Abstract
The role of type-2 astrocytes in the repair of central nervous system injury remains poorly understood. In this study, using a relatively simple culture condition in vitro, type-2 astrocytes, differentiated from oligodendrocyte precursor cells by induction with bone morphogenetic protein-4, were co-cultured with dorsal root ganglion neurons. We examined the effects of type-2 astrocytes differentiated from oligodendrocyte precursor cells on the survival and growth of dorsal root ganglion neurons. Results demonstrated that the number of dorsal root ganglion neurons was higher following co-culture of oligodendrocyte precursor cells and type-2 astrocytes than when cultured alone, but lower than that of neurons co-cultured with type-1 astrocytes. The length of the longest process and the length of all processes of a single neuron were shortest in neurons cultured alone, followed by neurons co-cultured with type-2 astrocytes, then neurons co-cultured with oligodendrocyte precursor cells, and longest in neurons co-cultured with type-1 astrocytes. These results indicate that co-culture with type-2 astrocytes can increase neuronal survival rate and process length. However, compared with type-1 astrocytes and oligodendrocyte precursor cells, the promotion effects of type-2 astrocytes on the growth of dorsal root ganglion neurons were weaker.
Keywords: nerve regeneration, spinal cord injury, oligodendrocyte, oligodendrocyte precursor cells, astrocytes, bone morphogenetic protein, neurons, neurites, dorsal root ganglion, NIH grant, neural regeneration
Introduction
Cell death of oligodendrocytes and subsequent demyelination are an important pathological hallmark of many neurological diseases, including multiple sclerosis and spinal cord injury[1]. Oligodendrocytes are myelin-forming cells in the central nervous system that differentiate from oligodendrocyte precursor cells[2]. Oligodendrocytes are also responsible for providing nutrition and support to neuronal axons[3]. Oligodendrocyte damage can cause axonal demyelination and secondary axonal degeneration and neuronal apoptosis[4], and contribute to the pathophysiology of many neurological diseases[5]. Therefore, enhancing oligodendrocyte remyelination is an important therapeutic approach for these diseases[6,7,8]. However, as they are terminally differentiated cells, intrinsic or transplanted mature oligodendrocytes are unable to initiate remyelination[9,10]. Remyelination could be induced by new oligodendrocytes differentiated from stem cells or precursor cells[11,12]. Oligodendrocyte precursor cells are immature progenitor cells that can directionally differentiate into mature oligodendrocytes, with certain proliferative and migratory capacity, and thus, are ideal cells for cell replacement therapy in the treatment of demyelinating diseases of the central nervous system[13]. Oligodendrocyte precursor cells exist in normal adult central nervous system and become activated after injuries[14]. However, endogenous remyelination is limited due to limited number of oligodendrocyte precursor cells, its inefficient migration and/or failure to differentiation and maturation due to the inhibiting environment[15]. For example, the number of oligodendrocyte precursor cells increases around the demyelinating lesion site[16], but they fail to differentiate into mature oligodendrocytes to remyelinate the demyelinated axons because of differentiation arrest[17,18,19,20]. Reactive astrocytes at the lesion site were often seen to express some factors that suppress the differentiation and maturation of oligodendrocytes[21,22] although oligodendrocyte precursor cells have certain characteristics of stem cells[23], and can differentiate into oligodendrocytes, astrocytes and neurons under different culture conditions[24]. The newly upregulated factors at the lesion site of the central nervous system possibly affect the directional differentiation and maturation of oligodendrocyte precursor cells, and thus interfere with therapeutic efficacy. Transplantation of oligodendrocyte precursor cells[25,26,27] or neural stem cells[6,28] approves an effective therapeutic approach to promote remyelination and functional recovery after many neurological diseases. But inhibiting signals in the injured environment may prevent the oligodendrocyte differentiation and maturation of grafted oligodendrocyte precursor cells or neural stem cells and blocking these inhibiting factors could further enhance therapeutic efficacy of cell transplantation.
The expression of bone morphogenetic proteins is significantly upregulated around the lesion site[22,29]. Bone morphogenetic proteins are member of the transforming growth factor superfamily, and play an important role in the neural development of vertebrates and nerve repair[30,31,32]. Many studies have verified that bone morphogenetic proteins effectively promote the astrocyte differentiation of oligodendrocyte precursor cells and glial-restricted precursor cells, and inhibit their differentiation into oligodendrocytes[33,34]. Glial-restricted precursor cells are one of the early progenitor cells of glial lineage during the development of the central nervous system of mammals[35]. Glial-restricted precursor cells can differentiate into astrocyte precursor cells and oligodendrocyte precursor cells under different differentiation conditions[35,36,37]. Bone morphogenetic proteins induce glial-restricted precursor cells to differentiate into type-1 astrocytes[35], and oligodendrocyte precursor cells into type-2 astrocytes[33]. Both type-1 and type-2 astrocytes express glial fibrillary acidic protein, but their morphologies are quite different. In addition, they are different regarding the expression of some surface antigens. For example, type-2 astrocytes express A2B5 antigen, but type-1 astrocytes do not[38]. Some previous studies indicate that type-1 astrocytes contribute to axonal regeneration and functional recovery after spinal cord injury[39,40,41]. However, few studies have examined the effects of type-2 astrocytes on regeneration.
In vitro studies have shown that the astrocyte differentiation of oligodendrocyte precursor cells or glial-restricted precursor cells is strongly associated with the concentration of bone morphogenetic proteins[42,43]. Therefore, increasing expression of bone morphogenetic proteins at the lesion site of the central nervous system possibly induces oligodendrocyte precursor cells to differentiate into type-2 astrocytes. While the restriction of oligodendrocyte differentiation could affect remyelination, it remains poorly understood how type-2 astrocytes regulate regeneration and functional recovery. Thus, examining the effects of type-2 astrocytes on neuronal growth is helpful in understanding the possible influential factors of oligodendrocyte precursor cells on axonal regeneration and remyelination, and may provide insights to develop a combined therapeutic strategy.
In this study, primary cultured oligodendrocyte precursor cells were purified from adult spinal cord. These cells are close to precursor cells from adult animals after spinal cord injury. Bone morphogenetic protein-4 was added to induce their differentiation into type-2 astrocytes, which were co-cultured with dorsal root ganglion neurons. We analyzed effects of oligodendrocyte precursor cells and type-2 astrocytes on neuronal survival and neurite growth.
Results
Morphology of oligodendrocyte precursor cells and oligodendrocytes identified by immunocytochemistry
A2B5 antigen is a cell surface ganglioside epitope expressed on developing oligodendrocyte precursor cells or glial-restricted precursor cells, and O1 is an antigen specifically expressed on oligodendrocytes. More than 90% of oligodendrocyte precursor cells immunopanned from the the spinal cord of adult rats using A2B5 antibody were positive for A2B5 (Figure 1A). These cells were most bipolar or tripolar with phase contrast bright cell body and a few thin processes. Few were positive for O1 or glial fibrillary acidic protein (data not shown). After passage, > 98% of cells were positive for A2B5, and most of them presented three or more long and thin processes (Figure 1B). Their appearance did not dramatically alter even after many passages. After differentiation for 3 days in oligodendrocyte medium, most cells differentiate into O1-positive oligodendrocytes with increasing numbers of processes (Figure 1C).
Figure 1.
