Abstract
Previous studies have demonstrated the protective effect of hypoxic preconditioning on acute cerebral infarction, but the mechanisms underlying this protection remain unclear. To investigate the protective mechanisms of hypoxic preconditioning in relation to its effects on angiogenesis, we induced a photochemical model of cerebral infarction in an inbred line of mice (BALB/c). Mice were then exposed to hypoxic preconditioning 30 minutes prior to model establishment. Results showed significantly increased vascular endothelial growth factor and CD31 expression in the ischemic penumbra at 24 and 72 hours post infarction, mainly in neurons and vascular endothelial cells. Hy-Hypoxic preconditioning increased vascular endothelial growth factor and CD31 expression in the ischemic penumbra and the expression of vascular endothelial growth factor was positively related to that of CD31. Moreover, hypoxic preconditioning reduced the infarct volume and improved rological function in mice. These findings indicate that the protective role of hypoxic preconditioning in acute cerebral infarction may possibly be due to an increase in expression of vascular endothelial growth factor and CD31 in the ischemic penumbra, which promoted angiogenesis.
Keywords: neural regeneration, brain injury, hypoxic preconditioning, acute cerebral infarction, ischemic penumbra, vascular endothelial growth factor, CD31, angiogenesis, neuroprotection, grants-supported paper, neuroregeneration
Research Highlights
(1) Previous studies have shown that hypoxic preconditioning had protective effects against acute cerebral infarction up to 24 hours after insult. However, 24 hours post acute cerebral infarction is not the peak period for damage following stroke. This study observed conditions at 72 hours after ischemia, the peak period for damage following stroke, to assess the protective effects of hypoxic preconditioning.
(2) Results showed that hypoxic preconditioning reduced infarct volume and attenuated the impairment of neurological function. These neuroprotective effects were related to increased vascular endothelial growth factor and CD31 expression, which promoted angiogenesis.
(3) This study lays a preliminary foundation for hypoxic preconditioning translational medicine.
INTRODUCTION
Hypoxic preconditioning is a condition where tissues and cells become tolerant to long periods of fatal ischemic/hypoxic injury following a short period of adaptation of non-fatal repetitive ischemia/hypoxia, mainly in the heart, brain, kidney, liver and intestine[1,2,3,4,5,6,7,8,9,10]. Previous studies from our group investigated the protective effects of hypoxic preconditioning and related mechanisms using behavior, physiology, morphology, neurochemistry and molecular biology. We found that the mechanism was complex, and involved animal behavior, metabolism, functional systems, neuromorphology, spinal cord activity, neural chemical composition, and molecular neurobiology[7,11,12,13,14,15,16,17,18,19,20]. In addition, hypoxic preconditioning has been demonstrated to protect against brain ischemic injury induced by acute cerebral infarction through detection of infarct volume, neurological function assessment and cell apoptosis[17]. However, the underlying mechanism still remains uncertain.
Angiogenesis is a focus in recent studies of acute cerebral infarction, as it can significantly increase blood and nutrition supply to ischemic neurons in the ischemic penumbra. This increased flow can promote neural regeneration and synapse formation, improve neurological function, and ameliorate ischemic brain injury[21,22,23,24,25]. The molecular mechanisms of vascular endothelial cell mitogen, lytic enzymes of the extracellular matrix and endothelial cell migration-related signaling have been shown to participate in angiogenesis[21]. Increasing the positive effects of angiogenesis while decreasing adverse reactions is significant for developing drugs that can promote angiogenesis to treat ischemic brain injury[22].
Vascular endothelial growth factor, also known as vascular permeability factor, is a specific mitogen of endothelial cells and has the strongest specificity, playing a major regulatory role in angiogenesis and formation[26,27,28,29,30]. Platelet endothelial cell adhesion molecule-1, i.e., CD31, expressed in blood vessel endothelium, is a commonly used index for angiogenesis[31,32,33,34,35]. Hu et al[31] reported that rehabilitation training of limbs significantly increased CD31 expression in the ischemic penumbra and improved neurological function following cerebral infarction, indicating that angiogenesis plays a critical role in recovery following cerebral infarction.
Based on previous studies, we observed vascular endothelial growth factor and CD31 changes in the ischemic penumbra following hypoxic preconditioning to investigate the protective mechanisms underlying this process during acute cerebral infarction and their relationship to angiogenesis.
