Abstract
Percutaneous microballoon compression of the trigeminal ganglion is a brand new operative technique for the treatment of trigeminal neuralgia. However, it is unclear how the procedure mediates pain relief, and there are no standardized criteria, such as compression pressure, compression time or balloon shape, for the procedure. In this study, percutaneous microballoon compression was performed on the rabbit trigeminal ganglion at a mean inflation pressure of 1,005 ± 150 mmHg for 2 or 5 minutes. At 1, 7 and 14 days after percutaneous microballoon compression, the large-diameter myelinated nerves displayed axonal swelling, rupture and demyelination under the electron microscope. Fragmentation of myelin and formation of digestion chambers were more evident after 5 minutes of compression. Image analyzer results showed that the diameter of trigeminal ganglion cells remained unaltered after compression. These experimental findings indicate that a 2-minute period of compression can suppress pain transduction. Immunohistochemical staining revealed that vascular endothelial growth factor expression in the ganglion cells and axons was significantly increased 7 days after trigeminal ganglion compression, however, the changes were similar after 2-minute compression and 5-minute compression. The upregulated expression of vascular endothelial growth factor in the ganglion cells after percutaneous microballoon compression can promote the repair of the injured nerve. These findings suggest that long-term compression is ideal for patients with recurrent trigeminal neuralgia.
Keywords: nerve regeneration, peripheral nerve injury, trigeminal neuralgia, percutaneous microballoon compression, trigeminal ganglion cell, demyelination, axons, vascular endothelial growth factor, neural regeneration
Introduction
Trigeminal neuralgia is a functional neurological disease defined as “sudden, unilateral, severe, brief, stabbing, recurrent episodes of pain in the distribution of one or more branches of the trigeminal nerve”[1,2,3,4]. Approximately 30–50% of patients have suicidal tendencies due to the pain. As a predominantly quinquagenarian ailment, the prevalence of trigeminal neuralgia increases with age. Recent epidemiological surveys[5,6] show higher incidences of trigeminal neuralgia, with 26.8 and 28.9 per 100,000 in the United Kingdom and the Netherlands, respectively.
Craniotomy followed by microvascular decompression is a conventional treatment approach, but is associated with severe complications, including morbidity and mortality. Percutaneous microballoon compression on the trigeminal ganglion is a brand new operation technique for trigeminal neuralgia treatment[7,8,9,10,11]. Because of minimal invasiveness, safety, high efficacy, shortened procedure time and no pain during operation, percutaneous microballoon compression has been widely accepted and preferred in many treatment centers.
Trigeminal nerve demyelination is regarded as the mechanistic cause of trigeminal neuralgia. In early 1934, Dandy reported that trigeminal neuralgia was caused by deformation of the trigeminal nerve under compression. Jannetta[12] found that trigeminal neuralgia is triggered by pulse compression of the root of the trigeminal nerve within the root entry zone, where the central and peripheral myelin sheaths connect with no Schwann cell encapsulation. This zone is extremely sensitive to pulse compression and susceptible to microvascular compression. With age, the cerebrum gradually descends, and the blood vessels begin to contact the root entry zone, thus resulting in microvascular compression. In addition, arteriosclerosis aggravates the compression. In 1967, Kerr[13] reported that trigeminal nerve demyelination was the main pathological change associated with trigeminal neuralgia. Trigeminal neuralgia mainly occurs in the middle-aged and aged populations, accompanying the arteriosclerosis. When the supporting artery of the trigeminal nerve becomes sclerotic or ischemic, the myelin sheaths deteriorate, causing demyelination, which results in a short circuit between the nerve fibers. As a consequence, nerve impulses are conducted centrally, where they induce pain sensation. Specifically, demyelination leads to a conduction fault between non-nociceptive Aβ fibers, nociceptive Aδ fibers and C fibers, thereby triggering neurons in the spinal nucleus of the trigeminal nerve. The afferent impulses then cause pain. In addition, efferent impulses can be converted to afferent impulses after demyelination; the spinal tract nucleus and thalamus receive an increased amount of activity, resulting in pain. The main pathological change in the peripheral nerve in patients with trigeminal neuralgia is Aβ fiber demyelination, which increases the proportion of Aδ and C fibers and decreases the inhibition on fine fibers. The sustained afferent impulses promote pain. However, there is no effective treatment for repairing the partially demyelinated nerve to relieve pain.
Percutaneous microcompression can selectively compress the demyelinated Aβ fiber without damaging the axons. It also blocks the abnormally discharging “short circuit”. Subsequently, new myelin sheath is generated, contributing to pain relief. However, there are no uniform criteria for percutaneous microcompression, such as compression pressure, compression time or balloon shape[14,15,16,17]. In particular, the links between compression duration and postoperative complications and pain recurrence are still under debate. An extended duration of compression would cause irreversible injury and drastic demyelination, and paresthesia and numbness with discomfort would ensue. In comparison, a short compression time may reduce postoperative complications. However, the rate of pain relief diminishes and the recurrence rate elevates. This may require a second operation and increase psychological and economic burdens on the patient. However, it remains a challenge to define optimal compression time, guarantee operative success, and reduce the complication rate and X-ray exposure.
Abdennebi and colleagues[18] operated on 200 patients using 6-minute percutaneous microballoon compression. The 51-month follow-up results showed that the incidence of severe sensory complications was 3%, and moderate dysesthesia was found in 15 patients (7.5%). Skirving and Dan[10] used compression times ranging between 2 and 7 minutes, but did not measure intraluminal balloon pressure. The recurrence rate was 19.2% (95 patients) within 5 years and 31.9% within 10 years. Early facial numbness occurred in 89% patients, and symptomatic dysesthesia in 3.8% patients. Seventy patients (3.4%) had symptomatic masseter weakness. Brown and Pilitsis[11] reported that immediately after percutaneous microballoon compression, 83% of patients developed mild facial numbness. At the most recent postoperative visit, 17% of patients reported persistent numbness, and no subjects described moderate or severe numbness. Two patients (4%) reported minor dysesthesia. Mild masseter muscle weakness was reported in 24% of patients.
The percutaneous microballoon compression procedure is generally performed using X-ray imaging. The operation requires the use of fluoroscopy to navigate the needle through the foramen ovale and to monitor balloon inflation and deflation. However, the safe level of radiation exposure for this technique remains unclear. Both surgeons and patients are exposed to the harmful radiation. The currently adopted time for compression is 3–6 minutes. Fransen[19] proposed that the dose and duration of radiation during percutaneous compression of the trigeminal ganglion should be seriously considered. However, he did not determine the optimal period of compression.
There is little research on the correlation between percutaneous microballoon compression duration and postoperative sensory complications and pain recurrence. Thus, this study aimed to determine the optimal compression duration for percutaneous microballoon compression, in an attempt to provide concrete evidence to establish an international standard for the technique.