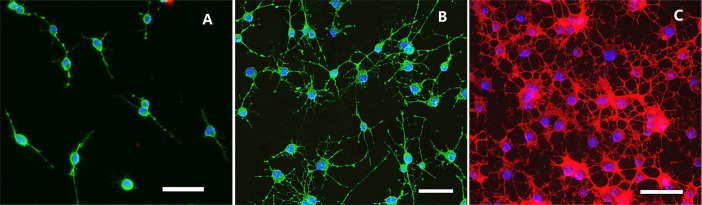
Immunofluorescence images of oligodendrocyte precursor cells from the spinal cord of adult rats and differentiated oligodendrocytes.
(A) Primary cultured oligodendrocyte precursor cells were double-stained with A2B5 (green fluorescence) and Hoechst 3442. (B) Oligodendrocyte precursor cells (passage 3) were double-stained with A2B5 (green fluorescence) and Hoechst 3442. (C) Oligodendrocytes appearing at 3 days of oligodendrocyte precursor cell differentiation were double-stained with O1 (red fluorescence) and Hoechst 3442. Blue fluorescence: Hoechst 3442-labeled nuclei. Scale bars: 50 μm.
Morphology of type-2 astrocytes formed by indution of bone morphogenetic protein-4 identified by immunocytochemistry
Oligodendrocyte precursor cells were induced to differentiate into type-2 astrocytes in the astrocyte differentiation medium containing bone morphogenetic protein-4. At 1 day after differentiation, immunocytochemical staining revealed some glial fibrillary acidic protein-positive cells, accounting for 8.1 ± 1.9% of total cells (Figure 2A). Three days later, the percentage of glial fibrillary acidic protein-positive cells was significantly increased to 78.1 ± 1.8% (Figure 2B). Five days later, most cells (96.3 ± 1.6%) were glial fibrillary acidic protein-positive astrocytes (Figure 2C). Seven days later, the bodies of the glial fibrillary acidic protein-positive cells became larger and processes became thicker (Figure 2D). These glial fibrillary acidic protein-positive cells presented many thin and long processes. The morphologies of type II astrocytes were evidently different from the fibroblastic morphology of type-1 astrocytes differentiated from glial-restricted precursor cells under the induction of bone morphogenetic protein-4 (Figure 2E), which was identical to previously studies[33,38]. The percentages of O1-positive cells were respectively 1.2 ± 1.8%, 0.1 ± 2.1%, 0, and 0 at 1, 3, 5 and 7 days after culture of oligodendrocyte precursor cells with bone morphogenetic protein-4.
Figure 2.
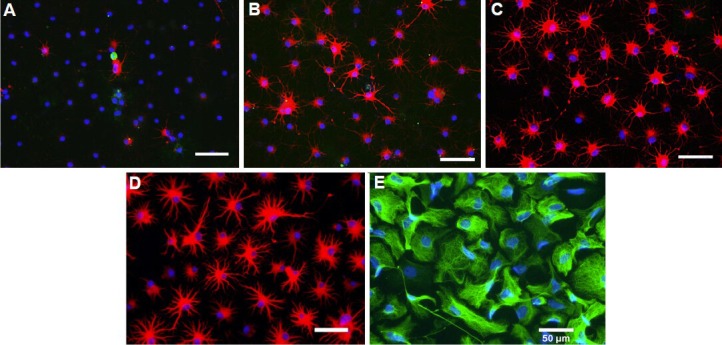
Immunofluorescence images of oligodendrocyte precursor cells after differentiation induced by bone morphogenetic protein-4.
Blue fluorescence: Hoechst 3442-labeled nuclei. (A–D) Cells were triple-stained with O1 (green fluorescence), glial fibrillary acidic protein (GFAP) (red fluorescence) and Hoechst 3442 at 1, 3, 5 and 7 days after differentiation of oligodendrocyte precursor cells. (E) Cells were triple-stained with A2B5 (red fluorescence), GFAP (green fluorescence) and Hoechst 3442 at 5 days after differentiation of glial-restricted precursor cells induced by bone morphogenetic protein-4. Scale bars: 50 μm.
Survival of dorsal root ganglion neurons and the length of processes following co-culture with type-2 astrocytes
After co-cultured with type-1 astrocytes, type-2 astrocytes, oligodendrocyte precursor cells or without any cells for 18 hours, embryonic dorsal root ganglion neurons and their processes were stained for NF-M, photographed under a fluorescence microscope, and were then counted and measured. Results showed that the number of dorsal root ganglion neurons was smaller in the blank control group compared with other groups (Figures 3A, 4A). The mean number of neurons in each plate was 242 ± 16, and majority of neurons were bipolar (Figure 3B). Of the four groups, the best survival of dorsal root ganglion neurons was observed in the type-1 astrocyte group (Figures 3C, 4A), and the mean number of surviving neurons in each plate was 636 ± 13. The number of surviving neurons was similar between the oligodendrocyte precursor cell group (524 ± 15) and the type-2 astrocyte group (516 ± 18) (Figures 3E, G, 4A), which were both lower than the type-1 astrocyte group (P < 0.05), but still higher than the blank control group (P < 0.01).
Figure 3.
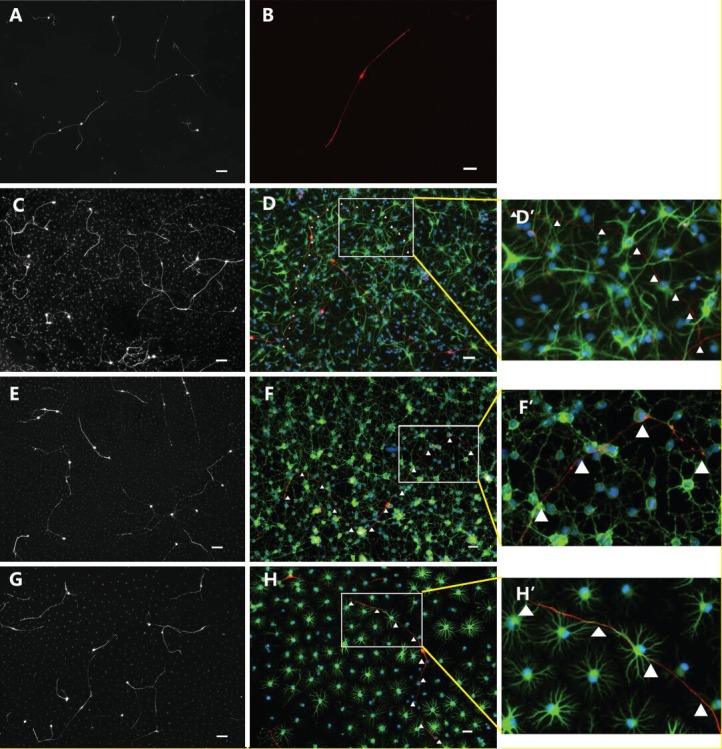
Immunofluorescence images of dorsal root ganglion neurons co-cultured with various cells for 18 hours.
Under the fluorescence microscope, cells in the type-1 and type-2 astrocyte groups were stained with NF-M, glial fibrillary acidic protein (GFAP) and Hoechst 3442. Cells in the oligodendrocyte precursor cell group were stained with NF-M, O4 and Hoechst 3442. Cells in the blank control group were stained with NF-M and Hoechst 3442. (A, C, E, G) Low-power images of cells in the blank control, type-1 astrocyte, oligodendrocyte precursor cell and type-2 astrocyte groups were co-cultured with dorsal root ganglion neurons for 18 hours (B, D, F, H respectively). High-power images of cells in the blank control, type-1 astrocyte, oligodendrocyte precursor cell and type-2 astrocyte groups were co-cultured with dorsal root ganglion neurons for 18 hours. D’, F’, H’: Magnified images of white panes of D, F, H, respectively; red fluorescence shows NF-M staining. Green fluorescence in F and F’ shows O4 staining. In D, D’, H, H’, green fluorescence shows GFAP staining, and blue fluorescence shows Hoechst 3442-labeled nuclei. Scale bars (A, C, E, G): 100 μm; scale bars (B, D, F, H): 50 μm.