RESULTS
Quantitative analysis of experimental animals
Ninety-six mice were randomly assigned to four groups: normal control (no treatment), sham-surgery (cold light irradiation with no rose Bengal), acute cerebral infarction (photochemical induction), and hypoxic preconditioning (hypoxic preconditioning + acute cerebral infarction) groups. All 96 mice were included in the final analysis.
Influence of hypoxic preconditioning on infarct volume in mice with acute cerebral infarction
Ischemic infarction foci were not observed in normal control and sham-surgery groups, but observed in acute cerebral infarction and hypoxic preconditioning groups 72 hours following cold light irradiation. The infarct volume was significantly smaller in the hypoxic preconditioning group (185.7 ± 11.7 mm3) compared with the acute cerebral infarction group (287.7 ± 12.6 mm3; t = −13.264, P = 0.000; Figure 1).
Figure 1.
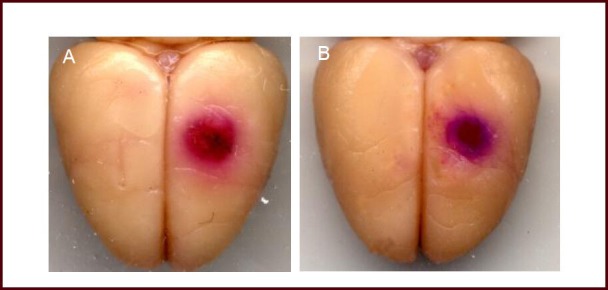
Influence of hypoxic preconditioning on infarct volume in mice with acute cerebral infarction.
Acute cerebral infarction group (A) revealed obvious infarction and a small ischemic penumbra; while the hypoxic preconditioning group (B) displayed a small infarction focus, and an increased ischemic penumbra. Red: Infarction focus; blue: ischemic penumbra.
Influence of hypoxic preconditioning on neurological function in mice with acute cerebral infarction
Seventy-two hours after cold light irradiation, neurological function scores showed that control mice had no neurological impairment.
No obvious neurological impairment was observed in the sham-surgery group as similar neurological function scores were obtained between this group and controls (P > 0.05). Symptoms of neurological impairment were found in the acute cerebral infarction and hypoxic preconditioning groups, and manifested as reduced spontaneous activity, asymmetric limb motion and creeping of the forelimbs, weakened grasping strength of the cage, and slowed reaction times. Neurological function scores were significantly reduced in the acute cerebral infarction and hypoxic preconditioning groups compared with normal control and sham-surgery groups (P < 0.01). Scores obtained for the hypoxic preconditioning group were higher than those for the acute cerebral infarction group (P < 0.05; Table 1).
Table 1.
Influence of hypoxic preconditioning on neurological function scores in mice with acute cerebral infarction
Influence of hypoxic preconditioning on vascular endothelial growth factor and CD31 expression in the ischemic penumbra in mice with acute cerebral infarction
Immunofluorescent staining showed that only a small amount of vascular endothelial growth factorand CD31 expression was observed in the cortex of control and sham-surgery groups 24 and 72 hours following cold light irradiation. Vascular endothelial growth factor expression was mainly distributed in neurons of the ischemic penumbra 24 hours after cold light irradiation and distributed in vascular endothelial cells of the ischemic penumbra 72 hours after cold light irradiation in the acute cerebral infarction and hypoxic preconditioning groups (Figure 2). CD31 expression was found in vascular endothelial cells of the ischemic penumbra 24 and 72 hours after cold light irradiation in the acute cerebral infarction and hypoxic preconditioning groups (Figure 3).
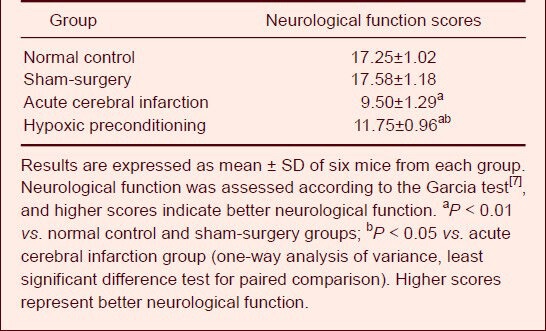
Figure 2.
