxiety, sleeping disturbances and decision-making abnormalities[3,4]. Functional magnetic resonance imaging (fMRI) studies have demonstrated that pain endurance alters the brain dynamics beyond the feeling of pain itself[5]. Pain processing is associated with pain perception, identification, memory, emotion functions, avoidance responses and a broad dysfunction of pain modulation[6,7]. Recent studies even suggest that prolonged pain can damage cortical areas that are unrelated to pain[8,9]. Therefore, it appears that low back pain may be a progressive disorder associated with changes in pain modulation and the integration of the sensory, affective and cognitive components of pain. Resting-state functional connectivity MRI is based on the observation that brain regions exhibit correlated slow fluctuations at rest, and has become a widely used tool for investigating spontaneous brain activity. Such resting-state studies have been employed in the investigation of the pathological mechanisms of several neuropathic diseases including fibromyalgia[10], migraine[11], complex regional pain syndrome[12] and diabetic neuropathic pain[13]. Thus, it is critical to take into account brain activity that occurs in the resting-state of pain stimulation to further understand how pain networks function. The default mode network is a key area in the resting state, involving the posterior cingulate cortex, precuneus, medial prefrontal and lateral temporal cortices, and is characterized by balanced positive and negative connections[14,15] classified as the “hubs” of structural and functional connectivity in brain studies[16,17]. Current concepts suggest that a broad pain processing dysfunction characterizes this common neurological disorder, and identify the default mode network as a known region linked to memory, attention and executive cognitive functions. Recent studies have also shown that functional default mode network connectivity is abnormal in patients with chronic back pain, and these changes may lead to behavioral dysfunctions[18,19].
The insula is typically activated during neuroimaging studies of acute experimental pain in healthy subjects and clinical pain syndromes, where the strength and range grow significantly as the pain threshold intensity is increased[20,21]. As a key region in the endogenous pain modulation system, it is likely that the functional association between the insula and other brain regions is changed when experiencing pain. Neuroimaging studies have shown that the insula is involved in multidimensional (sensory, affective and cognitive) pain status[3]. The insular cortex and limbic system are also closely linked, suggesting that the insula is associated with behavioral functions. Previous fMRI studies on pain reactivity indicate that the anterior insula have greater pain activation in the prefrontal regions, inferior parietal lobule and superior temporal gyrus, and that the posterior insula have higher activation in the medial prefrontal and cingulate cortex[22]. Resting state modeling has largely focused on the functional correlations among cortical structures, whereas the interactions between functional mechanisms in various brain regions remain unknown, particularly the changes in the resting functionality of the connected insula involved in low back pain.
To further explore the potential brain mechanisms underlying insular activation, we examined low back pain perception and pain-related processing over time. We hypothesized that the major regions of the default mode network would exhibit significant abnormal patterns in their functional activity and connectivity with the insula. Thus, we tested for differences between the anterior insula and posterior insula. Our analysis was focused on changes in the functional connectivity among regions that are known to be involved with the default mode network (i.e., medial prefrontal cortex, posterior cingulate cortex/precuneus and lateral temporal cortex), as resting connectivity within this network was found to be altered in chronic back pain patients compared with healthy subjects, while pain processing was shown to modulate resting default mode network connectivity[19]. Furthermore, multiple studies strongly suggest that the negative effects can be attenuated by directing the attentional focus away from certain aspects of a situation[23]. Thus, using fMRI and alterations in functional connectivity, we examined the functional connectivity changes to predict the extent of the attenuation of negative effects.
Results
Quantitative analysis of subjects
Twelve healthy subjects (six men and six women) aged 24.8 ± 3.3 years were included. Intramuscular injections of 0.3 mL hypertonic saline (5%) stimuli were performed at 2.0 cm on the right back muscle of the L4 spine to induce individual low back pain. For the muscle pain series, only the visual set of subjects who rated the pain intensity ≥ 3 using the visual analogue scale (0–10; 0 = “no pain” and 10 = “worst pain imaginable”) were used to compare the changes between the pain and non-pain status. All subjects’ rotation of head movements were within 2 mm in translation and 2° in rotation. All twelve subjects were included in the final analysis.
Pain perception and the questionnaire results in the low back pain models
Low back pain commenced within 10–15 seconds of the injection, peaked within < 20 seconds, and then subsided to pre-injection levels over ≥ 8 minutes. Muscle pain was described as dull and aching with mild numbness. The intramuscular injections of hypertonic saline (5%) caused moderate local pain. The individual average maximum pain scores were 6.25 ± 2.14 with a range of 3–9. In all subjects, pain stimuli elicited pain unpleasantness that ranged from mild distress to horror. “Distressing” (2.92 ± 1.78) and “horrible” (1.50 ± 1.62) scores were higher during the test than those before and after the test, although the differences were not statistically significant (one-way analysis of variance; P > 0.05).