In this study, percutaneous microballoon compression was performed on the rabbit trigeminal ganglion. The preliminary experiments and findings of Brown and colleagues[16] showed that the large myelinated nerve begins to demyelinate under a pressure of 133.7 ± 20.0 kPa (1,005 ± 150 mmHg). Two- and 5-minute periods of compression at this pressure could lead to damage to the trigeminal nerve. The rabbits were sacrificed at 1, 7 or 14 days after percutaneous microcompression to observe the pathological changes in the trigeminal ganglion, and to assess the diameter of trigeminal ganglion cells, changes in trigeminal nerve demyelination, and vascular endothelial growth factor expression in ganglion cells and axons.
This study has five major aims: (1) To determine if the rabbit is suitable for evaluating technical procedures in percutaneous microcompression technology; (2) To examine if nerve demyelination can be observed during percutaneous microcompression; (3) To assess if nerve demyelination can be affected by reducing compression time; (4) To examine changes in trigeminal cell diameter after compression; and (5) To evaluate changes in vascular endothelial growth factor expression in the trigeminal cell body and axon after compression.
In brief, we established an animal model of trigeminal neuralgia using percutaneous microballoon compression on the trigeminal ganglion, with the aim of observing changes in the trigeminal ganglion and the demyelination of myelinated nerve fibers under a compression pressure of 1,005 ± 150 mmHg. Vascular endothelial growth factor expression in the trigeminal cell bodies and axons was examined with immunohistochemical staining.
Results
Quantitative analysis of experimental animals
A total of 36 New Zealand white rabbits were randomly divided into normal control (n = 6), 2-minute compression (n = 15) and 5-minute compression (n = 15) groups. Percutaneous microballoon compression was applied to the rabbit trigeminal ganglion for 2 and 5 minutes at 1,005 ± 150 mmHg pressure. Three rabbits died due to infection and were replaced with additional rabbits. A total of 36 rabbits were included in the final analysis.
Histomorphological changes in the trigeminal ganglion and in the diameter of ganglion cells after percutaneous microballoon compression
Histological sections of the trigeminal ganglia and roots were prepared from rabbits at 1, 7 and 14 days after percutaneous microballoon compression, and were observed under the light microscope (Figure 1). The trigeminal ganglion cells were analyzed with an image analysis system. Because the myelin sheath contains phospholipids, it is stained negative by hematoxylin-eosin. In comparison, the ganglion cell bodies were round and dyed red, while the nuclei were dyed a deep red. Most perikarya in the ganglion appeared normal and were stained (basophilic). There was no significant difference in diameter of the trigeminal ganglion cells among the 2-minute compression group, the 5-minute compression group and the normal group (P > 0.05; Figure 2). Thus, the morphology of the trigeminal ganglion cells was not significantly affected by the 2- or 5-minute compression period.
Figure 1.
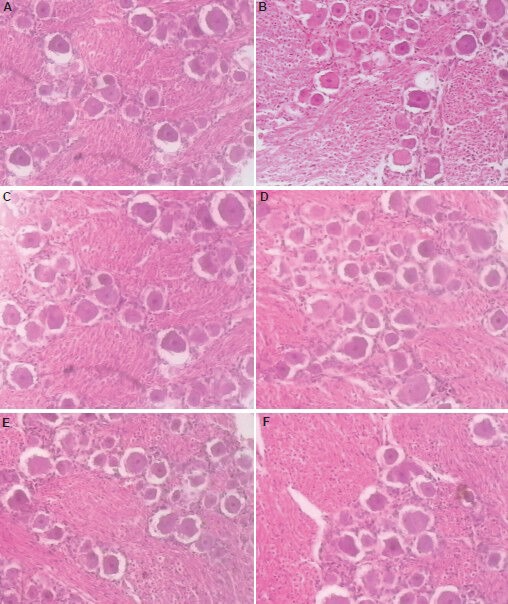
Morphology of trigeminal ganglion cells and axons after percutaneous microballoon compression (hematoxylin-eosin staining, × 100).
Normal group (A) and 2-minute compression group (B–D) at 1, 7 and 14 days after percutaneous microballoon compression; (E, F) 5-minute compression group at 7 and 14 days after percutaneous microballoon compression. Cell bodies are dyed red and the nucleus is deeply red, while the myelin is unstained. The cell bodies are round. Ganglion cell bodies and mean diameters showed no significant difference when compared with the normal group.
Figure 2.
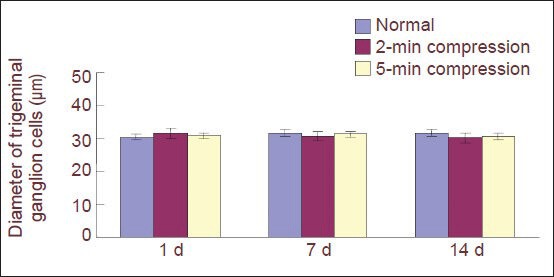
Effect of percutaneous microballoon compression on the diameter of trigeminal ganglion cells.
Data are expressed as mean ± SD. There are two, three and three rabbits, respectively, in the normal, 2-minute (min) compression and 5-min compression groups at 1, 7, 14 days (d) after percutaneous microballoon compression. No significant difference in diameter was observed among the 2-min compression, 5-min compression and normal groups (P> 0.05) using one-way analysis of variance.
Ultrastructure of the myelin sheath in the trigeminal nerve root after percutaneous microballoon compression
Normal myelin was arranged as homocentric circles, and the layering of the sheath was regular and orderly (Figure 3A). In the 2-minute compression group, the large-diameter myelinated axons were swollen, disrupted and demyelinated 7 days after percutaneous microballoon compression, as revealed under the electron microscope.
Figure 3.
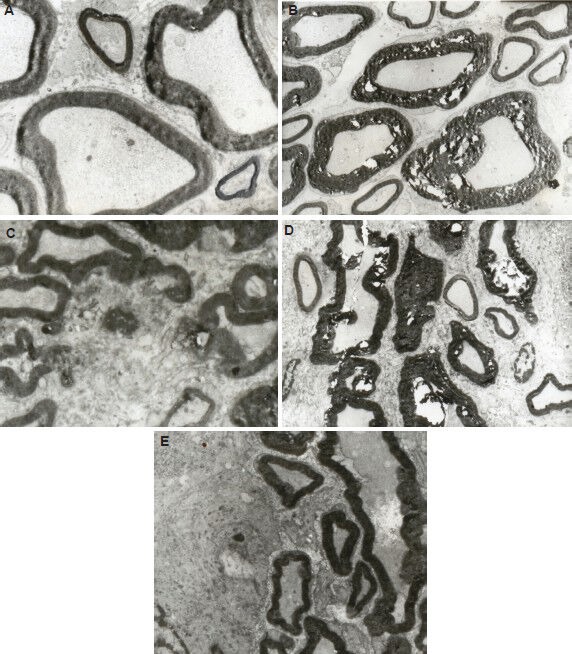
Effect of percutaneous microballoon compression on the ultrastructure of the trigeminal root at 7 and 14 days (d) after percutaneous microballoon compression (transmission electron microscope, × 3,000).
(A) In the normal group, myelin was arranged as homocentric circles and the stacking of the sheath was normal.