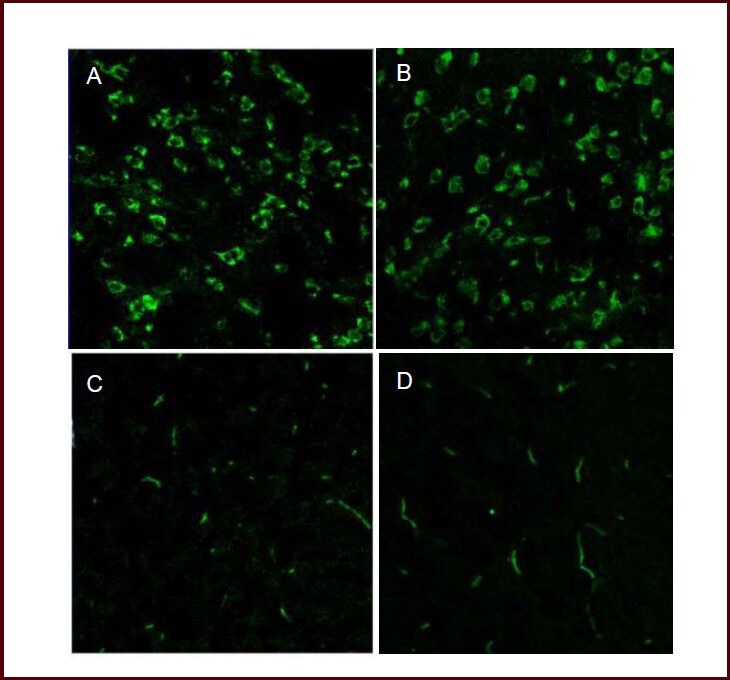
Influence of hypoxic preconditioning on vascular endothelial growth factor (VEGF) expression in the ischemic penumbra of mice with acute cerebral infarction (immunofluorescent staining, × 40).
Laser confocal microscopy showed that VEGF was mainly expressed in neurons of the ischemic penumbra in acute cerebral infarction (A) and hypoxic preconditioning (B) groups 24 hours following cold light irradiation. Seventy-two hours after cold light irradiation, the green fluorescence distribution migrated along blood vessels, mainly in vascular endothelial cells of the ischemic penumbra. VEGF fluorescence intensity was weaker in the acute cerebral infarction group (C) compared with hypoxic preconditioning group (D). Green: Fluorescein isothiocyanate (FITC)-labeled VEGF expression.
Figure 3.
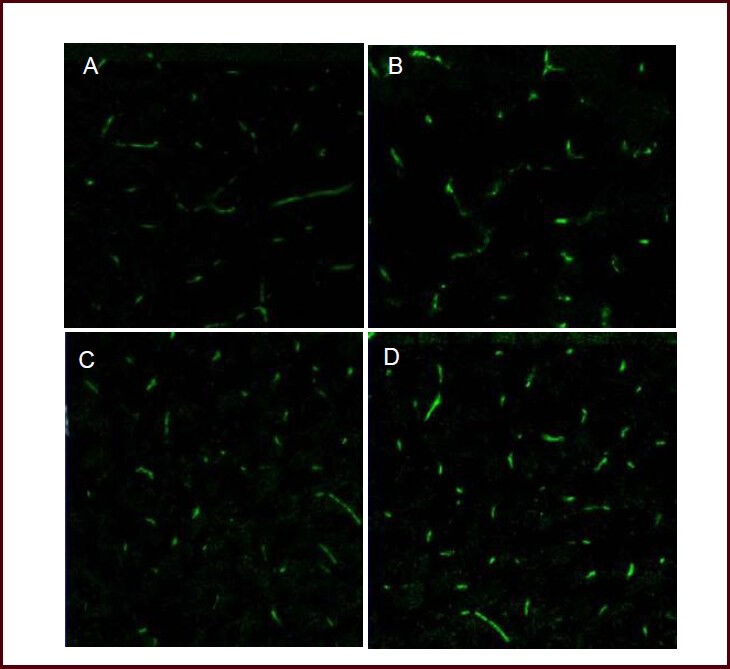
Influence of hypoxic preconditioning on CD31 expression in the ischemic penumbra of mice with acute cerebral infarction (immunofluorescent staining, × 40).