Functional connectivity alteration in the default mode network areas
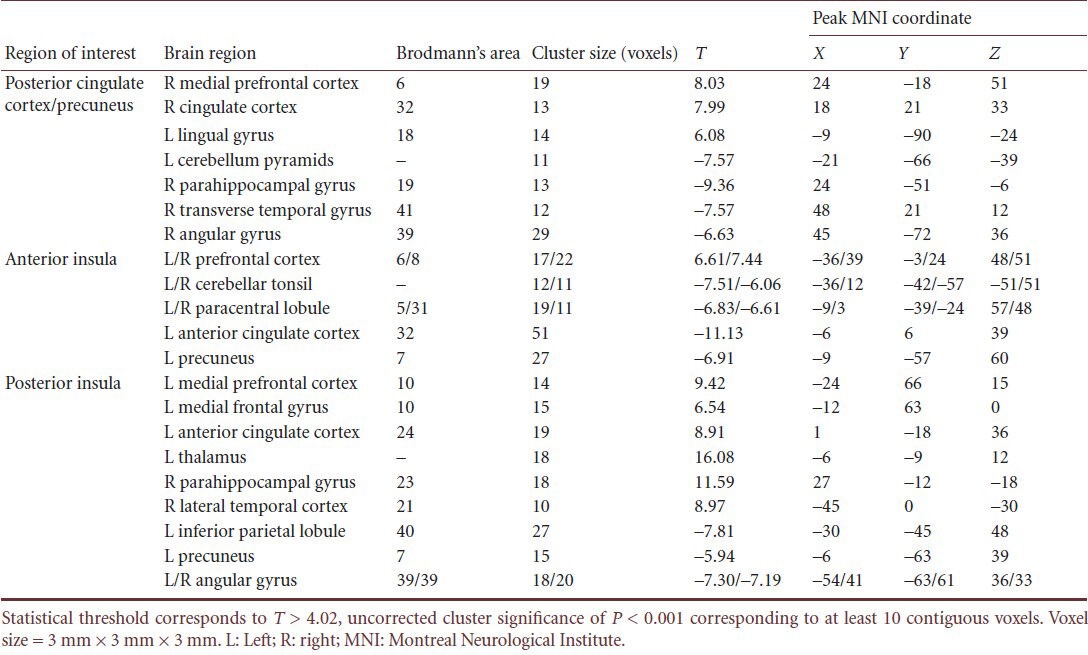
The results of the intrinsic connectivity in default mode network for the pain status are shown in Figure 1. The group analysis showed that low back pain was associated with significant functional connectivity alterations in various brain regions. Following intramuscular injection of hypertonic saline into the back muscle as painful stimuli, the posterior cingulate cortex/precuneus had greater intrinsic connectivity compared with the anterior cingulate cortex, lingual gyrus and prefrontal regions, particularly in the medial prefrontal cortex. However, there was a significant decrease in several regions of the cerebellum (mostly the pyramids), the parahippocampal and trans verse temporal gyri (Table 1) when compared with baseline (P < 0.001; uncorrected). Furthermore, the connection between the medial prefrontal and lateral temporal cortices was stronger during self experienced pain.
Figure 1.
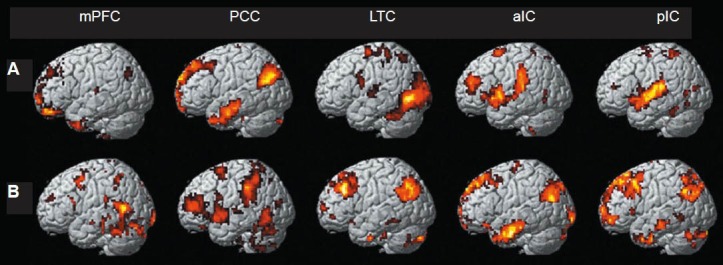
Resting-state functional connectivity map showing positively (A) and negatively (B) correlated voxels for seed regions of interest (mPFC, PCC, LTC, aIC and pIC) in low back pain subjects (P < 0.001, K ≥ 10, uncorrected).
Color area: Regions with differences between pain state and normal. mPFC: Medial prefrontal cortex; PCC: posterior cingulate cortex; LTC: lateral temporal cortex; aIC: anterior insula; pIC: posterior insula.
Table 1.
Brain areas with increased connectivity during resting-state functional magnetic resonance imaging in low back pain subjects
In addition, deactivated regions were observed outside the classical boundaries of the default mode network (i.e., the brainstem and cerebellum). These data show that the intrinsic connectivity within the default mode network and pain modulatory pathways was abnormal during low back pain.
Functional connectivity alteration in the insula in low back pain subjects during resting state
Images of specific changes in functional connectivity within the insula during pain are shown in Figure 2. Pain stimuli led to stronger connectivity between the insula and the medial prefrontal and lateral temporal cortices, whereas there was a significant decrease in the connectivity with the posterior cingulate cortex, precuneus and inferior parietal lobule (P < 0.001, uncorrected, K ≥ 10). Using the anterior insula as a seed, experimental low back pain increased the connectivity to the prefrontal cortex, but decreased connectivity to several cortical regions that were primarily involved with the anterior cingulate cortex, paracentral lobule and posterior lobe of the cerebellum (mainly the cerebellar tonsil).
Figure 2.