Figure 4.
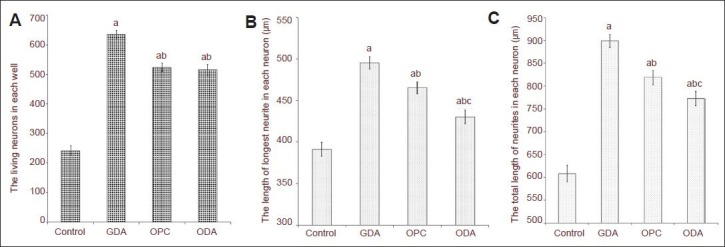
The number of living cells and the length of processes of dorsal root ganglion neurons co-cultured with various cells for 18 hours.
(A) Number of living neurons in each well in each group (cells double-stained with NF-M and Hoechst 3442). (B) Length of the longest processes of neurons in each group. (C) Length of all processes of neurons in each group. GDA: Type-1 astrocytes; OPC: oligodendrocyte precursor cells; ODA: type-2 astrocytes. Under the fluorescence microscope, neurons were observed, photographed, and quantified, and the number of living neurons was measured. The length of neuronal processes was measured using NIS-ELEMENT BR software self-contained by the microscope. The data are expressed as mean ± SD. Mean value of multiple groups was compared using one-way analysis of variance. Multiple comparisons among mean values were done using the Duncan's multiple range test. aP < 0.01, vs. blank control group; bP < 0.05, vs. type-1 astrocyte group; cP < 0.05, vs. oligodendrocyte precursor cell group.
The length of the longest process and the length of all processes of a single dorsal root ganglion neuron in each group were measured and statistically analyzed. Results showed that length of the longest process and the length of all processes of a neuron were shorter in the blank control group compared with the type-1 astrocyte, oligodendrocyte precursor cell and type-2 astrocyte groups (P < 0.01). Simultaneously, the length of the longest process and the length of all processes of a neuron were significantly longer in the type-1 astrocyte group compared with oligodendrocyte precursor cell and type-2 astrocyte groups (P < 0.05). The length of the longest process and the length of all processes of one neuron were shorter in the type-2 astrocyte group compared with the oligodendrocyte precursor cell group (P < 0.05; Figure 4B, C).
Discussion
Morphology of oligodendrocyte precursor cells and oligodendrocytes identified by immunocytochemistry
In this study, we purified oligodendrocyte precursor cells and glial-restricted precursor cells from adult and embryonic spinal cord, respectively, using immunopanning with A2B5. We then differentiated them into type-1 and type-2 astrocytes in the presence of bone morphogenetic protein 4. We directly compared their effects on the survival and neurite growth of co-cultured dorsal root ganglion neurons. Our results showed that the survival and neurite growth of dorsal root ganglion neurons were significantly better in the groups with cells than those without (blank control). These results are consistent with previous studies[44,45] suggesting that neural stem cells or precursors and their derivatives are able to promote the neuronal survival and axonal regeneration. The underneath mechanisms by which neural stem or precursor cells promote axonal regeneration and/or neuronal survival remain to be determined. The cells express the permissive substrates such as fibronectin or laminin which could promote the neurite growth in vitro or axonal regeneration in vivo[40,46]. They could also secrete the growth factors or neurotrophins by promoting neurite growth or neuronal survival. Neural stem or precursor cells expressed multiple neurotrophic or growth factors[47,48,49]. These cells can secrete growth or neruotrophic factors into the culture medium to promote neurite growth in vitro[40]. Following transplantation, the neural stem or precursor cells can release growth or neurotrophic factors to decrease the motor neuron loss in mouse models of amyotrophic lateral sclerosis neural[50,51] or oligodendrocyte death after spinal cord injury[49,52]. Similarly, the grafted neural stem or precursor cells promote axonal regeneration after spinal cord injury[40,53,54] or neuronal plasticity after stroke[55]. These studies suggest that, in addition to their potential to differentiation into neurons, oligodendrocytes or astrocytes for cell replacement, the neural stem or precursor cells could potentiate the endogenous repairing following transplantation after neurological diseases. However, different types of cells, including neural stem or precursor cells and their derivatives, may function differently on the repairing processes, such as axonal regeneration[56].
Our results showed different effects of oligodendrocyte precursor cells, type-1 and type-2 astrocytes on the survival and neurite growth of neurite of co-cultured dorsal root ganglion neurons. While no significant differences in the survival of co-cultured dorsal root ganglion neurons were detected between oligodendrocyte precursor cells and type-2 astrocytes, a much better dorsal root ganglion neuron survival was observed in the co-culture with type-1 astrocytes. Furthermore, both the length of the longest process and the length of all processes of co-cultured dorsal root ganglion neurons were significantly shorter in the type-2 astrocyte group compared with type-1 astrocyte group or oligodendrocyte precursor cell group. Results from this study indicate that relative to undifferentiated oligodendrocyte precursor cells, type-1 astrocytes promoted neuronal growth, but type-2 astrocytes suppressed neuronal growth. It is very interesting to note that astrocytes differentiated from embryonic glial-restricted precursor cells and adult oligodendrocyte precursor cells using the same differentiation factor, bone morphogenetic protein 4, function very differently on neurite growth. These results are consistent with previous studies using astrocytes from different developmental stages. For example, astrocytes in the early developing central nervous system (CNS) promote oligodendrogenesis[57]. Astrocytes from the developing CNS enhance remyelination after transplantation into the demyelinated spinal cord[58,59]. The reactive astrocytes from the injured spinal cord, however, inhibit remyelination[58]. Astrocytes directly from the embryonic CNS promote axon regeneration after transplantation into adult CNS injuries[60,61]. However, when the same astrocytes become aging during the prolonged in vitro culturing, they are less supportive[62], which has also resulted in minimal axon growth after their transplantation to adult spinal cord injuries[63]. Astrocytes from distinct regions of adult CNS may also behave differentially. For example, astrocytes in the hippocampus may promote neural stem cells to differentiate into neurons while astrocytes in the adult spinal cord may induce the astrocyte differentiation and inhibit neurogenesis from neural stem cells[64]. In fact, astrocytes differentiated from the same embryonic glial-restricted precursor cells using different differentiation factors also have different, or opposite effects on regeneration. Astrocytes induced from glial-restricted precursor cells with bone morpho- genetic protein 4 promote axonal regeneration and functional recovery after transplantation following spinal cord inury[40,46,53,54]. But transplantation of astrocytes induced from glial-restricted precursor cells with ciliary neurotrophin factor fails to promote axonal regeneration and functional recovery after transplantation[54,65]. Furthermore, transplantation of ciliary neurotrophin factor-induced astrocytes, but not bone morphogenetic protein 4-induced astrocytes, causes allodynia after spinal cord inury[65]. It is worthy to note that bone morphogenetic protein 4-induced astrocytes are the type-1 astrocytes in this study. The ciliary neurotrophin factor-induced astrocytes resemble the type-2 astrocytes described in this study in many aspects, such as morphology and expression of A2B5. These studies indicate that not all astrocytes have similar effects on axonal regeneration or oligodendrocyte differentiation and myelination, highlighting the importance of cell types, resources and developing stages on the therapies of neurological diseases.