(B) In the 2-minute compression group at 7 days, large-diameter myelinated nerve axons were swollen, disrupted and demyelinated. Fragmentation of myelin and formation of digestion chambers were evident.
(C) In the 5-minute compression group at 7 days, part of the myelin had completely disintegrated into fragments. Significant demyelination was apparent.
(D) In the 2-minute compression group at 14 days, large-diameter myelinated nerve axons were strongly demyelinated and deformed. Large digestion chambers were evident.
(E) In the 5-minute compression group at 14 days, the myelin fragments were partially resorbed. Deformation of the large-diameter axons was apparent.
Axonal swelling and fragmentation were uniformly present (Figure 3B). At 14 days after compression, fragmentation of myelin and formation of digestion chambers were evident (Figure 3D). In the 5-minute compression group, gross demyelination in the root was detected 7 days after percutaneous microballoon compression. A portion of the myelin had completely disintegrated into fragments, indicating significant demyelination (Figure 3C). At 14 days after compression, some of these fragments had been absorbed, but deformation of the large myelinated fibers could still be observed (Figure 3E). These changes were not seen in the roots or ganglia in the normal group. These changes are indicative of Wallerian degeneration and retrograde axonal degeneration.
Vascular endothelial growth factor immunoreactivity in trigeminal ganglion cells after percutaneous microballoon compression
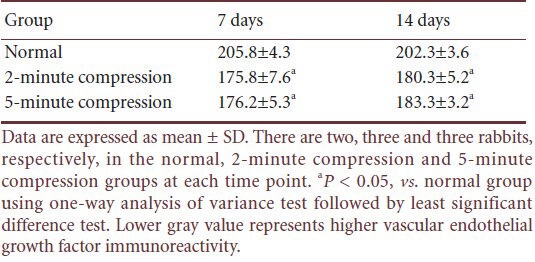
There was negative or weak vascular endothelial growth factor immunoreactivity in normal trigeminal ganglion cells. Immunohistochemical staining showed that vascular endothelial growth factor immunoreactivity in the ganglion cell bodies was reduced 7 and 14 days after trigeminal ganglion compression. There was no statistically significant difference between the 2- and 5-minute compression groups (P > 0.05). In the normal group, vascular endothelial growth factor immunoreactivity in the ganglion cell body was lower than in the compression groups (P < 0.05). These experimental findings indicate that vascular endothelial growth factor immunoreactivity is upregulated following compression (Figure 4; Table 1).
Figure 4.
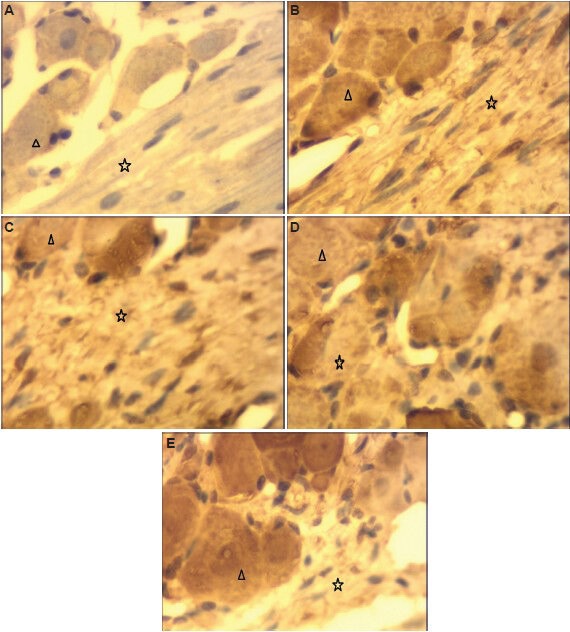
Effect of percutaneous microballoon compression on vascular endothelial growth factor immunoreactivity in trigeminal ganglion cells (immunohistochemical staining, light microscope, × 400).
Trigeminal ganglion cell bodies and axons were negative (no brown spots) in the normal group (A), but there were numerous brown spots in cell bodies and axons in the 2-minute compression group (B: at 7 days; D: at 14 days) and in the 5-minute compression group (C: at 7 days; E: at 14 days). Triangle and star represent cell body and axon, respectively.
Table 1.
Vascular endothelial growth factor immunoreactivity (gray value) in the trigeminal ganglion cell bodies in rabbits at 7 and 14 days after percutaneous microballoon compression
Discussion
Percutaneous microballoon compression was first reported in 1983 by Mullan and Lichtor[20]. Subsequently, it became one of the most widely used alternative therapeutic approaches because of its high (93–99%) initial pain relief[21,22,23,24,25], easy application, and low morbidity[24,26,27,28]. Unlike glycerol injection and radiofrequency lesioning, which require a cooperative patient[29,30], percutaneous balloon compression is based on timed balloon inflation guided with radiographic imaging, and may be performed under general anesthesia.
Because the surgery may be performed under general anesthesia, it is painless to the patient during the entire procedure and is reassuring to the surgeons, irrespective of their familiarity with transforamen ovale long needle techniques. Some researchers[31,32,33,34] have proposed that percutaneous microballoon compression is suitable for patients with ophthalmic division involvement, whereas others recommend it as a first-line treatment in patients who are reluctant to have a major surgical operation, such as microvascular decompression. Therefore, percutaneous balloon compression is widely preferred for treating trigeminal neuralgia, especially for elderly patients, because of the high success rate, technical simplicity and relative safety[35,36,37,38,39].
Percutaneous microballoon compression is generally performed with the guidance of X-ray fluoroscopy[40,41,42,43,44,45]. This operation requires the use of fluoroscopy to navigate the needle through the foramen ovale and to monitor balloon inflation and deflation. However, the level of radiation exposure for this technique has not been adequately described. Both surgeons and patients are exposed to the radiation. The currently used compression duration ranges from 3 to 6 minutes. Fransen[19] suggested use of lead-coated protective clothing and careful use of the fluoroscope mandatory.
Scientists[46,47,48] have modified and reduced the compression time to 2 minutes while maintaining good treatment outcome. Our findings indicate that the large myelinated fibers underwent uniform demyelination under the different periods of compression. Swelling, unraveling, disintegration of myelin and digestion chambers were evident. Gross demyelination in the trigeminal root was detected. These changes were not seen in the roots or ganglia of rabbits in the normal group. These changes represented Wallerian degeneration and retrograde axonal degeneration. There was significant demyelination in the longer-term compression group. For the recurrent patient, an extended duration of compression would be required to obtain a high rate of pain relief and prolong the recurrence interval.