Laser confocal microscopy showed that green fluorescence migrated along the distribution of blood vessels 24 hours (A, B) and 72 hours (C, D) following cold light irradiation, and CD31 was mainly expressed in vascular endothelial cells of the ischemic penumbra. Fluorescence intensity was weaker in the acute cerebral infarction group (A, C) compared with the hypoxic preconditioning group (B, D). Green: Fluorescein isothiocyanate (FITC)-labeled CD31 expression.
Influence of hypoxic preconditioning on vascular endothelial growth factor and CD31 fluorescence intensity in the ischemic penumbra of mice with acute cerebral infarction
Vascular endothelial growth factor fluorescence intensity
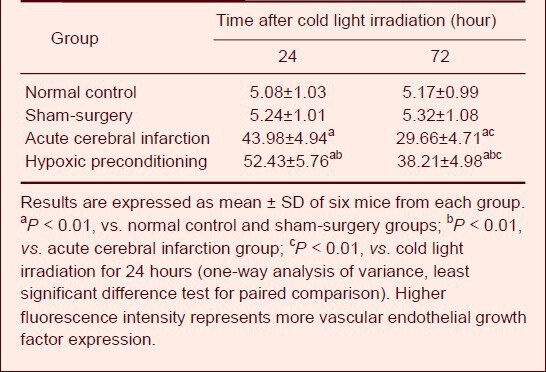
Statistical analysis showed that vascular endothelial growth factor fluorescence intensity in the ischemic penumbra was significantly stronger in acute cerebral infarction and hypoxic preconditioning groups compared with normal control and sham-surgery groups (P < 0.01) 24 and 72 hours after cold light irradiation. Vascular endothelial growth factor fluorescence intensity in the ischemic penumbra was significantly increased in the hypoxic preconditioning group compared with the acute cerebral infarction group 24 and 72 hours after cold light irradiation (P < 0.01).
Moreover, vascular endothelial growth factor fluorescence intensity in the ischemic penumbra was significantly stronger in the acute cerebral infarction and hypoxic preconditioning groups 24 hours after cold light irradiation compared with 72 hours after cold light irradiation (Table 2).
Table 2.
Influence of hypoxic preconditioning on vascular endothelial growth factor expression (fluorescence intensity) in ischemic penumbra of mice with acute cerebral infarction
CD31 fluorescence intensity
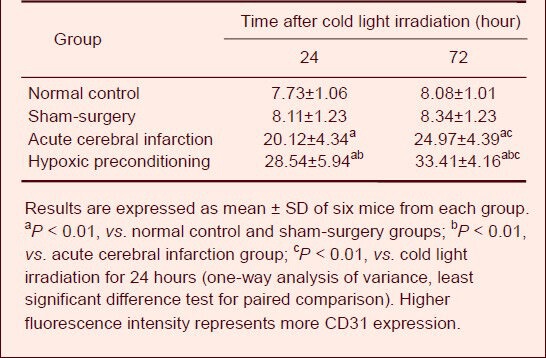
Statistical analysis showed that CD31 fluorescence intensity in the ischemic penumbra was significantly stronger in the acute cerebral infarction and hypoxic preconditioning groups compared with the control and sham-surgery groups 24 and 72 hours after cold light irradiation (P < 0.01). CD31 fluorescence intensity in the ischemic penumbra was significantly increased in the hypoxic preconditioning group compared with the acute cerebral infarction group 24 and 72 hours after cold light irradiation (P < 0.01). Moreover, CD31 fluorescence intensity in the ischemic penumbra was significantly weaker in the acute cerebral infarction and hypoxic preconditioning groups 24 hours after cold light irradiation compared with 72 hours after cold light irradiation (Table 3).
Table 3.
Influence of hypoxic preconditioning on CD31 expression (fluorescence intensity) in the ischemic penumbra of mice with acute cerebral infarction
Correlation between vascular endothelial growth factor and CD31 expression in the ischemic penumbra post cerebral infarction
Pearson correlation analysis showed that vascular endothelial growth factor expression was positively correlated with CD31 expression in the ischemic penumbra of the acute cerebral infarction and hypoxic preconditioning groups (r = 0.571, P < 0.01; Figure 4).
Figure 4.