Reactive astrogliosis and the subsequent formation of a glial scar are robust phenomena that occur following diverse CNS injuries[66,67]. However, their functions in vivo are not well established. Both harmful and beneficial effects have been attributed to reactive astrogliosis[68,69]. For example, reactive astrocytes could protect CNS cells and tissue by limiting inflammatory cells[70,71], protection from oxidative stress via glutathione production[72], uptake of potentially excitotoxic glutamate[73] and facilitating blood brain barrier repair[74]. On the other hand, glial scars at the injury site could restrict the regeneration of neuronal axons[75,76], and suppress remyelination[77]. Multiple types of cells in the adult CNS, such as astrocytes, neural stem cells or oligodendrocyte precursor cells, contribute to astrogliois after CNS injuries[78,79]. However, it is unknown whether reactive astrocytes from different resources will have different roles after CNS injuries. Our results showed that astrocytes derived from different precursor cells may function differently. Type-1 astrocytes from glial-restricted precursor cells promote neurite growth while type-2 astrocytes from oligodendrocyte precursor cells are much less supportive for neurite growth. Oligodendrocyte precursor cells widely distribute in the adult CNS[80,81]. Its numbers are dramatically increased after CNS injuries[82]. Importantly, expression of bone morphogenetic proteins is significantly increased in the reactive astrocytes around the injured area following neurological diseases[22,83,84]. The up-regulated bone morphogenetic proteins in the injury area may inhibit the oligodendrocyte differentiation and remyeliantion of endogenous or grafted oligodendrocyte precursor cells and promote their astrocyte differentiation[22,85,86]. This study suggests that type-2 astrocytes derived from oligodendrocyte precursor cells by bone morphogenetic protein signaling may be less supportive for axonal regeneration. Increased bone morphogenetic protein signaling in the injured area following CNS insults may inhibit axonal remyelination by blocking oligodendrocyte differentiation and maturation. It may also inhibit axonal regeneration by inducing oligodendrocyte precursor cells into lesser supportive type-2 astrocyte. Therefore, manipulation of bone morphogenetic protein signaling in the injured area may represent a novel therapeutic strategy to enhance axonal regeneration and remyelination[30].
In summary, our results show that glial precursor cells and their derivatives promote the neuronal survival and neurite regrowth likely by releasing growth factors and providing supportive substrates. These data suggest that transplantation of glial precursor cells could promote functional recovery by replacing the lost oligodendrocytes and enhancing the endogenous repairing. Our results also show that astrocytes differentiated from embryonic glial-restricted precursor cells and adult oligodendrocyte precursor cells by bone morphogenetic protein 4 have different effects on neurite growth in vitro. These results highlight the importance of choosing the optimal cell types or the proper developmental stage of cells for treating neurological diseases. Manipulating the bone morphogenetic protein signaling in the injured area may be critical to improve the therapeutic efficacy of endogenous or grafted oligodendrocyte precursor cells.
Materials and Methods
Design
A parallel controlled in vitro experiment.
Time and setting
Experiments were performed at the Laboratory of Neurosurgery, Medical School at Houston, University of Texas, USA from November 2009 to April 2010 and the Department of Human Anatomy and Neurobiology, Xiangya School of Medicine, Central South University, China from March to October 2012.
Materials
Five adult female Fisher 344 rats, weighing 140–160 g and aged 3 months, and Fisher 344 pregnant rats at embryonic day 14 (a brood of 12 rats) were purchased from Jackson Lab (Sacramento, CA, USA). All animals were housed at 25°C and relative humidity of 50–60% under a 12-hour light/dark cycle, and allowed free access to food and water. One week later, they were used for culture of primary cells. Animal experiments were approved by the Animal Welfare Committee and Biosafety Committee of the University of Texas Health Science Center, Houston, USA, and Animal Ethics Committee of Central South University, China.
Methods
Isolation and culture of oligodendrocyte precursor cells
Primary oligodendrocyte precursor cells were isolated from adult rat spinal cord using an immunopanning method as described previously[22,87]. A 100 mm diameter Petri dish (Corning Costar, Keller, TX, USA) was washed with Dulbecco phosphate-buffered saline (DPBS) in the culture hood. Donkey anti-mouse IgM or donkey anti-rabbit IgG (10 µg/mL dissolved in 0.05 mol/L Tris-HCl; Jackson Immuno Research West Grove, PA, USA) was added to ensure the bottom was completely covered, followed by coating at 4°C overnight. The next day, after removal of antibody and three washes with DPBS, A2B5 or RAN-2 antibody was added (stock solution; Research Center for Spinal Cord Injury, Louisville, KY, USA), incubated on a swing bed at room temperature for 2 hours, and washed three times with DPBS.
Adult Fisher 344 rats were intraperitoneally anesthetized with sodium pentobarbital (Nembutal, 50 mg/kg). The spinal cord was dissected and placed in an aseptic D-Hanks solution. Under an anatomical microscope (Olympus, Center Valley, PA, USA), blood vessels on the surface of spinal dura mater and spinal cord were carefully peeled off. The spinal cord was digested in Hanks solution supplemented with 0.1% papain, 0.1% neutral protease and 0.01% DNase at 37°C for 30 minutes. The digestion was terminated by adding 10% fetal bovine serum. After centrifugation at 500 × g for 15 minutes, digestion solution was removed, and 2 mL oligodendrocyte precursor cell culture medium was added. The sample was triturated with a 1 mL-sample-loading pipette tip. The oligodendrocyte precursor cell culture medium was composed of Dulbecco's modified Eagle's medium (DMEM)/F12 (Life Technologies, Grand Island, NY, USA) containing 0.5% N2 (Invitrogen, Carlsbad, CA, USA), 1% B27 (Invitrogen), 0.1% BSA (Sigma, St. Louis, MO, USA), 5 mg/mL insulin (Sigma), 20 ng/mL fibroblast growth factor-2 (Invitrogen), and 10 ng/mL PDGF-AA (Sigma). After addition to RAN-2-coated Petri dishes, the cell suspension was incubated in RAN2-coated Petri dishes in a swing bed at room temperature for 1 hour to get rid of astrocytes and fibrocytes and then in A2B5-coated Petri dishes in a swing bed at room temperature for 1 hour. After cell suspension was removed, the dishes were washed with DPBS to remove unconjugated floating cells. Oligodendrocyte precursor cells that adhered to the bottom of the Petri dish were collected with cell lifter, plated in PDL-laminin-coated cell culture dishes and cultured in oligodendrocyte precursor cell medium. The medium was replaced every other day. After cells were confluent, they were passaged. Passages 2 and 3 oligodendrocyte precursor cells were used for co-culture.
Differentiation of oligodendrocyte precursor cells in vitro
Passage 3 oligodendrocyte precursor cells were seeded in PDL/laminin-coated 24-well plates at 1 × 104 cells/well for 3 days. At 60% confluency, they were divided into two groups, which were differentiated in oligodendrocyte and type-2 astrocyte differentiation medium, respectively. Oligodendrocyte differentiation medium was composed of DMEM/F12 containing 0.5% N2, 1% B27, 0.1% bovine serum albumin, 5 mg/mL insulin, 30 ng/mL T3 (Sigma), and 10 ng/mL NT3 (Upstates, Billerica, MA, USA). Type-2 astrocyte differentiation medium (induced by bone morphogenetic protein-4) consisted of DMEM/F12 containing 0.5% N2, 1% B27, 0.1% bovine serum albumin, 5 mg/mL insulin, and 20 ng/mL bone morphogenetic protein-4 (R&D Systems Inc., Minneapolis, MN, USA).