Unfortunately, it remains unclear how percutaneous microballoon compression provides pain relief, although many investigations have been performed[49,50]. This is due, in part, to the complex anatomical structure of the trigeminal nerve. Trigeminal ganglia contain sensory and movement components. The ganglia are formed by a combination of neural crest cells and cells derived from a thickening (placode) of the ectoderm. The trigeminal cell bodies of the first-order trigeminal sensory neurons are located in the semilunar (Gasserian) ganglion of the trigeminal nerve. The first-order neurons for the facial nerve lie in the sensory ganglion for that nerve. The ganglia are, therefore, homologous to the dorsal root ganglia of the spinal nerves. The central processes of the axons of the first-order neurons descend up the brainstem via the spinal trigeminal tract to the spinal trigeminal nucleus, where they synapse with the second-order neurons. The axons of the second-order neurons decussate to the contralateral side and ascend in the ventral trigeminal tract to synapse in the ventral posteromedial nucleus of the thalamus with the third-order neurons. The ventral trigeminal tract or ventral secondary ascending tract of the trigeminal nerve is analogous to the lateral spinothalamic tract. The axons of the third-order neurons pass through the internal capsule to the postcentral gyrus. The entry of the nerve fibers into the spinal tract and nucleus of the trigeminal nerve is highly organized.
It can be described in terms of an imaginary picture of the head being projected onto the nucleus like a slide on a screen. This is called a somatotopic organization and is common in sensory systems. If the nucleus is envisaged as a fingerlike object lying parallel to the neuraxis, sensations from successive strips of tissue in the head are fed into successive slices of the nucleus. This is called the onion skin concept. In addition, in a transverse section of that part of the nucleus connected to the trigeminal nerve itself, the three different tiers of cells are selectively associated with the ophthalmic, maxillary and mandibular divisions of the nerve. However, there is little research on the association between the complex anatomical structure and the histopathological changes after percutaneous microballoon compression, especially for different durations of compression. Neurosurgeons from different countries have adopted varying compression periods that range from 3 to 6 minutes[40,41,42,43,44,45].
To further improve pain relief, many surgical procedures have emerged to analyze the impact of balloon shape, balloon position, balloon volume and duration on the therapeutic effect of percutaneous balloon compression. Our research team modified the compression time to 2 minutes and obtained satisfactory results[46,47,48]. Mild to severe facial numbness accompanied by hypoesthesia and transient masticatory weakness was found in most of the patients postoperatively; however, most of these resolved within the first 3–10 months. Corneal anesthesia and keratitis were observed in 1% of patients.
Most technical modifications have been based on the surgeon's personal experience, with no solid experimental foundation. No histological changes have been reported after acute trigeminal nerve compression. A study of Rydevik and colleagues[51] showed that an external pressure of 2.66 kPa (20 mmHg) reduced epineurial venule blood flow. A pressure of 3.99 kPa (30 mmHg) inhibited both antegrade and retrograde axonal transport, and with 10.64 kPa (80 mmHg) pressure, all endoneurial blood flow ceased. Dyck et al.[52] examined histopathologic changes with acute cuff compression (6.65 kPa [50 mmHg] for 2 minutes) and found an alteration in the shape of the myelin sheath. Szabo and Chidgey[53] and Gelberman et al.[54] demonstrated that pressures within the carpal tunnel were elevated in patients. These authors increased the external pressure within the carpal tunnel for a maximum of 4 hours and studied neurological function. Some neurological functions were lost at 5.32 kPa (40 mmHg), and all functions were blocked at 6.65 kPa (50 mmHg). In volunteers with different blood pressures, the critical extraneural pressure threshold above which function was blocked was 3.99 kPa (30 mmHg) less than the diastolic pressure and 5.98 kPa (45 mmHg) below mean arterial blood pressure. The correlation between systemic blood pressure and neurological function in response to acute compression supports the hypothesis that decreased microvascular blood flow plays an important role in nerve compression[55,56].
What about the histological changes in the trigeminal ganglion after compression injury? In the present study, we performed percutaneous microballoon compression in rabbits with an inflation pressure for two different periods of compression (2 and 5 minutes). The results revealed that there was no significant change in ganglion cell morphology and diameter after percutaneous microballoon compression. However, the larger myelinated fibers were more likely to be injured and demyelinated. These findings are similar to those of Baker and Kerr[28], who performed Shelden's procedure for ganglion compression through a temporal craniectomy in cats. The demyelination was more severe with prolonged compression. This observation is in accordance with the findings in many patients with a weak zygomatic reflex (which is related to the A-alpha fibers) after percutaneous microballoon compression, which suggests that the compression selectively damages the large myelinated fibers. This is likely due to the greater sensitivity of large fibers to mechanical compression. The present study shows that most ganglion cells can withstand compression injury.
Accumulating evidence[57,58,59] shows that trigeminal root demyelination is the main pathological change in trigeminal neuralgia. However, the mechanism of demyelination remains unclear. Some researchers speculate that it is related to nerve nutrition and metabolism[59]. This would be in accordance with the histopathologic findings of fibrosis, which manifests as a thickening of the external epineurium and perineurium. These changes interfere with blood flow in the vessels that pass through the epineurium and perineurium, resulting in ischemia of the nerve fibers. Numerous experimental studies have demonstrated that nerve injury is related to the degree and duration of compression, with both mechanical and ischemic factors contributing to neurological dysfunction[49,50]. Nukada and Dyck[59] inferred that regional hypoxia causes axonal stasis as a primary event. Demyelination was found in fibers showing swollen dark and attenuated axons. Their findings suggest that axons are selectively vulnerable to acute ischemia and dependent on its severity; the fibers either undergo axonal degeneration or transitory structural alterations without axonal degeneration; the latter consisting of axonal changes and secondary demyelination. Kupers et al.[60] showed clear evidence of the demyelination of large myelinated fibers after compression. Their data indicate that the main histopathological change in the ischemic peripheral nerve after compression is demyelination.
Very little is known of the changes in cytokine levels after percutaneous microballoon compression injury of the trigeminal nerve. A number of researchers[60,61,62] have shown that vascular endothelial growth factor plays an important role in neural regeneration. Vascular endothelial growth factor is an endothelium-specific growth factor that promotes endothelial cell proliferation, differentiation and survival, mediates endothelium-dependent vasodilatation, induces microvascular hyperpermeability. Hypoxia stimulates the secretion of vascular endothelial growth factor and other angiogenic factors, leading to neovascularization and protection against ischemic injury. Arany et al.[63] showed that vascular endothelial growth factor mRNA activates and controls the progression of angiogenesis. The new vascularization improves blood supply in the ischemic tissue. Ischemic neurons would regain normal function when the oxygen and nutrient supply is restored.
In the present study, there were more vascular endothelial growth factor-positive cells in the compressed ganglion cells, which illustrates that vascular endothelial growth factor expression is upregulated and plays an important role in nerve repair in the early stages of nerve injury. The increased vascular endothelial growth factor expression helps repair the nerve defects. Thus, vascular endothelial growth factor may have a potential as a therapeutic agent for nerve injury repair.
In summary, there were histopathological changes after percutaneous trigeminal ganglion compression in the rabbits, including significant axon demyelination. High vascular endothelial growth factor expression was also observed. Further experiments are needed to determine if the higher vascular endothelial growth factor levels have a beneficial effect.
Materials and Methods
Design
A neuropathological, observational, animal study.
Time and setting
This experiment was performed at the Laboratory of the People's Hospital of Liaoning Province and the Laboratory Center of Liaoning Medical College, Liaoning, China between April and September 2012.