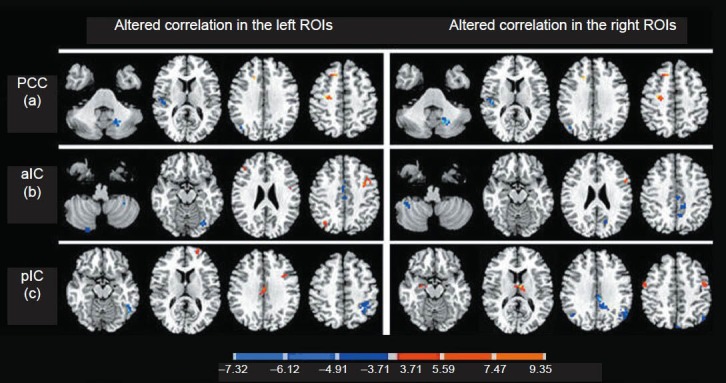
Resting-state group functional activation of positively and negatively correlated voxels for posterior cingulate cortex (a), anterior insula (b) and posterior insula (c) seeds (n = 12).
Areas with significant differences in connectivity with the left and right posterior cingulate cortex, anterior insula and posterior insula that were changed during pain compared with the baseline. Areas with decreased changes in connectivity are shown in blue, whereas areas where the connectivity change was high are shown in red (significance threshold of P < 0.001, uncorrected; paired t-test). A minimum of 10 contiguous voxels was used. The color of each activated voxel corresponds to its T-value, as shown in the color bar scales.
By contrast, there was significant functional connectivity with the posterior insula including the prefrontal regions, thalamus and parahippocampal gyrus, but negative connectivity to the lingual gyrus and inferior parietal lobule (Table 1). Of interest, the anterior cingulate cortex had enhanced negative correlations with the anterior insula at rest, but positive correlations with the posterior insula, suggesting that it had different connectivity with the anterior insula and posterior insula. Our results demonstrate that pain changed the connectivity between the insula and medial prefrontal and posterior cingulate cortices, precuneus and inferior parietal lobule, consistent with studies suggesting that changes in the experience of pain arise from differences in pain processing based on the known organization of the insula[22].
Discussion
Functional connectivity measurements based on low- frequency BOLD signal fluctuations are used to define correlations between spatially remote neurophysiological events[24,25,26,27]. Recent studies have shown that correlated low-frequency fluctuations can be detected, and can identify the brain functional regions where signal changes are associated with seed regions of interest[27].
To examine the hypothesis that chronic pain stimuli can leave a mark beyond the cortical circuits involved with perception, we identified a matrix of cortical regions that support interoceptive cognition and emotion involving the bilateral medial prefrontal cortex, posterior cingulate cortex/precuneus, lateral temporal cortex, anterior insula and posterior insula. In addition, we observed that pain-processing pathways were unaffected compared with healthy subjects. Thus, these data suggest that using back muscle pain to infer the status of pain in the brains of patients with low back pain is fully justified. Group analysis revealed that the experimental low back pain caused an abnormal default mode network and other cortical and subcortical areas, i.e., the frontal and temporal cortices, parietal lobe, cingulate gyrus and insular cortex. It is not surprising that the default mode network as a dynamic system would converge toward a state in which the major hubs of connectivity were preferentially represented. In agreement with previous studies[4,19], the experimental low back pain produced significant dysfunctions in the functional default mode network connectivity. The posterior cingulate cortex has dense structural connectivity as a major node in the component brain regions, where it appears to be involved with the formation of cognitive activities and internally directed thought[28]. In the present study, the posterior cingulate cortex/precuneus had significant connectivity in the superior frontal and anterior cingulate cortices, but decreased connectivity in the posterior lobe of the cerebellum, parahippocampal, lingual and transverse temporal gyri.
These changes may reflect abnormalities during the experience-dependent maturation of cortical functional specialization, and simultaneously deactivated regions may be observed as a result of increased inhibitory drive or asynchronous neuronal firing. Muscle pain produced stronger connectivity between the bilateral medial prefrontal cortex and most seed regions of interest, including the posterior cingulate and lateral temporal cortices, anterior insula and posterior insula. The higher medial prefrontal cortex activation suggests that this region plays a specific role in mediating the attenuation of pain perception via cognitive control mechanisms[29] and may be related to symptoms of anergia and the loss of motivated behaviors[30]. Furthermore, a previous investigation showed that affective instability may be caused by a pattern of limbic hyperreactivity paired with dysfunctional prefrontal regulation mechanisms[31], where painful stimulation was found to decrease affective arousal at the neural level, possibly underlying the effect of lessening pain.