Isolation and culture of glial-restricted precursor cells
Pregnant rats at embryonic day 14 were intraperitoneally anesthetized, and sterilized with 70% alcohol. The abdomen skin was cut and all embryos were obtained and placed in a Petri dish containing aseptic Leibovitz's L 15 medium (Gibco, Buffalo, NY, USA). The spinal cords of all fetal rats were dissected within 1 hour. Under an anatomical microscope, spinal dura mater was carefully peeled off. All spinal cords were cut into 1 mm-length segments, digested in 37°C preheated 0.05% trypsin (trypsin-ethylenediamine tetraacetic acid) for about 15 minutes, which was terminated by adding 10% fetal bovine serum. Following centrifugation at 500 × g for 15 minutes, the supernatant was removed. A total of 1 mL of culture solution for glial-restricted precursor cells composed of DMEM/F12 containing 0.5% N2, 1% B27, 20 ng/mL fibroblast growth factor-2 and 10 ng/mL PDGF-AA was added. The sample was triturated with a 1 mL-sample-loading pipette tip. Cell suspension was incubated in a RAN-2-coated Petri dish in a swing bed at room temperature for 1 hour and then in an A2B5-coated Petri dish in a swing bed at room temperature for another 1 hour. The unconjugated floating cells were removed by repeated washing of DPBS. The culture solutions for glial-restricted precursor cells were added, and cells that adhered to the bottom of the Petri dish were collected with cell lifter and proliferated in a FL-laminin-coated cell culture dish in an incubator. The medium was replaced every other day. After cells were confluent, they were passaged. Passages 1, 2 and 3 glial-restricted precursor cells were used in this study.
Harvesting of type-1 astrocyte progenitor cells
Type-1 astrocytes were differentiated from glial-restricted precursor cells using the same differentiation medium of type-2 astrocytes in the presence of bone morphogenetic protein-4, as described previously[46,88].
Isolation of dorsal root ganglion neurons
Embryos were obtained from pregnant rats, and the spinal column was opened to expose the spinal cord and bilateral dorsal root ganglions. Dorsal root ganglions were carefully removed with eye scissors, and the spinal dura mater was peeled off. Then dorsal root ganglions were digested in 0.25% trypsin (trypsin-ethylenediamine tetraacetic acid) at 37°C for 20 minutes, which was terminated by adding 10% fetal bovine serum. After centrifugation at 500 × g for 5 minutes and removal of trypsin, 1 mL dorsal root ganglion neuron culture medium was added. The dorsal root ganglion neuron culture medium was composed of neurobasal medium containing 1% B27, 100 mmol/L L-glutamine (Sigma), and 25 ng/mL nerve growth factor (R&D Systems Inc.). The sample was triturated with a 1 mL-sample-loading pipette tip and the sediment was discarded. Cell suspension was obtained and enough culture solution was added.
Co-culture of dorsal root ganglion neurons and cells
Dorsal root ganglion neurons were co-cultured with type-1 astrocytes, oligodendrocyte precursor cells, type-2 astrocytes, or without any cells, respectively. Dorsal root ganglion neurons were seeded in 24-well plates with one dorsal root ganglion in each well after the respective cells were 75–85% confluent. After 18 hours of co-culture in dorsal root ganglion neuron culture medium, the cells were fixed and then stained. The dorsal root ganglion neuron culture medium was composed of neurobasal medium supplemented with 1% B27, 100 mmol/L L-glutamine (Sigma), and 25 ng/mL nerve growth factor (R&D Systems Inc.). This culture medium was used in all groups. In the blank control group, dorsal root ganglion neurons were added into the wells without other cells. In the oligodendrocyte precursor cell group, after passage or thawing, cells were cultured in oligodendrocyte precursor cell medium for 2–3 days to reach the optimal confluence before co-culture.
Immunofluorescence technique
Living cells were incubated with the specific mouse antibodies, anti-A2B5, anti-O1, and anti-O4 (stock solution; Research Center for Spinal Cord Injury) at room temperature for 1 hour, fixed with 4% paraformaldehyde, at room temperature for 10–15 minutes, washed three times with PBS-T (0.01 mol/L PBS containing 0.1% Triton X-100), each for 5 minutes, and blocked in 10% donkey serum at room temperature for 1 hour. After washed with PBS-T, the cells were incubated with primary antibody (prepared with PBS-T containing 5% donkey serum) rabbit or chicken anti-glial fibrillary acidic protein (1:500; Chemicon, Billerica, MA, USA), rabbit anti-NF-M (1:200; Chemicon) at 4°C overnight. The cells were placed at room temperature for 10 minutes, washed four times with PBS-T, each for 10 minutes, and then incubated with fluorescently-labeled secondary antibody and Hoechst 3342 (prepared by PBS-T containing 5% donkey serum). Secondary antibodies were Texas Red- or FITC-labeled donkey anti-mouse IgM (mouse anti-A2B5, O1 or O4) (1:100; Jackson Immuno Research), Rhodamine-labeled donkey anti-rabbit IgG (rabbit anti-NF-M and rabbit anti-glial fibrillary acidic protein) (1:200; Jackson Immuno Research), Rhodamine- or FITC-labeled donkey anti-chicken IgG (chicken anti-glial fibrillary acidic protein) (1:200; Jackson Immuno Research) at room temperature in the dark for 1 hour. The cells were washed with 0.01 mol/L PBS, mounted with mounting medium, and then imaged with a fluorescence microscope (NIKON TE2000, Melville, NY, USA). All cells were quantified under a microscope using a 10 × eyepiece lens, moving in “S”-shape order. The percentage of positive cells was calculated by the number of positive cells by immunostaining/the number of cells by nuclear staining with Hoechst 3342 dye (i.e., total number of cells) × 100%. The length of neuronal processes was measured using NIS-ELEMENT BR software self-contained by the microscope.
Statistical analysis
The data were expressed as mean ± SD, and analyzed with SPSS 16.0 software (SPSS, Chicago, IL, USA). The mean value of many groups was compared with one-way analysis of variance. Multiple comparison among mean values was performed using the Duncan method. A value of P < 0.05 was considered statistically significant.
Footnotes
Funding: This study was supported by the NIH Foundation of the USA, No. R01 NS061975, and the Natural Science Foundation of Hunan Province in China, No. 11JJ6077.
Conflicts of interest: None declared.
Peer review: This study investigated effects of type-2 astrocytes differentiated from oligodendrocyte precursor cells induced by bone morphogenetic protein-4 on the growth of dorsal root ganglion neurons. Results suggest that type-2 astrocytes exhibited a weak promoting effect on the growth of dorsal root ganglion neurons. This study innovatively explored the source and type of proliferating astrocytes required for the repair of spinal cord injury, and the influential factors for oligodendrocyte precursor cells in remyelination. This study provided insight into a potential direction for the study of myelin reformation and glial cell proliferation after spinal cord injury.