Materials
A total of 36 healthy adult, New Zealand rabbits, aged 12–24 months, of either gender, weighing 2.4–4.3 kg, were provided by the Animal Center of Liaoning Medical College (license No. SCXK (Liao) 2003-0007). The experimental animals were sacrificed according to the Guidance Suggestions for the Care and Use of Laboratory Animals, published by the Ministry of Science and Technology of China[64]. All rabbits were housed in an air-conditioned room and were allowed to freely feed.
Methods
Establishment of percutaneous microballoon compression model
Percutaneous trigeminal ganglion compression was performed on 30 adult New Zealand White rabbits. After rabbits were anesthetized with ketamine (25 mg/kg intramuscularly), the unilateral foramen ovale and trigeminal ganglion and root were located to determine the optimal percutaneous puncture pathway. An 18-gauage needle (Edwards Lifesciences, Irvine, CA, USA) was positioned at the foramen ovale by fluoroscopic guidance. When the foramen ovale was identified on the fluoroscopic image, a No. 2 Fogarty embolectomy catheter (Edwards Lifesciences) was positioned in Meckel's cave and advanced 3 to 5 mm. The position of the catheter was confirmed by lateral radiographs (Figure 5). The microballoon was gradually inflated with an Omnipaque (Ansheng Corporation, Shanghai, China), with an inflation pressure of 1,005 ± 150 mmHg, for 2 or 5 minutes by means of a micrometer (Allwell Medical Corporation, Raleigh, USA) and then removed. The rabbits were intramuscularly injected gentamicin (2.5 mg/kg daily) after percutaneous microballoon compression for 3 days post-surgery to prevent infection.
Figure 5.
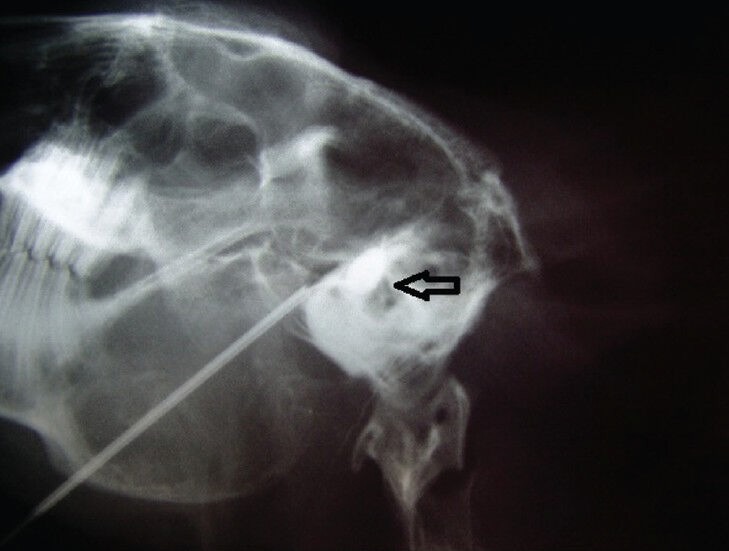
X-ray of the rabbit skull.
Lateral view of a rabbit skull showing percutaneous microcompression of the trigeminal ganglion with a No. 2 Fogarty catheter inflated (arrow) in Meckel's cave.
Preparation of tissue specimens
At 1, 7 and 14 days after percutaneous microballoon compression, rabbits were deeply anesthetized with 20% ethyl carbamate (1.2 g/kg, intraperitoneal), and transcardially perfused with 500 mL PBS, followed by 500 mL of 4% paraformaldehyde (w/v), and then the trigeminal ganglia were removed. Tissue specimens within 4 mm of the trigeminal ganglion were resected, dehydrated in a graded alcohol series, and paraffin-embedded. The tissue was cut using a Paraffin microtome (Leica, Solms, Germany) into 4-µm-thick sections for hematoxylin-eosin and immunohistochemical staining.
Hematoxylin-eosin staining
The tissue sections were stained in Harris Hematoxylin for 8 minutes. Excess stain was removed by rinsing in running tap water for 2 minutes. Sections were dipped in 0.3% ammonia water or saturated in lithium carbonate water until specimens were jean blue (3–6 dips). Subsequently, tissue sections were rinsed in tap water for 1 hour and immersed in tap water for 2 minutes, then hydrated in a graded alcohol series for 1 minute each and in water for 2 minutes. Alcoholic Eosin Y staining was performed for 2 minutes. Tissue sections were dehydrated in 100% alcohol, placed in xylene, cover-slipped with neutral gum and marked. The morphology of the compressed and normal ganglia and roots was observed under light microscopy (Olympus, Osaka, Japan).
Diameter of trigeminal ganglion cells measured using image analysis
Cell imaging software (Daheng Corporation, Beijing, China) was used to quantify cell diameter. The trigeminal ganglion specimen sections were photographed using an optical microscope (Olympus) at 400 × magnification. The normal ganglion cell structure was clearly visible, and the cell membranes had an even thickness and distinct margins. The total area of the trigeminal ganglion cells was set as a measurement reference. Three sections for each specimen were randomly selected from every group for observation. Four visual fields for each section were randomly selected and a sufficient number (≥ 5) of trigeminal cells were measured to reduce error. The dot number of the trigeminal ganglion cell area under a 10 × objective was set as A. According to the systematic setting, 639 dots amount to 400 µm. The acquired area dot A was converted to equivalent diameter dot B. Then, the actual trigeminal ganglion cell diameter was B × 0.626. The mean diameter of every time point after percutaneous microballoon compression was acquired and analyzed statistically. The steps were as follows: (1) Image capture: Sections were placed under the microscope and observed under a 10 × objective lens. Four sections were selected. The image analysis system automatically recorded and displayed three images on the monitor; (2) selection of measurement parameters: The total area of the trigeminal ganglion cells was set as the measurement reference; (3) image enhancement: The obscured and distorted regions were stroked out to enhance the quality of the image by adjusting the contrast and shadow, and by smoothening and sharpening; (4) image segmentation: The gray value was selected based on the image characteristics. Five axon images were selected randomly and converted to binary images; (5) image binary management: The binary image was adjusted to remove objects of no interest; (6) image recognition and analysis process: All images were processed and analyzed according to the instructions provided with the image analysis system, which converted the data to ganglion cell diameter and performed the analysis.
Ultrastructure of the trigeminal ganglion under the electron microscope
The trigeminal ganglion tissues were dissected out quickly and divided into several small pieces (< 1 mm × 1 mm × 1 mm) at low temperature (0–4°C). All the tissues were placed in 2.5% glutaraldehyde fixing solution for more than 2 hours, rinsed with PBS, fixed in 1% osmium tetroxide solution for 1.5 hours, pH 7.3–7.4, and rinsed with PBS for 20 minutes. After washing, the tissue was dehydrated with 70% acetone for 15 minutes, 80% acetone for 15 minutes, 90% acetone for 15 minutes, and 100% acetone for 10 minutes twice. Tissues were then placed in a dilute solution of plastic embedding medium epoxy resin mixture, and then polymerized in an oven at 45°C (12 hours), and thereafter at 60°C (36 hours). When the plastic was hard, the block was trimmed for sectioning on the ultramicrotome. Specimens were cut into 50–70-nm thick sections with a glass knife using an LKB-NOVE ultramicrotome (Leica). The sections were floated off the edge of the knife onto the surface of a water trough, and picked off the surface with a copper grid of 3 mm diameter covered with support Formvar film (10 nm thickness). After the sections were dried, they were stained with heavy metal solutions. Changes in ultrastructure were observed using a JEM-1200EX transmission electron microscope (JEOL, Tokyo, Japan).