The insula does not simply process pain signals, and has been associated with multiple aversive or otherwise salient experiential states, both interoceptive[32] and exteroceptive[33]. The anterior insula was proposed to receive nociceptive information via direct inputs from the ventromedial thalamic nucleus, whereas the posterior insula receives direct projections from the secondary somatosensory cortex and a pain-specific region in the thalamus (i.e., the posterior portion of the ventromedial thalamic nucleus)[34]. Using functional neuroimaging, the endurance of painful stimulation was found to produce dysfunction of the anterior insula, which showed a significantly decreased functional connectivity with cortical regions such as the anterior cingulate cortex, precuneus, paracentral lobule and cerebellum, suggesting that inhibitory systems may be a possible pathogenic mechanism during prolonged low back pain[35]. The present study found that anterior insula had increased resting connectivity to the inferior and middle frontal gyri, similar to the results described by Tagliazucchi et al.[19]. In addition, we identified the bilateral posterior insula as the seed regions of interest, which changed posterior insula functional connectivity to several cortical and subcortical regions involved primarily with the prefrontal cortex, thalamus, parahippocampal gyrus, precuneus, anterior cingulate and lateral temporal cortices, lingual gyrus and inferior parietal lobule. These areas modulate attention, memory, execution and other cognition function by different projections. These findings are consistent with previous reports that pain endurance may disrupt functional connectivity between the cortical regions of the default mode network and the insula[4,19], which is a key region implicated in internally generated thought processes[14]. Moreover, the right posterior insula had a higher intrinsic connectivity to the left thalamus and right parahippocampal gyrus, suggesting that the pain stimuli led to more nociceptive input into the thalamus via the spinothalamic tract. In particular, the parahippocampal gyrus plays an important role in the evaluation of pain intensity and other somatic signals[36], and it was highly activated during pain, which may reflect that pain disrupts various cognitive processes that mediate the attenuation of pain perception via cognitive control mechanisms[29].
Previous functional imaging studies have detected the pain-related activation of the parasylvian cortical structures (such as the insula), medial frontal cortical structures (anterior cingulate and middle cingulate cortices and supplementary motor area) and the primary somatosensory cortex, which are often described as modules in a “pain network”[1,37]. The anterior cingulate cortex and insula exhibited abnormal intrinsic connectivity in this pain network during experimental low back pain. We found that the anterior cingulate cortex had enhanced negative correlations with the anterior insula, and positive correlations with the posterior insula, suggesting that the anterior insula is more likely to lower the response to peripheral nociceptive stimuli via a self-control function. Similarly, the emotional components of nociceptive stimuli are processed within the insula as lesions encompassing the insula can result in pain asymbolia, a condition where the intensity and quality of nociceptive stimuli are preserved Thus, it seems reasonable to suggest that the persistent, self-reflective thoughts that occur during pain may have led to disruption of the modular organization and increased the number of connections with the anterior cingulate cortex[31]. In addition, the subjects all suffered mild anxiety and horror during the test, which further supported a hypothesis that the anterior cingulate cortex is involved mainly with affective responses to pain[38,39]. Of interest, it was recently shown that both the posterior lobes of the cerebellum and brainstem had a lower intrinsic connectivity with most brain regions, including the medial prefrontal and posterior cingulate cortices, precuneus, and insular and lateral temporal cortices. In terms of the midbrain in the resting state and its correlation with the pain unpleasantness level, our findings are inconsistent with those of previous studies suggesting that self-generated feelings of sadness, anger, happiness and fear produce midbrain activation[40]. An investigation of the functional connectivity in the cerebellum also suggested that this region is involved with emotional modulation[41]. Interactions between the cerebellum and frontoparietal cortex are involved with cognition via the cerebellothalamocortical or corticopontocerebellar circuits, which were deactivated in a study that showed they have important roles in mood-cognitive processing and self-awareness mechanisms; these results were consistent with the findings replicated by position emission tomography[42]. Furthermore, the brain regions with decreased functional connectivity were concentrated in the left hemisphere, which suggests that the left hemisphere corresponds to the affective consequences of pain, whereas the right hemisphere corresponds mainly to homeostatic and autonomic control[43].
In conclusion, this study clearly demonstrated alterations in the resting-state functional connectivity in a range of cortical and subcortical areas during experimental low back pain. The three main findings were as follows: (1) the default mode network was dysfunctional; (2) the insula had significant connectivity to the medial prefrontal and lateral temporal cortices, but decreased connectivity to posterior cingulate cortex, precuneus and inferior parietal lobule; and (3) the anterior insula and posterior insula may be involved with attention, memory, execution and cognition functions (especially the anterior insula, which has a significant role in pain processing). These findings suggest that the brain affected by low back pain is not healthy during the processing of pain information, but rather is changed by persistent pain, particularly with respect to the higher mental activities associated with cognition and emotion.
Our data further support the concept that prolonged muscle pain, representing a sustained pain, is encoded primarily in the insular cortex[35]. These results may help to improve our understanding of abnormal neural activities in brain regions and the mechanisms of pain.
Subjects and Methods
Design
A non-randomized, controlled study.
Time and setting
The experiment was performed in the Department of Radiology of Zhujiang Hospital, Southern Medical University, China, from July 2011 and March 2012.
Subjects
Twelve healthy subjects recruited by advertising in Zhujiang Hospital, with similar residence (southern region, Guangzhou, Guangdong Province, China), age (21–30 years old, average 24.8 ± 3.3 years), education (14–21 years, average 17 years) and gender (6 men and 6 women) participated in this study. All subjects were right-handed. Inclusion criteria included: (1) Body mass index range for standard ± 10%; (2) no psychiatric illnesses or medical illnesses (i.e., multiple sclerosis, epilepsy); (3) no pain (including dysmenorrhea) and drug (i.e., antipyretics, sleeping pills) experience within the last 1 month; and (4) self-rating anxiety scale and self-rating depression scale scores < 50 (lower than 50 represents “normal”). The general exclusion criteria were organic brain disease, a history of skull or brain damage, substance dependency, severe neurological illnesses, metallic components in the body, claustrophobia and analgesic medication (during the last 4 weeks). All experiments and protocols were approved by the Ethics Committee of Zhujiang Hospital Affiliated to Southern Medical University, China. According to the State Council of China[44], each subject provided written informed consent after detailed instructions on the experimental procedures and risks were fully explained.