Copyedited by Wallace M, Huang F, Jin CZ, Mu WJ, Qiu Y, Li CH, Song LP, Liu WJ, Zhao M
References
- [1].Dutta R, Trapp B. Mechanisms of neuronal dysfunction and degeneration in multiple sclerosis. Prog Neurobiol. 2011;93:1–12. doi: 10.1016/j.pneurobio.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Asou H, Hamada K, Miyazaki T. CNS myelin genesis in vitro: time course and pattern of rat oligodendrocyte development. J Neurosci Res. 1995;40:519–534. doi: 10.1002/jnr.490400411. [DOI] [PubMed] [Google Scholar]
- [3].Zoupi L, Sawaki M, Karagogeos D. Axons and myelinating glia: An intimate contact. IUBMB Life. 2011;63:730–735. doi: 10.1002/iub.513. [DOI] [PubMed] [Google Scholar]
- [4].Trapp BD, Peterson J, Ransohoff RM, et al. Axonal transection in the lesions of multiple sclerosis. N Engl J Med. 1998;338:278–285. doi: 10.1056/NEJM199801293380502. [DOI] [PubMed] [Google Scholar]
- [5].Bradl M, Lassmann H. Oligodendrocytes: biology and pathology. Acta neuropathologica. 2010;119:37–53. doi: 10.1007/s00401-009-0601-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Jadasz JJ, Aigner L, Rivera FJ, et al. The remyelination Philosopher's Stone: stem and progenitor cell therapies for multiple sclerosis. Cell Tissue Res. 2012;349:331–347. doi: 10.1007/s00441-012-1331-x. [DOI] [PubMed] [Google Scholar]
- [7].Watson RA, Yeung TM. What is the potential of oligodendrocyte progenitor cells to successfully treat human spinal cord injury? BMC Neurol. 2011;11:113. doi: 10.1186/1471-2377-11-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Mekhail M, Almazan G, Tabrizian M. Oligodendrocyte-protection and remyelination post-spinal cord injuries: A review. Prog Neurobiol. 2012;96:322–339. doi: 10.1016/j.pneurobio.2012.01.008. [DOI] [PubMed] [Google Scholar]
- [9].Targett M, Sussman J, Scolding N, et al. Failure to achieve remyelination of demyelinated rat axons following transplantation of glial cells obtained from the adult human brain. Neuropathol Appl Neurobiol. 1996;22:199–206. [PubMed] [Google Scholar]
- [10].Keirstead HS, Blakemore WF. Identification of post-mitotic oligodendrocytes incapable of remyelination within the demyelinated adult spinal cord. J Neuropathol Exp Neurol. 1997;56:1191–1201. doi: 10.1097/00005072-199711000-00003. [DOI] [PubMed] [Google Scholar]
- [11].Einstein O, Friedman-Levi Y, Grigoriadis N, et al. Transplanted neural precursors enhance host brain-derived myelin regeneration. J Neurosci. 2009;29:15694–15702. doi: 10.1523/JNEUROSCI.3364-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Gensert JA, Goldman JE. Endogenous progenitors remyelinate demyelinated axons in the adult CNS. Neuron. 1997;19:197–203. doi: 10.1016/s0896-6273(00)80359-1. [DOI] [PubMed] [Google Scholar]
- [13].Pluchino S, Quattrini A, Brambilla E, et al. Injection of adult neurospheres induces recovery in a chronic model of multiple sclerosis. Nature. 2003;422:688–694. doi: 10.1038/nature01552. [DOI] [PubMed] [Google Scholar]
- [14].Levine JM, Reynolds R, Fawcett JW. The oligodendrocyte precursor cell in health and disease. Trends Neurosci. 2001;24:39–47. doi: 10.1016/s0166-2236(00)01691-x. [DOI] [PubMed] [Google Scholar]
- [15].Kremer D, Aktas O, Hartung HP, et al. The complex world of oligodendroglial differentiation inhibitors. Ann Neurol. 2011;69:602–618. doi: 10.1002/ana.22415. [DOI] [PubMed] [Google Scholar]
- [16].Cao Q, He Q, Wang Y, et al. Transplantation of Ciliary Neurotrophic Factor-Expressing Adult Oligodendrocyte Precursor Cells Promotes Remyelination and Functional Recovery after Spinal Cord Injury. J Neurosci. 2010;30:2989–3001. doi: 10.1523/JNEUROSCI.3174-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Hansmann F, Pringproa K, Ulrich R, et al. Highly malignant behavior of a murine oligodendrocyte precursor cell line following transplantation into the demyelinated and nondemyelinated central nervous system. Cell Transplant. 2012;21:1161–1175. doi: 10.3727/096368911X627444. [DOI] [PubMed] [Google Scholar]
- [18].Plemel JR, Chojnacki A, Sparling JS, et al. Platelet‐derived growth factor‐responsive neural precursors give rise to myelinating oligodendrocytes after transplantation into the spinal cords of contused rats and dysmyelinated mice. Glia. 2011;59:1891–1910. doi: 10.1002/glia.21232. [DOI] [PubMed] [Google Scholar]
- [19].Uccelli A, Mancardi G. Stem cell transplantation in multiple sclerosis. Curr Opin Neurol. 2010;23:218–225. doi: 10.1097/WCO.0b013e328338b7ed. [DOI] [PubMed] [Google Scholar]
- [20].Ishii K, Toda M, Nakai Y, et al. Increase of oligodendrocyte progenitor cells after spinal cord injury. J Neurosci Res. 2001;65:500–507. doi: 10.1002/jnr.1180. [DOI] [PubMed] [Google Scholar]
- [21].Kotter MR, Stadelmann C, Hartung HP. Enhancing remyelination in disease—can we wrap it up? Brain. 2011;134:1882–1900. doi: 10.1093/brain/awr014. [DOI] [PubMed] [Google Scholar]
- [22].Kuhlmann T, Miron V, Cuo Q, et al. Differentiation block of oligodendroglial progenitor cells as a cause for remyelination failure in chronic multiple sclerosis. Brain. 2008;131:1749–1758. doi: 10.1093/brain/awn096. [DOI] [PubMed] [Google Scholar]
- [23].Miron VE, Kuhlmann T, Antel JP. Cells of the oligodendroglial lineage, myelination, and remyelination. Biochim Biophys Acta. 2011;1812:184–193. doi: 10.1016/j.bbadis.2010.09.010. [DOI] [PubMed] [Google Scholar]
- [24].Piaton G, Williams A, Seilhean D, et al. Remyelination in multiple sclerosis. Prog Brain Res. 2009;175:453–464. doi: 10.1016/S0079-6123(09)17530-1. [DOI] [PubMed] [Google Scholar]
- [25].John GR, Shankar SL, Shafit-Zagardo B, et al. Multiple sclerosis: re-expression of a developmental pathway that restricts oligodendrocyte maturation. Nat Med. 2002;8:1115–1121. doi: 10.1038/nm781. [DOI] [PubMed] [Google Scholar]
- [26].Wang Y, Cheng X, He Q, et al. Astrocytes from the contused spinal cord inhibit oligodendrocyte differentiation of adult oligodendrocyte precursor cells by increasing the expression of bone morphogenetic proteins. J Neurosci. 2011;31:6053–6058. doi: 10.1523/JNEUROSCI.5524-09.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Kondo T, Raff M. Oligodendrocyte precursor cells reprogrammed to become multipotential CNS stem cells. Science. 2000;289:1754–1757. doi: 10.1126/science.289.5485.1754. [DOI] [PubMed] [Google Scholar]
- [28].Nishiyama A, Yu M, Drazba J, et al. Normal and reactive NG2+ glial cells are distinct from resting and activated microglia. J Neurosci Res. 1997;48:299–312. doi: 10.1002/(sici)1097-4547(19970515)48:4<299::aid-jnr2>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- [29].Hall AK, Miller RH. Emerging roles for bone morphogenetic proteins in central nervous system glial biology. J Neurosci Res. 2004;76:1–8. doi: 10.1002/jnr.20019. [DOI] [PubMed] [Google Scholar]
- [30].Hampton DW, Asher RA, Kondo T, et al. A potential role for bone morphogenetic protein signalling in glial cell fate determination following adult central nervous system injury in vivo. Eur J Neurosci. 2007;26:3024–3035. doi: 10.1111/j.1460-9568.2007.05940.x. [DOI] [PubMed] [Google Scholar]