Vascular endothelial growth factor immunoreactivity in trigeminal ganglion cells detected by immunohistochemical staining
The tissue specimens were dewaxed and hydrated prior to microwave antigen retrieval in citrate buffer solution, followed by incubation in 3% H2O2 to block endogenous peroxidase. After rinsing three times for 5 minutes each, tissue specimens were incubated with normal goat serum for 15 minutes at room temperature, with primary mouse anti-vascular endothelial growth factor polyclone antibody (1:100; Boaosen Corporation, Beijing, China) at 4°C for 24 hours, and with biotinylated goat anti-mouse IgG (1:200; Solarbio Corporation, Beijing, China) for 10 minutes at 37°C. Specimens were then incubated in 3,3′-diaminobenzidine (Beyotime Corporation, Beijing, China) in PBS for 1–2 minutes to reveal labeling. The stained sections were mounted on slides, dehydrated in a graded ethanol series, cleared with xylene, and cover-slipped with neutral gum. Using the Paxinos atlas[65] as a guide, five visual fields for each specific region were randomly selected from each of the four sections. The trigeminal ganglion cell bodies and axons labeled brown were positive. Cell imaging software (Daheng Corporation) was used to quantify vascular endothelial growth factor immunoreactivity, and trigeminal cells and axons were photographed using an optical microscope at 400 × magnification. The ganglion cell bodies and axons in each section were photographed. HPIAS-1000 high definition color image analysis system (Image Engineering Corporation, Wuhan, Hubei Province, China) was used to assay the gray value of each section.
Statistical analysis
All data were expressed as mean ± SD. One-way analysis of variance followed by least significant difference test was performed using SPSS 10.0 software (SPSS, Chicago, IL, USA). P < 0.05 was considered statistically significant.
Footnotes
Funding: This study was supported by a grant from Shengjing Hospital, China Medical University, China, No. 201010252.
Conflicts of interest: None declared.
Peer review: Extracellular recording was used to examine response properties in auditory neurons of the inferior colliculus. The results help to explain the difficulty in sound localization and speech perception that patients with hearing aids exhibit in complex sound environments.
Copyedited by Patel B, Stow A, Norman C, Zou JJ, Wang LM, Yang Y, Li CH, Song LP; Liu WJ, Zhao M
References
- [1].Merskey H, Bogduk N. 2nd ed. Seattle: IASP Press; 1994. Classification of Chronic Pain. Descriptors of Chronic Pain Syndromes and Definitions of Pain Terms. [Google Scholar]
- [2].Sweet WH. 3rd ed. Philadelphia: WB Saunders; 1995. Oprative Neurosurgery Techniques: Indications, Methods, and Results. [Google Scholar]
- [3].Zakrzewska JM, McMillan R. Trigeminal neuralgia: the diagnosis and management of this excruciating and poorly understood facial pain. Postgrad Med J. 2011;87(1028):410–416. doi: 10.1136/pgmj.2009.080473. [DOI] [PubMed] [Google Scholar]
- [4].Headache Classification Subcommittee of the International Headache Society. The International Classification of Headache Disorders: 2nd edition. Cephalalgia. 2004;24(Suppl 1):9–160. doi: 10.1111/j.1468-2982.2003.00824.x. [DOI] [PubMed] [Google Scholar]
- [5].Dieleman JP, Kerklaan J, Huygen FJ, et al. Incidence rates and treatment of neuropathic pain conditions in the general population. Pain. 2008;137(3):681–688. doi: 10.1016/j.pain.2008.03.002. [DOI] [PubMed] [Google Scholar]
- [6].Chen JF, Tu PH, Lee ST. Repeated percutaneous balloon compression for recurrent trigeminal neuralgia: a long-term study. World Neurosurg. 2012;77(2):352–356. doi: 10.1016/j.wneu.2011.06.013. [DOI] [PubMed] [Google Scholar]
- [7].Chen JF, Tu PH, Lee ST. Long-term follow-up of patients treated with percutaneous balloon compression for trigeminal neuralgia in Taiwan. World Neurosurg. 2011;76(6):586–591. doi: 10.1016/j.wneu.2011.05.021. [DOI] [PubMed] [Google Scholar]
- [8].Campos WK, Linhares MN. A prospective study of 39 patients with trigeminal neuralgia treated with percutaneous balloon compression. Arq Neuropsiquiatr. 2011;69(2A):221–226. doi: 10.1590/s0004-282x2011000200016. [DOI] [PubMed] [Google Scholar]
- [9].Omeis I, Smith D, Kim S, et al. Percutaneous balloon compression for the treatment of recurrent trigeminal neuralgia: long-term outcome in 29 patients. Stereotact Funct Neurosurg. 2008;86(4):259–265. doi: 10.1159/000138770. [DOI] [PubMed] [Google Scholar]
- [10].Skirving JD, Dan NG. A 20-year review of percutaneous balloon compression of the trigeminal ganglion. J Neurosurg. 2001;94(6):913–917. doi: 10.3171/jns.2001.94.6.0913. [DOI] [PubMed] [Google Scholar]
- [11].Brown JA, Pilitsis JG. Percutaneous balloon compression for the treatment of trigeminal neuralgia: results in 56 patients based on balloon compression pressure monitoring. Neurosurg Focus. 2005;18(5):E10. doi: 10.3171/foc.2005.18.5.11. [DOI] [PubMed] [Google Scholar]
- [12].Jannetta PJ. Treatment of trigeminal neuralgia for micro-operative decompression. In: Youmans JR, editor. Neurological Surgery. Philadelphia: Saunders; 1982. [Google Scholar]
- [13].Kerr FW. Pathology of trigeminal neuralgia: light and electron microscopic observations. J Neurosurg. 1967;26(1 Suppl):151–156. doi: 10.3171/jns.1967.26.1part2.0151. [DOI] [PubMed] [Google Scholar]
- [14].Natarajan M. Percutaneous trigeminal ganglion balloon compression: experience in 40 patients. Neurol India. 2000;48(4):330–332. [PubMed] [Google Scholar]
- [15].Li YF, Ma Y, Huang HT, et al. Percutaneous microcompression for bilateral trigeminal neuralgia treatment. Jieru Fangshe Xue Zazhi. 2010;19(6):502–504. [Google Scholar]