Methods
Hypertonic saline as pain stimulus (experimental induced pain) and 3.0 T MRI for data acquisition
Prior to scanning for 10 minutes, a fine plastic cannula (24 gauge) was attached to a 1 mL syringe containing a sterile hypertonic (5%) saline stimuli that was inserted 2 cm into the right-sided erector spinae muscle at the level of lumbar vertebra 4 (Figure 3). The pain caused by the needle subsided to pre-injection levels within approximately 20 seconds under the non-anesthesia condition. The imaging volume extended from the dorsal side of the pons to the primary somatosensory cortices, covering most of the cortical and subcortical regions associated with pain perception. T2-weighted functional images were acquired in a single-shot gradient echo-planar imaging sequence (24 axial slices, repetition time/echo time = 3,000/40 ms, in-plane resolution = 3.54 mm × 3.54 mm, field of view = 220 mm × 220 mm, slice thickness = 5 mm and interslice gap = 0.5 mm) using a 3.0 T Philips Achieva scanner (Royal Philips Electronics, Eindhoven, the Netherlands) equipped with an eight- channel head coil. The subjects were instructed to close their eyes and rest comfortably throughout scans, without moving or falling asleep. Prior to obtaining the functional measurements, high-resolution anatomical T1-weighted images were collected using a fast-spin-echo scan (repetition time/echo time = 500/14 ms, matrix = 256 × 256 and flip angle = 90°). Each subject underwent two identical fMRI of 318 seconds at baseline and after 5% hypertonic saline as the pain stimulus.
Figure 3.
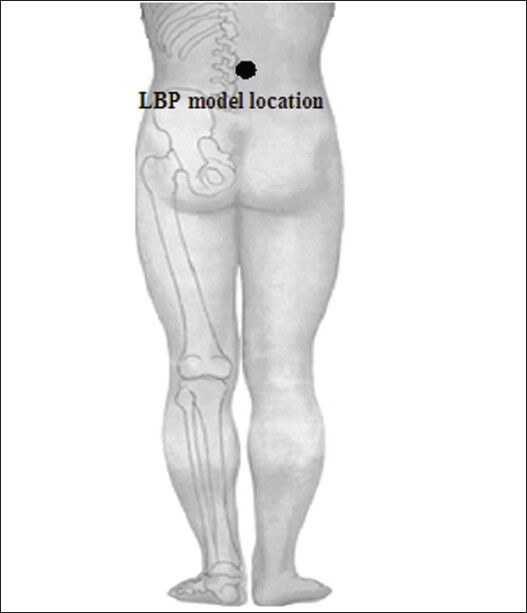
Area of the low back pain (LBP) model during the hypertonic saline (5%) infusion projected into the muscle.
A fine plastic cannula (24-gauge) was attached to a 1 mL syringe containing sterile hypertonic (5%) saline stimuli that was inserted 2 cm into the right-sided erector spinae muscle at the level of lumbar vertebra 4.
After the first fMRI, each of the 12 subjects received an intramuscular injection of 5% hypertonic saline (0.3 mL) in the right-sided erector spinae muscle at the level of lumbar vertebra 4, which was followed by the second scan at 20 seconds after the injection[45,46]. The subjects remained inside the scanner after the scans and were asked to describe when (1) they felt the onset of pain, (2) the pain began to subside from its peak and (3) the pain ceased. Furthermore, the subjects were asked to complete a linear 10-point visual analogue scale to record the maximum pain intensity (0 = no pain and 10 = maximum imaginable pain) and an in-house mood scale to measure the pain unpleasantness (i.e., distressing and horrible, ranging from 0–10, where 0 = infinitely small and 10 = excruciating). The pain differences in unpleasantness were compared before, during and after the test.
Preprocessing of experimental functional MRI data
The fMRI image data were preprocessed and analyzed using the Data Processing Assistant for Resting-State fMRI (DPARSF, http://www.restfmri.net) by routines in MATLAB R2010a. The blood oxygen level-dependent (BOLD) time series preprocessing steps included removal of the first 10 volumes, slice-time correction, motion correction, intensity normalization, spatial smoothing and linear high-pass temporal filtering. The first 10 volumes of each scan were discarded to eliminate any non-equilibrium effects of magnetization and to allow subjects to become familiar with the scanning environment. The motion time courses were used to select subjects head movements of < 2 mm in translation and 2° in rotation, who were used for further analysis (no subjects were excluded). For the muscle pain series, only the image sets of subjects who rated the pain intensity ≥ 3 were used in subsequent analysis (no subjects were excluded). Each individual's functional images were normalized using the symmetric echo-planar imaging templates and resampled at a resolution of 3 mm × 3 mm × 3 mm. The normalized functional images were smoothed spatially using a Gaussian kernel of full-width half-maximum 6 mm. Finally, voxel-wise linear trend removal and temporal high-pass filtering (0.01 Hz < f < 0.08 Hz) were applied.