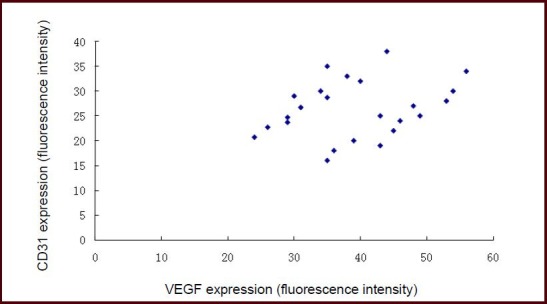
Correlation between vascular endothelial growth factor (VEGF) expression and CD31 expression in the ischemic penumbra of mice with acute cerebral infarction.
Pearson correlation analysis showed positive correlation between VEGF and CD31 expression (r = 0.571, P < 0.01).
DISCUSSION
Acute ischemic stroke can be severe and is associated with a high death and disability rate. Salvaging ischemic injured tissue after acute cerebral infarction is the key for treatment. Thus, research into protection methods or drugs for ischemic brain injury has become a large area of research focus[36,37,38,39,40,41].
Hypoxic preconditioning is an endogenous protective mechanism. It can attenuate hypoxic injury through a series of complex steps to protect the body[7]. Models of cardiac ischemia/reperfusion, cerebral ischemia/reper- fusion, ex vivo neurons and hippocampal brain section cultures showed that hypoxic preconditioning is protective to secondary injuries[1,2,3,4,5,6,7,8,9,10,11,12,13]. However, the mechanisms underlying hypoxic preconditioning are very complex, involving animal behavior and metabolism, neuromorphology, spinal cord activity and nerve chemical composition, as well as molecular neurobiology[1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20].
The present study established a photochemical model of acute cerebral infarction in mice because the cranium of mice is thin, so light irradiation can pass though the bone, preventing craniotomy and increasing the survival rate. Additionally, foci of focal cerebral infarction are more consistent and the infarction process is similar to that following cerebral arterial thrombosis in humans[42]. Cerebral infarction was indeed noted in the acute cerebral infarction and hypoxic preconditioning groups, demonstrating the success of model establishment.
In this study, we prolonged observation duration post ischemia because 72 hours post acute cerebral infarction is the peak stage of cerebral edema, infarct volume and neurological function impairment[43]. Results from this study showed that hypoxic preconditioning reduced infarct volume and improved neurological function at this time point.
However, the neuroprotective mechanism of hypoxic preconditioning remains poorly understood. Some studies used general hypoxic preconditioning in mice and a middle cerebral artery occlusion model to investigate the influence of hypoxic preconditioning on ischemic brain injury. Results showed that hypoxic preconditioning can improve behaviors in model mice and reduce infarct volume and degree of edema. Hypoxic preconditioning can antagonize the reduction of new protein kinase C membrane translocation in the infarct area and reduce cAMP response element binding protein phosphorylation in the ischemic cortex. Preconditioning can also reduce cAMP response element binding protein 2 hydrolysis fragments in the ischemic penumbra, and increase phosphorylation of mitogen- and stress-activated kinase 1 and cAMP response element binding protein, thereby protecting the brain against middle cerebral artery occlusion-induced injury[44,45]. The present study also demonstrated the protective effects of hypoxic preconditioning through its influence on angiogenesis.
Angiogenesis followed by blood vessel formation is an area of focus in ischemic cerebrovascular disease research[21,22,23,24,25]. Vascular endothelial growth factor is a multifunctional cytokine. It can promote vasopermeability of arteriole and arterial blood vessels, proliferation and migration of vascular endothelial cells and induce angiogenesis[25,26,27,28,29,30]. Vascular endothelial growth factor and receptor expression in brain tissues is increased after ischemia, especially in vascular endothelial cells and neurons of the ischemic penumbra, thereby protecting ischemic brain tissue[25,26,27,28,29,30]. CD31 is a platelet endothelial cell adhesion factor and is expressed in blood vessel endothelium. It has been regarded as a clinical index to reflect angiogenesis[31,32,33,34,35]. Thus, the present study observed changes of vascular endothelial growth factor and CD31 to investigate the protective mechanism of hypoxic preconditioning.