- [16].Brown JA, McDaniel MD, Weaver MT. Percutaneous trigeminal nerve compression for treatment of trigeminal neuralgia: results in 50 patients. Neurosurgery. 1993;32(4):570–573. doi: 10.1227/00006123-199304000-00012. [DOI] [PubMed] [Google Scholar]
- [17].Pickett GE, Bisnaire D, Ferguson GG. Percutaneous retrogasserian glycerol rhizotomy in the treatment of tic douloureux associated with multiple sclerosis. Neurosurgery. 2005;56(3):537–545. doi: 10.1227/01.neu.0000153907.43563.ff. [DOI] [PubMed] [Google Scholar]
- [18].Abdennebi B, Mahfouf L, Nedjahi T. Long-term results of percutaneous compression of the gasserian ganglion in trigeminal neuralgia (series of 200 patients) Stereotact Funct Neurosurg. 1997;68(1-4 Pt 1):190–195. doi: 10.1159/000099922. [DOI] [PubMed] [Google Scholar]
- [19].Fransen P. Fluoroscopic exposure during percutaneous balloon compression of the Gasserian ganglion. J Neurointerv Surg. doi: 10.1136/neurintsurg-2012-010370. in press. [DOI] [PubMed] [Google Scholar]
- [20].Mullan S, Lichtor T. Percutaneous microcompression of the trigeminal ganglion for trigeminal neuralgia. J Neurosurg. 1983;59(6):1007–1012. doi: 10.3171/jns.1983.59.6.1007. [DOI] [PubMed] [Google Scholar]
- [21].Revuelta R, Nathal E, Balderrama J, et al. External carotid artery fistula due to microcompression of the gasserian ganglion for relief of trigeminal neuralgia. Case report. J Neurosurg. 1993;78(3):499–500. doi: 10.3171/jns.1993.78.3.0499. [DOI] [PubMed] [Google Scholar]
- [22].De Siqueira SR, da Nobrega JC, de Siqueira JT, et al. Frequency of postoperative complications after balloon compression for idiopathic trigeminal neuralgia: prospective study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;102(5):e39–45. doi: 10.1016/j.tripleo.2006.03.028. [DOI] [PubMed] [Google Scholar]
- [23].Urculo E, Alfaro R, Arrazola M, et al. Trochlear nerve palsy after repeated percutaneous balloon compression for recurrent trigeminal neuralgia: case report and pathogenic considerations. Neurosurgery. 2004;54(2):505–509. doi: 10.1227/01.neu.0000103675.32713.a9. [DOI] [PubMed] [Google Scholar]
- [24].Langford P, Holt ME, Danks RA. Cavernous sinus fistula following percutaneous balloon compression of the trigeminal ganglion. Case report. J Neurosurg. 2005;103(1):176–178. doi: 10.3171/jns.2005.103.1.0176. [DOI] [PubMed] [Google Scholar]
- [25].Jellish WS, Benedict W, Owen K, et al. Perioperative and long-term operative outcomes after surgery for trigeminal neuralgia: microvascular decompression vs percutaneous balloon ablation. Head Face Med. 2008;4:11. doi: 10.1186/1746-160X-4-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Lopez BC, Hamlyn PJ, Zakrzewska JM. Systematic review of ablative neurosurgical techniques for the treatment of trigeminal neuralgia. Neurosurgery. 2004;54(4):973–983. doi: 10.1227/01.neu.0000114867.98896.f0. [DOI] [PubMed] [Google Scholar]
- [27].Sweet WH, Polleti CE. Complications of percutaneous rhizotomy and microvascular decompression operations for facial pain. In: Schmidek H, Sweet W, editors. Current Techniques in Operative Neurosurgery. Orlando: Grune & Stratton; 1988. [Google Scholar]
- [28].Baker GS, Kerr FW. Structural changes in the trigeminal system following compression procedures. J Neurosurg. 1963;20:181–184. doi: 10.3171/jns.1963.20.3.0181. [DOI] [PubMed] [Google Scholar]
- [29].Kanpolat Y, Ugur HC. Systematic review of ablative neurosurgical techniques for the treatment of trigeminal neuralgia. Neurosurgery. 2005;57(3):E601. doi: 10.1227/01.neu.0000181084.00290.7a. [DOI] [PubMed] [Google Scholar]
- [30].Hannerz J, Linderoth B. Neurosurgical treatment of short-lasting, unilateral, neuralgiform hemicrania with conjunctival injection and tearing. Br J Neurosurg. 2002;16(1):55–58. doi: 10.1080/026886902753512600. [DOI] [PubMed] [Google Scholar]
- [31].Sweet WH. The treatment of trigeminal neuralgia (tic douloureux) N Engl J Med. 1986;315(3):174–177. doi: 10.1056/NEJM198607173150307. [DOI] [PubMed] [Google Scholar]
- [32].Broggi G, Ferroli P, Franzini A. Treatment strategy for trigeminal neuralgia: a thirty years experience. Neurol Sci. 2008;29(Suppl 1):S79–82. doi: 10.1007/s10072-008-0893-6. [DOI] [PubMed] [Google Scholar]
- [33].Liu HB, Ma Y, Zou JJ, et al. Percutaneous microballoon compression for trigeminal neuralgia. Chin Med J (Engl) 2007;120(3):228–230. [PubMed] [Google Scholar]
- [34].Lee ST, Chen JF. Percutaneous trigeminal ganglion balloon compression for treatment of trigeminal neuralgia, part II: results related to compression duration. Surg Neurol. 2003;60(2):149–154. doi: 10.1016/s0090-3019(03)00253-2. [DOI] [PubMed] [Google Scholar]
- [35].Bergenheim AT, Linderoth B. Diplopia after balloon compression of retrogasserian ganglion rootlets for trigeminal neuralgia: technical case report. Neurosurgery. 2008;62(2):E533–534. doi: 10.1227/01.neu.0000316025.58915.10. [DOI] [PubMed] [Google Scholar]
- [36].Lobato RD, Rivas JJ, Sarabia R, et al. Percutaneous microcompression of the gasserian ganglion for trigeminal neuralgia. J Neurosurg. 1990;72(4):546–553. doi: 10.3171/jns.1990.72.4.0546. [DOI] [PubMed] [Google Scholar]
- [37].Lee ST, Chen JF. Percutaneous trigeminal ganglion balloon compression for treatment of trigeminal neuralgia-part I: pressure recordings. Surg Neurol. 2003;59(1):63–67. doi: 10.1016/s0090-3019(02)00899-6. [DOI] [PubMed] [Google Scholar]
- [38].Brown JA. Percutaneous trigeminal nerve compression. In: Batjer HH, Loftus CM, editors. Textbook of Neurological Surgery: Principles and Practice. Philadelphia: Lippincott Williams & Wilkins; 2002. [Google Scholar]
- [39].Wilkinson HA. Percutaneous trigeminal ganglion balloon compression for treatment of trigeminal neuralgia--part I: pressure recordings. Surg Neurol. 2003;60(5):470. doi: 10.1016/s0090-3019(03)00514-7. [DOI] [PubMed] [Google Scholar]