Seed regions of interest for the functional connectivity analysis
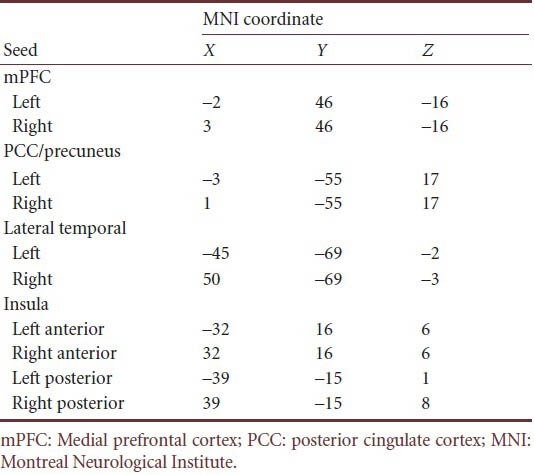
DPARSF was used to create individual subject seed-to-voxel connectivity maps. The seed regions of interest were spheres of 6 mm radius that were centered on the Montreal Neurological Institute coordinates used to identify the corresponding networks in previous resting-state functional connectivity studies[4,24,25,26]. We extracted the individual time course of activity from the regions of interest relative to the standard echo-planar imaging template for the insular cortex that contained the anterior insula and posterior insula, and the default mode network that included the medial prefrontal cortex, posterior cingulate cortex/precuneus and lateral temporal cortex (Table 2). Regressors corresponding to the six motion correction parameters and their global gray matter, white matter and cerebrospinal fluid were also included to remove variance related to motion, the global, white matter and the cerebrospinal fluid signals, respectively. Regressors were created by extracting the BOLD time courses from the tissue class segmented images, averaged across all voxels within each tissue class. The modeled group effect size and standard error were then divided to produce a volume where the voxels were T scores, which were subsequently transformed to Z scores. The resulting Z distribution had a zero mean and a standard deviation of unity.
Table 2.
Regions of interest of the functional connectivity analysis
Statistical analysis
SPSS 13.0 software (SPSS, Chicago, IL, USA) was used to calculate descriptive statistics (mean ± SD) for age, gender, pain intensity and pain unpleasantness (data obeyed a normal distribution). One-way analysis of variance was used to compare the unpleasantness ratings before, during and after the injection (data obeyed a normal distribution). Random effects analysis was used to create intragroup statistical parametric mapping (SPM8, http://www.fil.ion.ucl.ac.uk) for the regions of interest and to explore to evaluate functional connectivity differences between pain and healthy conditions. Regions that were positively correlated with the seed regions of interest in each status were identified at the cluster level because of P < 0.05, after correcting for multiple comparisons using the false discovery rate and applying a minimum cluster size of 10 contiguous voxels.
The intrinsic brain connectivity differences between baseline and pain status were calculated using two-tailed paired t-tests at P < 0.001 (uncorrected) with a minimum cluster size of 10 voxels for regions of interest (anterior insula, posterior insula, medial prefrontal cortex, posterior cingulate cortex/precuneus and lateral temporal cortex).
Acknowledgments:
All MRI scanning was conducted at the MRI Clinical Imaging Department at Zhujiang Hospital (Guangzhou, China). We thank Yang JM from the Department of Neurology, Zhujiang Hospital, Southern Medical University in China for assistance. We thank all volunteers for the assistance in the scanning.
Footnotes
Funding: This study was supported by the Science and Technology Foundation of Guangdong Province of China, No. 2012B031800305.
Conflicts of interest: None declared.
Peer review: Hypertonic saline was used as a low back pain stimulus. This study explored potential brain mechanisms underlying insular activation, and made observations over time based on low back pain perception and pain-related processing. Furthermore, using functional magnetic resonance imaging and alterations in functional connectivity, this study found functional connectivity changes that predict the extent of the attenuation of negative effects.