Results showed that vascular endothelial growth factor was mainly expressed in neurons in the early ischemic period (24 hours), and in endothelial cells in later ischemia (72 hours). It is likely that after ischemia or other stress conditions, the body first protects tissues and cells with important functions, but poor tolerance. Collateral circulation is then constructed through protecting endothelial cells and promoting endothelial cell proliferation to increase blood supply and stably protect the ischemic tissues for a long period of time[25,26,27,28,29,30]. In the present study, little vascular endothelial growth factor and CD31 was expressed in the normal control and sham-surgery groups, indicating that angiogenesis is not active under normal conditions. However, vascular endothelial growth factor and CD31 expression was significantly increased after acute cerebral infarction, indicating that angiogenesis is increased post ischemia to promote recovery of ischemic injury. Further results showed that vascular endothelial growth factor and CD31 expression was significantly increased in hypoxic preconditioning compared with the acute cerebral infarction group, and vascular endothelial growth factor expression was positively correlated with CD31 expression, indicating that hypoxic preconditioning can enhance angiogenesis in the ischemic penumbra after acute cerebral infarction and promote functional recovery. This finding suggests that hypoxic preconditioning may have protective effects on brain injury in patients with acute cerebral infarction.
Vascular endothelial growth factor functions by binding to receptors. After binding to the receptors, vascular endothelial growth factor is phosphorylated and regulates Ca2+ influx to function via the protein tyrosine kinase pathway: Vascular endothelial growth factor specifically acts on vascular endothelial cells to promote division. vascular endothelial growth factor binds receptors on vascular endothelial cells, flt-1 and flk-1 to construct collateral circulation in ischemic tissues, thereby increasing reperfusion and oxygen supply and attenuating ischemic injury. In addition, vascular endothelial growth factor directly protects nerve cells and vascular endothelial cells to promote neural regeneration[46,47]. Palmer et al[48] found newly generated neurons around blood vessels of the dentate gyrus and proposed neurogenesis due to vascular endothelial growth factor. Another study showed that vascular endothelial growth factor could stimulate neurogenesis in brain cell culture or in the dentate gyrus and subependymal region of mice[49]. vascular endothelial growth factor has also been shown to protect cortical neurons against hypoxic injury, stimulate axon growth and improve survival of ganglion cell in rat neck and dorsal root ganglion[50]. An in vitro HN33 cell model of brain ischemia showed that vascular endothelial growth factor could increase the number of surviving cells deprived of oxygen and glucose for 24 hours, but no angiogenesis was observed. These findings demonstrate the direct protective effects of vascular endothelial growth factor[51], and may indicate a novel molecule that could be used for the treatment of ischemic injury.
In summary, hypoxic preconditioning promoted angiogenesis by increasing vascular endothelial growth factor and CD31 expression in the ischemic penumbra after acute cerebral infarction, thereby protecting brain tissues against ischemic injury. However, further studies are needed to observe the long-term effects of hypoxic preconditioning as we only observed vascular endothelial growth factor and CD31 expression 24 and 72 hours after acute cerebral infarction.
MATERIALS AND METHODS
Design
A randomized and controlled animal study.
Time and setting
Experiments were conducted in the Institute of Cerebrovascular Disease, Xuanwu Hospital, Capital Medical University, China from June to September 2012.
Materials
Ninety-six BALB/c mice of clean grade, aged 6–8 weeks, weighing 18–22 g, of either gender, were provided by the Animal Department of Capital Medical University, China (license No. SCXK (Army) 2007-004). All procedures were in accordance with the Guidance Suggestions for the Care and Use of Laboratory Animals, formulated by the Ministry of Science and Technology of China[52].
Methods
Establishment of the hypoxic preconditioning model
Mice in the hypoxic preconditioning group were placed in a 125-mL wide-mouthed bottle. The bottle was immediately sealed with the rubber stopper until the animals exhibited a first gasp as a tolerance limit of hypoxia. This was regarded as the first hypoxic exposure. The procedures were conducted in triplicate to reproduce a model of hypoxic preconditioning[1,2,3,4,5,6,7,8,9,10]. Mice from other groups were not exposed to hypoxic preconditioning.