- [31].Sabo JK, Aumann TD, Merlo D, et al. Remyelination is altered by bone morphogenic protein signaling in demyelinated lesions. J Neurosci. 2011;31:4504–4510. doi: 10.1523/JNEUROSCI.5859-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Sabo J, Kilpatrick T, Cate H. Effects of bone morphogenic proteins on neural precursor cells and regulation during central nervous system injury. Neurosignals. 2009;17:255–264. doi: 10.1159/000231892. [DOI] [PubMed] [Google Scholar]
- [33].Mabie P, Mehler M, Marmur R, et al. Bone morphogenetic proteins induce astroglial differentiation of oligodendroglial-astroglial progenitor cells. J Neurosci. 1997;17:4112–4120. doi: 10.1523/JNEUROSCI.17-11-04112.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Hu JG, Lü HZ, Wang YX, et al. BMP signaling mediates astrocyte differentiation of oligodendrocyte progenitor cells. Tohoku J Exp Med. 2010;222:195–200. doi: 10.1620/tjem.222.195. [DOI] [PubMed] [Google Scholar]
- [35].Gregori N, Pröschel C, Noble M, et al. The tripotential glial-restricted precursor (GRP) cell and glial development in the spinal cord: generation of bipotential oligodendrocyte-type-2 astrocyte progenitor cells and dorsal-ventral differences in GRP cell function. J Neurosci. 2002;22:248–256. doi: 10.1523/JNEUROSCI.22-01-00248.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Herrera J, Yang H, Zhang SC, et al. Embryonic-derived glial-restricted precursor cells (GRP cells) can differentiate into astrocytes and oligodendrocytes in vivo. Exp Neurol. 2001;171:11–21. doi: 10.1006/exnr.2001.7729. [DOI] [PubMed] [Google Scholar]
- [37].Rao MS, Noble M, Mayer-Pröschel M. A tripotential glial precursor cell is present in the developing spinal cord. Proc Natl Acad Sci U S A. 1998;95:3996–4001. doi: 10.1073/pnas.95.7.3996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Raff M, Abney E, Cohen J, et al. Two types of astrocytes in cultures of developing rat white matter: differences in morphology, surface gangliosides, and growth characteristics. J Neurosci. 1983;3:1289–1300. doi: 10.1523/JNEUROSCI.03-06-01289.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Davies J, Huang C, Proschel C, et al. Astrocytes derived from glial-restricted precursors promote spinal cord repair. J Biol. 2006;5:7. doi: 10.1186/jbiol35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Fan C, Zheng Y, Cheng X, et al. Transplantation of D15A-expressing glial-restricted-precursor-derived astrocytes improves anatomical and locomotor recovery after spinal cord injury. Int J Biol Sci. 2013;9:78–93. doi: 10.7150/ijbs.5626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Miller RH. Building bridges with astrocytes for spinal cord repair. J Biol. 2006;5:6. doi: 10.1186/jbiol40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Lü HZ, Wang YX, Zou J, et al. Differentiation of neural precursor cell-derived oligodendrocyte progenitor cells following transplantation into normal and injured spinal cords. Differentiation. 2010;80:228–240. doi: 10.1016/j.diff.2010.09.179. [DOI] [PubMed] [Google Scholar]
- [43].Cate HS, Sabo JK, Merlo D, et al. Modulation of bone morphogenic protein signalling alters numbers of astrocytes and oligodendroglia in the subventricular zone during cuprizone-induced demyelination. J Neurochem. 2010;115:11–22. doi: 10.1111/j.1471-4159.2010.06660.x. [DOI] [PubMed] [Google Scholar]
- [44].Ketschek AR, Haas C, Gallo G, et al. The roles of neuronal and glial precursors in overcoming chondroitin sulfate proteoglycan inhibition. Exp Neurol. 2012;235:627–637. doi: 10.1016/j.expneurol.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Haas C, Neuhuber B, Yamagami T, et al. Phenotypic analysis of astrocytes derived from glial restricted precursors and their impact on axon regeneration. Exp Neurol. 2012;233:717–732. doi: 10.1016/j.expneurol.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Davies JE, Huang C, Proschel C, et al. Astrocytes derived from glial-restricted precursors promote spinal cord repair. J Biol. 2006;5:7. doi: 10.1186/jbiol35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Wyatt TJ, Keirstead HS. Stem cell-derived neurotrophic support for the neuromuscular junction in spinal muscular atrophy. Expert Opin Biol Ther. 2010;10:1587–1594. doi: 10.1517/14712598.2010.529895. [DOI] [PubMed] [Google Scholar]
- [48].Sharp J, Frame J, Siegenthaler M, et al. Human embryonic stem cell-derived oligodendrocyte progenitor cell transplants improve recovery after cervical spinal cord injury. Stem Cells. 2010;28:152–163. doi: 10.1002/stem.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Rossi SL, Nistor G, Wyatt T, et al. Histological and functional benefit following transplantation of motor neuron progenitors to the injured rat spinal cord. PLoS One. 2010;5:e11852. doi: 10.1371/journal.pone.0011852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Lepore AC, O’Donnell J, Kim AS, et al. Human glial-restricted progenitor transplantation into cervical spinal cord of the SOD1 mouse model of ALS. PLoS One. 2011;6:e25968. doi: 10.1371/journal.pone.0025968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Lepore AC, Rauck B, Dejea C, et al. Focal transplantation-based astrocyte replacement is neuroprotective in a model of motor neuron disease. Nat Neurosci. 2008;11:1294–1301. doi: 10.1038/nn.2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Sharp J, Frame J, Siegenthaler M, et al. Human embryonic stem cell-derived oligodendrocyte progenitor cell transplants improve recovery after cervical spinal cord injury. Stem Cells. 2010;28:152–163. doi: 10.1002/stem.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Haas C, Fischer I. Human astrocytes derived from glial restricted progenitors support regeneration of the injured spinal cord. J Neurotrauma. 2013;30:1035–1052. doi: 10.1089/neu.2013.2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Davies SJ, Shih CH, Noble M, et al. Transplantation of specific human astrocytes promotes functional recovery after spinal cord injury. PLoS One. 2011;6:e17328. doi: 10.1371/journal.pone.0017328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Andres RH, Horie N, Slikker W, et al. Human neural stem cells enhance structural plasticity and axonal transport in the ischaemic brain. Brain. 2011;134:1777–1789. doi: 10.1093/brain/awr094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Noble M, Mayer-Proschel M, Davies JE, et al. Cell therapies for the central nervous system: how do we identify the best candidates? Curr Opin Neurol. 2011;24:570–576. doi: 10.1097/WCO.0b013e32834cd4c9. [DOI] [PubMed] [Google Scholar]
- [57].Liedtke W, Edelmann W, Bieri PL, et al. GFAP is necessary for the integrity of CNS white matter architecture and long-term maintenance of myelination. Neuron. 1996;17:607–615. doi: 10.1016/s0896-6273(00)80194-4. [DOI] [PubMed] [Google Scholar]
- [58].Franklin RJ, Crang AJ, Blakemore WF. The role of astrocytes in the remyelination of glia-free areas of demyelination. Adv Neurol. 1993;59:125–133. [PubMed] [Google Scholar]