Copyedited by Dean J, Stow A, Chen F, Sun XJ, Wang J, Qiu Y, Li CH, Song LP, Liu WJ, Zhao M
References
- [1].Casey KL. Concepts of pain mechanisms: the contribution of functional imaging of the human brain. Prog Brain Res. 2000;129:277–287. doi: 10.1016/S0079-6123(00)29020-1. [DOI] [PubMed] [Google Scholar]
- [2].Linnman C, Beucke JC, Jensen KB, et al. Sex similarities and differences in pain-related periaqueductal gray connectivity. Pain. 2012;153(2):444–454. doi: 10.1016/j.pain.2011.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Apkarian AV, Bushnell MC, Treede RD, et al. Human brain mechanisms of pain perception and regulation in health and disease. Eur J Pain. 2005;9(4):463–484. doi: 10.1016/j.ejpain.2004.11.001. [DOI] [PubMed] [Google Scholar]
- [4].Baliki MN, Geha PY, Apkarian AV, et al. Beyond feeling: chronic pain hurts the brain, disrupting the default-mode network dynamics. J Neurosci. 2008;28(6):1398–1403. doi: 10.1523/JNEUROSCI.4123-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Baliki MN, Chialvo DR, Geha PY, et al. Chronic pain and the emotional brain: specific brain activity associated with spontaneous fluctuations of intensity of chronic back pain. J Neurosci. 2006;26(47):12165–12173. doi: 10.1523/JNEUROSCI.3576-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Derbyshire SW. Exploring the pain “neuromatrix”. Curr Rev Pain. 2000;4(6):467–477. doi: 10.1007/s11916-000-0071-x. [DOI] [PubMed] [Google Scholar]
- [7].Biswal B. Functional connectivity in the motor cortex of resting human brain using echoplanar MRI. Magn Reson Med. 1995;34(4):537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- [8].Apkarian AV, Sosa Y, Sonty S, et al. Chronic back pain is associated with decreased prefrontal and thalamic gray matter density. J Neurosci. 2004;24(46):10410–10415. doi: 10.1523/JNEUROSCI.2541-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Acerra NE, Moseley GL. Dysynchiria: watching the mirror image of the unaffected limb elicits pain on the affected side. Neurology. 2005;65(3):751–753. doi: 10.1212/01.wnl.0000178745.11996.8c. [DOI] [PubMed] [Google Scholar]
- [10].Napadow V, LaCount L, Park K, et al. Intrinsic brain connectivity in fibromyalgia is associated with chronic pain intensity. Arthritis Rheum. 2010;62(8):2545–2555. doi: 10.1002/art.27497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Mainero C, Boshyan J, Hadjikhani N. Altered functional magnetic resonance imaging resting-state connectivity in periaqueductal gray networks in migraine. Ann Neurol. 2011;70(5):838–845. doi: 10.1002/ana.22537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Becerra L, Schwartzman RJ, Kiefer RT, et al. CNS measures of pain responses pre- and post-anesthetic ketamine in a patient with complex regional pain syndrome. Pain Med. 2009;25:1–8. doi: 10.1111/pme.12939. [DOI] [PubMed] [Google Scholar]
- [13].Cauda F, Sacco K, D’Agata F, et al. Low-frequency BOLD fluctuations demonstrate altered thalamocortical connectivity in diabetic neuropathic pain. BMC Neurosci. 2009;138(10):1–14. doi: 10.1186/1471-2202-10-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Raichle ME, MacLeod AM, Snyder AZ, et al. A default mode of brain function. Proc Natl Acad Sci U S A. 2001;98(2):676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Wu JT, Wu HZ, Yan CG, et al. Aging-related changes in the default mode network and its anti-correlated networks: a resting-state fMRI study. Neurosci Lett. 2011;504(1):62–67. doi: 10.1016/j.neulet.2011.08.059. [DOI] [PubMed] [Google Scholar]
- [16].Buckner RL, Andrews-Hanna JR, Schacter DL. The brain's default network: anatomy, function, and relevance to disease. Ann NY Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- [17].Tomasi D, Volkow ND. Association between functional connectivity hubs and brain networks. Cereb Cortex. 2011;21(9):2003–2013. doi: 10.1093/cercor/bhq268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Balenzuela P, Chernomoretz A, Fraiman D, et al. Modular organization of brain resting state networks in chronic back pain patients. Front Neuroinform. 2010;4:116. doi: 10.3389/fninf.2010.00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Tagliazucchi E, Balenzuela P, Fraiman D, et al. Brain resting state is disrupted in chronic back pain patients. Neurosci Lett. 2010;485(1):26–31. doi: 10.1016/j.neulet.2010.08.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Owen DG, Clarke CF, Bureau Y, et al. Measuring the neural response to continuous intramuscular infusion of hypertonic saline by perfusion MRI. J Magn Reson Imaging. 2012;35(3):669–677. doi: 10.1002/jmri.22814. [DOI] [PubMed] [Google Scholar]
- [21].Schweinhardt P, Glynn C, Brooks J, et al. An fMRI study of cerebral processing of brush-evoked allodynia in neuropathic pain patients. Neuroimage. 2006;32(1):256–265. doi: 10.1016/j.neuroimage.2006.03.024. [DOI] [PubMed] [Google Scholar]
- [22].Yi HM, Zhou Y, Zhang Q, et al. Functional MRI study of insula in the task-state and resting-state. Zhongguo Yixue Yingxiang Jishu. 2010;26(3):439–443. [Google Scholar]