Establishment of the acute cerebral infarction model
After establishment of the hypoxic preconditioning model for 30 minutes, the acute cerebral infarction model was induced using the photochemistry method[42]. Briefly, mice were anesthetized by intraperitoneal injection of 0.35 g/kg chloral hydrate at 25°C, followed by slow injection of 100 mg/kg rose Bengal (5%; Sigma, St. Louis, MO, USA) via the tail vein. The mouse head was fixed onto stereotaxic apparatus (type 51600; Stoelting Co., Illinois, USA), and a median incision was made at the head to expose the left cranium. The optical fiber probe of cold light [150 W, 24 V metal halide lamp (LG-150; Xuzhou Hengda Optical Electronic Instrument, Jiangsu, China); ultra-violet ray and infrared ray were filtered, with single green beam at 530 nm] was perpendicularly attached to the exposed cranium at the site, 2 mm left of the sagittal suture and 2 mm posterior to the coronal suture. The irradiation field was 3 mm in diameter. The cold light was started 5 minutes after rose Bengal injection at an intensity of 2 lx. The incision was sutured after irradiation for 10 minutes, and disinfected with iodine tincture. The sham-surgery group was irradiated with cold light, but not injected with rose Bengal. Evans blue (1%) was injected 1 hour prior to collecting the brain tissue to allow for observation of infarct volume.
Determination of infarct volume
Six mice from each group were sacrificed 72 hours after establishment of the photochemical model. The infarct volume (length × width × height, mm3)[17] was measured with vernier calipers (precision, 0.02 mm; Santo 8012, Shanghai Santo, Shanghai, China).
Assessment of neurological function
Six mice from each group were sacrificed 72 hours after establishment of the photochemical model. Neurological function was assessed according to the Garcia test including spontaneous activity; symmetry in four-limb movement; forepaw outstretching; climbing; body proprioception; and response to whisker touch. Each item was rated 0–3, and the sum of six tests gave a total score[7]. Higher scores indicate better neurological function.
Immunofluorescent staining for vascular endothelial growth factor and CD31 expression in the ischemic penumbra
Six mice from each group were sacrificed at 24 and 72 hours after establishment of the photochemical model. Brain tissue in the ischemic penumbra was harvested, frozen in liquid nitrogen, and sectioned (10 μm) using a freezing microtome (CM1900; Leica, Heerbrugg, Switzerland). Briefly, sections were washed with 0.1 mol/L PBS three times, 5 minutes each, and incubated with blocking goat serum (1:10 dilution; Wuhan Boster, Wuhan, Hubei Province, China). Subsequently, sections were incubated with mouse anti-human vascular endothelial growth factor monoclonal antibody and mouse anti-mouse CD31 monoclonal antibody (1:50 dilution; Santa Cruz Biotechnology, Santa Cruz, CA, USA) overnight at 4°C after discarding the blocking serum. Sections were washed with 0.1 mol/L PBS twice, 5 minutes each, and incubated with fluorescein isothiocyanate-labeled goat anti-mouse IgG (1:100; Santa Cruz Biotechnology) at 37°C for 30 minutes. Sections were then washed with 0.1 mol/L PBS twice, 5 minutes each, and mounted with PBS glycerol (pH 8.5–9.5). The edges of the cover slip were smeared with nail polish. A Bio-Rad Radiance 2100 laser scanning confocal microscope (Hercules, CA, USA) was used to scan positive staining. Scanning parameters included excitation light wavelength 554 nm, observation light wavelength 575 nm, objective 40 × magnification with spot scanning (Zoom, 1.0). Lasersharp 2000 software (4.5.3; Bio-Rad) was used to obtain images. Fluorescence intensity of 30 randomly selected sections from each group was determined using LaserSharp 2000 software (Bio-Rad, Hercules, CA, USA) and the mean value was calculated.
Statistical analysis
Results were expressed as mean ± SD. Comparisons were conducted using one-way analysis of variance and paired comparison was performed with least significant difference test using SPSS 10.0 (SPSS, Chicago, IL, USA). Correlation between vascular endothelial growth factor and CD31 was analyzed using Pearson correlation analysis. A value of P < 0.05 was considered statistically significant.
Footnotes
Funding: This study was supported by the National Natural Science Foundation of China, No. 30870854; the Natural Science Foundation of Beijing, No. 7111003; and the Natural Science Foundation of Shandong Province, No. ZR2010HM029.
Conflicts of interest: None declared.
Ethical approval: This study received permission from the Animal Ethics Committee of Capital Medical University, China.