- [40].Trojnik T, Ŝmigoc T. Percutaneous trigeminal ganglion balloon compression rhizotomy: experience in 27 patients. ScientificWorldJournal 2012. 2012 doi: 10.1100/2012/328936. 328936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Chen JF, Lee ST. Comparison of percutaneous trigeminal ganglion compression and microvascular decompression for the management of trigeminal neuralgia. Clin Neurol Neurosurg. 2003;105(3):203–208. doi: 10.1016/s0303-8467(03)00012-x. [DOI] [PubMed] [Google Scholar]
- [42].Correa CF, Teixeira MJ. Balloon compression of the Gasserian ganglion for the treatment of trigeminal neuralgia. Stereotact Funct Neurosurg. 1998;71(2):83–89. doi: 10.1159/000029651. [DOI] [PubMed] [Google Scholar]
- [43].Goerss SJ, Atkinson JL, Kallmes DF. Variable size percutaneous balloon compression of the gasserian ganglion for trigeminal neuralgia. Surg Neurol. 2009;71(3):388–391. doi: 10.1016/j.surneu.2007.09.040. [DOI] [PubMed] [Google Scholar]
- [44].Park SS, Lee MK, Kim JW, et al. Percutaneous balloon compression of trigeminal ganglion for the treatment of idiopathic trigeminal neuralgia: experience in 50 patients. J Korean Neurosurg Soc. 2008;43(4):186–189. doi: 10.3340/jkns.2008.43.4.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Abdennebi B, Amzar Y. Treatment of essential trigeminal neuralgia by Gasserian compression using balloon (50 cases) Neurochirurgie. 1991;37(2):115–118. [PubMed] [Google Scholar]
- [46].Li FY, Ma Y, Zou JJ, et al. Related pain free after percutaneous microcompression treatment for trigeminal neuralgia. Zhongguo Wei Qinxi Shenjing Waike Zazhi. 2010;15(3):105–106. [Google Scholar]
- [47].Wang B, Ma Y, Zou JJ, et al. Clinical experience of percutaneous microcompression treatment for trigeminal neuralgia. Zhonghua Shenjing Waike Zazhi. 2008;24(5):330. [Google Scholar]
- [48].Li YF, Ma Y, Zou JJ, et al. Percutaneous microcompression for trigeminal neuralgia treatment. Zhongguo Wei Qinxi Shenjing Waike Zazhi. 2008;13(11):514–515. [Google Scholar]
- [49].Hilton DA, Love S, Gradidge T, et al. Pathological findings associated with trigeminal neuralgia caused by vascular compression. Neurosurgery. 1994;35(2):299–303. doi: 10.1227/00006123-199408000-00017. [DOI] [PubMed] [Google Scholar]
- [50].Devor M, Govrin-Lippmann R, Rappaport ZH. Mechanism of trigeminal neuralgia: an ultrastructural analysis of trigeminal root specimens obtained during microvascular decompression surgery. J Neurosurg. 2002;96(3):532–543. doi: 10.3171/jns.2002.96.3.0532. [DOI] [PubMed] [Google Scholar]
- [51].Rydevik B, Lundborg G, Bagge U. Effects of graded compression on intraneural blood blow. An in vivo study on rabbit tibial nerve. J Hand Surg Am. 1981;6(1):3–12. doi: 10.1016/s0363-5023(81)80003-2. [DOI] [PubMed] [Google Scholar]
- [52].Dyck PJ, Lais AC, Giannini C, et al. Structural alterations of nerve during cuff compression. Proc Natl Acad Sci U S A. 1990;87(24):9828–9832. doi: 10.1073/pnas.87.24.9828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Szabo RM, Chidgey LK. Stress carpal tunnel pressures in patients with carpal tunnel syndrome and normal patients. J Hand Surg Am. 1989;14(4):624–627. doi: 10.1016/0363-5023(89)90178-0. [DOI] [PubMed] [Google Scholar]
- [54].Gelberman RH, Szabo RM, Williamson RV, et al. Sensibility testing in peripheral-nerve compression syndromes. An experimental study in humans. J Bone Joint Surg Am. 1983;65(5):632–638. [PubMed] [Google Scholar]
- [55].Jeon HJ, Han SR, Park MK, et al. A novel trigeminal neuropathic pain model: compression of the trigeminal nerve root produces prolonged nociception in rats. Prog Neuropsychopharmacol Biol Psychiatry. 2012;38(2):149–158. doi: 10.1016/j.pnpbp.2012.03.002. [DOI] [PubMed] [Google Scholar]
- [56].Mackinnon SE. Pathophysiology of nerve compression. Hand Clin. 2002;18(2):231–241. doi: 10.1016/s0749-0712(01)00012-9. [DOI] [PubMed] [Google Scholar]
- [57].Kerr FW. Evidence for a peripheral etiology of trigeminal neuralgia. 1967. J Neurosurg. 2007;107(1):225–231. [PubMed] [Google Scholar]
- [58].Zhou JH, Jiang XZ, Sun WG, et al. The correlation between Trigeminal nerve demyelination and pathological change of intraneural microcirculation. Xiandai Kouqiang Yixue Zazhi. 2004;18(6):483–485. [Google Scholar]
- [59].Nukada H, Dyck PJ. Acute ischemia causes axonal stasis, swelling, attenuation, and secondary demyelination. Ann Neurol. 1987;22(3):311–318. doi: 10.1002/ana.410220306. [DOI] [PubMed] [Google Scholar]
- [60].Kupers R, Yu W, Persson JK, et al. Photochemically-induced ischemia of the rat sciatic nerve produces a dose-dependent and highly reproducible mechanical, heat and cold allodynia, and signs of spontaneous pain. Pain. 1998;76(1-2):45–59. doi: 10.1016/s0304-3959(98)00022-0. [DOI] [PubMed] [Google Scholar]
- [61].Wang LX, Yin RX, Sun JB. Effect of Tongxinluo on nestin and vascular endothehal growth factor mRNA expression in rat brain tissue after cerebral ischemia-reperfusion injury. Nan Fang Yi Ke Da Xue Xue Bao. 2008;28(12):2131–2135. [PubMed] [Google Scholar]
- [62].Hall GC, Carroll D, Parry D, et al. Epidemiology and treatment of neuropathic pain: the UK primary care perspective. Pain. 2006;122(1-2):156–162. doi: 10.1016/j.pain.2006.01.030. [DOI] [PubMed] [Google Scholar]
- [63].Arany Z, Foo SY, Ma Y, et al. HIF-independent regulation of VEGF and angiogenesis by the transcriptional coactivator PGC-1alpha? Nature. 2008;451(7181):1008–1012. doi: 10.1038/nature06613. [DOI] [PubMed] [Google Scholar]
- [64].The Ministry of Science and Technology of the People's Republic of China. Guidance Suggestions for the Care and Use of Laboratory Animals. 2006-09-30 [Google Scholar]
- [65].Urban I, Richard P. Springfield, IL: Charles C Thomas Publisher Ltd; 1972. A Stereotaxic Atlas of the New Zealand Rabbit's Brain. [Google Scholar]