- [23].Sheppes G, Meiran N. Better late than never. On the dynamics of online regulation of sadness using distraction and cognitive reappraisal? Pers Soc Psychol Bull. 2007;33(11):1518–1532. doi: 10.1177/0146167207305537. [DOI] [PubMed] [Google Scholar]
- [24].Ichesco E, Quintero A, Clauw DJ, et al. Altered functional connectivity between the insula and the cingulate cortex in patients with temporomandibular disorder: a pilot study. Headache. 2012;52(3):441–454. doi: 10.1111/j.1526-4610.2011.01998.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Vincent JL, Snyder AZ, Fox MD, et al. Coherent spontaneous activity identifies a hippocampal parietal memory network. J Neurophysiol. 2006;96(6):3517–3531. doi: 10.1152/jn.00048.2006. [DOI] [PubMed] [Google Scholar]
- [26].Vincent JL, Kahn I, Snyder AZ, et al. Evidence for a frontoparietal control system revealed by intrinsic functional connectivity. J Neurophysiol. 2008;100(6):3328–3342. doi: 10.1152/jn.90355.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Linnman C, Beucke JC, Jensen KB, et al. Sex similarities and differences in pain-related periaqueductal gray connectivity. Pain. 2012;153(2):444–454. doi: 10.1016/j.pain.2011.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Leech R, Braga R, Sharp DJ. Echoes of the brain within the posterior cingulate cortex. J Neurosci. 2012;32(1):215–222. doi: 10.1523/JNEUROSCI.3689-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Wiech K, Ploner M, Tracey I. Neurocognitive aspects of pain perception. Trends Cognit Sci. 2008;12(8):306–313. doi: 10.1016/j.tics.2008.05.005. [DOI] [PubMed] [Google Scholar]
- [30].Milak MS, Parsey RV, Keilp J, et al. Neuroanatomic correlates of psychopathologic components of major depressive disorder. Arch Gen Psychiatry. 2005;62(4):397–408. doi: 10.1001/archpsyc.62.4.397. [DOI] [PubMed] [Google Scholar]
- [31].Banks SJ, Eddy KT, Angstadt M, et al. Amygdala-frontal connectivity during emotion regulation. Soc Cogn Affect Neurosci. 2007;2(4):303–312. doi: 10.1093/scan/nsm029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Craig AD. How do you feel? Interoception: The sense of the physiological condition of the body. Nat Rev Neurosci. 2002;3(8):655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- [33].Seeley WW, Menon V, Schatzberg AF, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27(9):2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Craig AD, Zhang ET. Retrograde analyses of spinothalamic projections in the macaque monkey: input to posterolateral thalamus. J Comp Neurol. 2006;499(6):953–964. doi: 10.1002/cne.21155. [DOI] [PubMed] [Google Scholar]
- [35].Owen DG, Clarke CF, Ganapathy S, et al. Using perfusion MRI to measure the dynamic changes in neural activation associated with tonic muscular pain. Pain. 2010;148(3):375–386. doi: 10.1016/j.pain.2009.10.003. [DOI] [PubMed] [Google Scholar]
- [36].Critchley HD, Wiens S, Rotshtein P, et al. Neural systems supporting interoceptive awareness. Nat Neurosci. 2004;7(2):189–195. doi: 10.1038/nn1176. [DOI] [PubMed] [Google Scholar]
- [37].Jones EG, Lenz F, Willis WD. Cambridge: Cambridge University Press, UK; 2009. The Human Pain System: Experimental and Clinical Perspectives. [Google Scholar]
- [38].Mechias ML, Etkin A, Kalisch R. A meta-analysis of instructed fear studies: implications for conscious appraisal of threat. Neuroimage. 2010;49(2):1760–1768. doi: 10.1016/j.neuroimage.2009.09.040. [DOI] [PubMed] [Google Scholar]
- [39].Shackman AJ, Salomons TV, Slagter HA, et al. The integration of negative affect, pain and cognitive control in the cingulate cortex. Nat Rev Neurosci. 2011;12(3):154–167. doi: 10.1038/nrn2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Damasio AR, Grabowski TJ, Bechara A, et al. Subcortical and cortical brain activity during the feeling of self-generated emotions. Nat Neurosci. 2000;3(10):1049–1056. doi: 10.1038/79871. [DOI] [PubMed] [Google Scholar]
- [41].Habas C, Guillevin R, Abanou A. Functional connectivity of the superior human temporal sulcus in the brain resting state at 3T. Neuroradiology. 2011;53(2):129–140. doi: 10.1007/s00234-010-0775-5. [DOI] [PubMed] [Google Scholar]
- [42].Nusbaum F, Redouté J, Le Bars D, et al. Chronic low-back pain modulation is enhanced by hypnotic analgesic suggestion by recruiting an emotional network: a PET imaging study. Int J Clin Exp Hypn. 2011;59(1):27–44. doi: 10.1080/00207144.2011.522874. [DOI] [PubMed] [Google Scholar]
- [43].Craig A. Forebrain emotional asymmetry: a neuroanatomical basis? Trends Cognit Sci. 2005;9(12):566–571. doi: 10.1016/j.tics.2005.10.005. [DOI] [PubMed] [Google Scholar]
- [44].State Council of the People's Republic of China. Administrative Regulations on Medical Institution. 1994-09-01 [Google Scholar]
- [45].Nash PG, Macefield VG, Klineberg IJ, et al. Bilateral activation of the trigeminothalamic tract by acute orofacial cutaneous and muscle pain in humans. Pain. 2010;151(2):384–393. doi: 10.1016/j.pain.2010.07.027. [DOI] [PubMed] [Google Scholar]
- [46].Owen DG, Clarke CF, Ganapathy S, et al. Using perfusion MRI to measure the dynamic changes in neural activation associated with tonic muscular pain. Pain. 2010;148(3):375–386. doi: 10.1016/j.pain.2009.10.003. [DOI] [PubMed] [Google Scholar]