- [59].Franklin RJ. Why does remyelination fail in multiple sclerosis? Nat Rev Neurosci. 2002;3:705–714. doi: 10.1038/nrn917. [DOI] [PubMed] [Google Scholar]
- [60].Kliot M, Smith GM, Siegal JD, et al. Astrocyte-polymer implants promote regeneration of dorsal root fibers into the adult mammalian spinal cord. Exp Neurol. 1990;109:57–69. doi: 10.1016/s0014-4886(05)80008-1. [DOI] [PubMed] [Google Scholar]
- [61].Wunderlich G, Stichel CC, Schroeder WO, et al. Transplants of immature astrocytes promote axonal regeneration in the adult rat brain. Glia. 1994;10:49–58. doi: 10.1002/glia.440100107. [DOI] [PubMed] [Google Scholar]
- [62].Smith GM, Rutishauser U, Silver J, et al. Maturation of astrocytes in vitro alters the extent and molecular basis of neurite outgrowth. Dev Biol. 1990;138:377–390. doi: 10.1016/0012-1606(90)90204-v. [DOI] [PubMed] [Google Scholar]
- [63].Wang JJ, Chuah MI, Yew DT, et al. Effects of astrocyte implantation into the hemisected adult rat spinal cord. Neuroscience. 1995;65:973–981. doi: 10.1016/0306-4522(94)00519-b. [DOI] [PubMed] [Google Scholar]
- [64].Song H, Stevens CF, Gage FH. Astroglia induce neurogenesis from adult neural stem cells. Nature. 2002;417:39–44. doi: 10.1038/417039a. [DOI] [PubMed] [Google Scholar]
- [65].Davies JE, Proschel C, Zhang N, et al. Transplanted astrocytes derived from BMP- or CNTF-treated glial-restricted precursors have opposite effects on recovery and allodynia after spinal cord injury. J Biol. 2008;7:24. doi: 10.1186/jbiol85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Sofroniew MV, Vinters HV. Astrocytes: biology and pathology. Acta Neuropathol. 2010;119:7–35. doi: 10.1007/s00401-009-0619-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Verkhratsky A, Sofroniew MV, Messing A, et al. Neurological diseases as primary gliopathies: a reassessment of neurocentrism. ASN Neuro. 2012:4. doi: 10.1042/AN20120010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Hamby ME, Sofroniew MV. Reactive astrocytes as therapeutic targets for CNS disorders. Neurotherapeutics. 2010;7:494–506. doi: 10.1016/j.nurt.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Verkhratsky A, Rodriguez JJ, Parpura V. Astroglia in neurological diseases. Future Neurol. 2013;8:149–158. doi: 10.2217/fnl.12.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Herrmann JE, Imura T, Song B, et al. STAT3 is a critical regulator of astrogliosis and scar formation after spinal cord injury. J Neurosci. 2008;28:7231–7243. doi: 10.1523/JNEUROSCI.1709-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Li L, Lundkvist A, Andersson D, et al. Protective role of reactive astrocytes in brain ischemia. Cereb Blood Flow Metab. 2008;28:468–481. doi: 10.1038/sj.jcbfm.9600546. [DOI] [PubMed] [Google Scholar]
- [72].Vargas MR, Johnson DA, Sirkis DW, et al. Nrf2 activation in astrocytes protects against neurodegeneration in mouse models of familial amyotrophic lateral sclerosis. J Neurosci. 2008;28:13574–13581. doi: 10.1523/JNEUROSCI.4099-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Rothstein JD, Dykes-Hoberg M, Pardo CA, et al. Knockout of glutamate transporters reveals a major role for astroglial transport in excitotoxicity and clearance of glutamate. Neuron. 1996;16:675–686. doi: 10.1016/s0896-6273(00)80086-0. [DOI] [PubMed] [Google Scholar]
- [74].Bush TG, Puvanachandra N, Horner CH, et al. Leukocyte infiltration, neuronal degeneration, and neurite outgrowth after ablation of scar-forming, reactive astrocytes in adult transgenic mice. Neuron. 1999;23:297–308. doi: 10.1016/s0896-6273(00)80781-3. [DOI] [PubMed] [Google Scholar]
- [75].Silver J, Miller JH. Regeneration beyond the glial scar. Nat Rev Neurosci. 2004;5:146–156. doi: 10.1038/nrn1326. [DOI] [PubMed] [Google Scholar]
- [76].Fawcett JW, Asher RA. The glial scar and central nervous system repair. Brain Res Bull. 1999;49:377–391. doi: 10.1016/s0361-9230(99)00072-6. [DOI] [PubMed] [Google Scholar]
- [77].Marignier R, Nicolle A, Watrin C, et al. Oligodendrocytes are damaged by neuromyelitis optica immunoglobulin G via astrocyte injury. Brain. 2010;133:2578–2591. doi: 10.1093/brain/awq177. [DOI] [PubMed] [Google Scholar]
- [78].Buffo A, Rite I, Tripathi P, et al. Origin and progeny of reactive gliosis: A source of multipotent cells in the injured brain. Proc Natl Acad Sci U S A. 2008;105:3581–3586. doi: 10.1073/pnas.0709002105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Guo F, Maeda Y, Ma J, et al. Macroglial plasticity and the origins of reactive astroglia in experimental autoimmune encephalomyelitis. J Neurosci. 2011;31:11914–11928. doi: 10.1523/JNEUROSCI.1759-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Nishiyama A. Polydendrocytes: NG2 cells with many roles in development and repair of the CNS. Neuroscientist. 2007;13:62–76. doi: 10.1177/1073858406295586. [DOI] [PubMed] [Google Scholar]
- [81].Dawson MRL, Polito A, Levine JM, et al. NG2-expressing glial progenitor cells: an abundant and widespread population of cycling cells in the adult rat CNS. Mol Cell Neurosci. 2003;24:476–488. doi: 10.1016/s1044-7431(03)00210-0. [DOI] [PubMed] [Google Scholar]
- [82].Horner PJ, Power AE, Kempermann G, et al. Proliferation and differentiation of progenitor cells throughout the intact adult rat spinal cord. J Neurosci. 2000;20:2218–2228. doi: 10.1523/JNEUROSCI.20-06-02218.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Ara J, See J, Mamontov P, et al. Bone morphogenetic proteins 4, 6, and 7 are up-regulated in mouse spinal cord during experimental autoimmune encephalomyelitis. J Neurosci Res. 2008;86:125–135. doi: 10.1002/jnr.21462. [DOI] [PubMed] [Google Scholar]
- [84].Sahni V, Mukhopadhyay A, Tysseling V, et al. BMPR1a and BMPR1b signaling exert opposing effects on gliosis after spinal cord injury. J Neurosci. 2010;30:1839–1855. doi: 10.1523/JNEUROSCI.4459-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Mabie PC, Mehler MF, Marmur R, et al. Bone morphogenetic proteins induce astroglial differentiation of oligodendroglial-astroglial progenitor cells. J Neurosci. 1997;17:4112–4120. doi: 10.1523/JNEUROSCI.17-11-04112.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Grinspan JB, Edell E, Carpio DF, et al. Stage-specific effects of bone morphogenetic proteins on the oligodendrocyte lineage. J Neurobiol. 2000;43:1–17. [PubMed] [Google Scholar]
- [87].Cheng X, Wang Y, He Q, et al. Bone morphogenetic protein signaling and olig1/2 interact to regulate the differentiation and maturation of adult oligodendrocyte precursor cells. Stem Cells. 2007;25:3204–3214. doi: 10.1634/stemcells.2007-0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Cao Q, Xu XM, DeVries WH, et al. Functional recovery in traumatic spinal cord injury after transplantation of multineurotrophin-expressing glial-restricted precursor cells. J Neurosci. 2005;25:6947–6957. doi: 10.1523/JNEUROSCI.1065-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]