(Reviewed by Apricò K, Robens J, Xu WH, Li XG)
(Edited by Wang LM, Su LL, Li CH, Song LP, Liu WJ, Zhao M)
REFERENCES
- [1].Choukèr A, Ohta A, Martignoni A, et al. In vivo hypoxic preconditioning protects from warm liver ischemia-reperfusion injury through the adenosine A2B receptor. Transplantation. 2012;94(9):894–902. doi: 10.1097/TP.0b013e31826a9a46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Wacker BK, Perfater JL, Gidday JM. Hypoxic preconditioning induces stroke tolerance in mice via a cascading HIF, sphingosine kinase, and CCL2 signaling pathway. J Neurochem. 2012;123(6):954–962. doi: 10.1111/jnc.12047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Autheman D, Sheldon RA, Chaudhuri N, et al. Glutathione peroxidase overexpression causes aberrant ERK activation in neonatal mouse cortex after hypoxic preconditioning. Pediatr Res. 2012;72(6):568–575. doi: 10.1038/pr.2012.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Lu HS, Chen HP, Wang S, et al. Hypoxic preconditioning up-regulates DJ-1 protein expression in rat heart-derived H9c2 cells through the activation of extracellular-regulated kinase 1/2 pathway. Mol Cell Biochem. 2012;370(1-2):231–240. doi: 10.1007/s11010-012-1414-8. [DOI] [PubMed] [Google Scholar]
- [5].Yan F, Yao Y, Chen L, et al. Hypoxic preconditioning improves survival of cardiac progenitor cells: role of stromal cell derived factor-1α-CXCR4 axis. PLoS One. 2012;7(7):e37948. doi: 10.1371/journal.pone.0037948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Naparus A, Ashrafpour H, Huang N, et al. Combination of hypoxic preconditioning and postconditioning does not induce additive protection of ex vivo human skeletal muscle from hypoxia/reoxygenation injury. J Cardiovasc Pharmacol. 2012;60(4):347–356. doi: 10.1097/FJC.0b013e318262c961. [DOI] [PubMed] [Google Scholar]
- [7].Shao G, Lu GW. Hypoxic preconditioning in an autohypoxic animal model. Neurosci Bull. 2012;28(3):316–320. doi: 10.1007/s12264-012-1222-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Lu YZ, Wu CC, Huang YC, et al. Neutrophil priming by hypoxic preconditioning protects against epithelial barrier damage and enteric bacterial translocation in intestinal ischemia/reperfusion. Lab Invest. 2012;92(5):783–796. doi: 10.1038/labinvest.2012.11. [DOI] [PubMed] [Google Scholar]
- [9].Yeh CH, Hsu SP, Yang CC, et al. Hypoxic preconditioning reinforces HIF-alpha-dependent HSP70 signaling to reduce ischemic renal failure-induced renal tubular apoptosis and autophagy. Life Sci. 2010;86(3-4):115–123. doi: 10.1016/j.lfs.2009.11.022. [DOI] [PubMed] [Google Scholar]
- [10].Sims B, Clarke M, Francillion L, et al. Hypoxic preconditioning involves system Xc- regulation in mouse neural stem cells. Stem Cell Res. 2012;8(2):285–291. doi: 10.1016/j.scr.2011.09.002. [DOI] [PubMed] [Google Scholar]
- [11].Shao G, Gao CY, Lu GW. Alterations of hypoxia-inducible factor-1 alpha in the hippocampus of mice acutely and repeatedly exposed to hypoxia. Neurosignals. 2005;14(5):255–261. doi: 10.1159/000088641. [DOI] [PubMed] [Google Scholar]
- [12].Shao G, Zhang R, Wang ZL, et al. Hypoxic preconditioning improves spatial cognitive ability in mice. Neurosignals. 2006-2007;15(6):314–321. doi: 10.1159/000121368. [DOI] [PubMed] [Google Scholar]
- [13].Shao G, Gong KR, Li J, et al. Antihypoxic effects of neuroglobin in hypoxia-preconditioned mice and SH-SY5Y cells. Neurosignals. 2009;17(3):196–202. doi: 10.1159/000209867. [DOI] [PubMed] [Google Scholar]
- [14].Niu JZ, Zhang YB, Li MY, et al. Lessening effect of hypoxia-preconditioned rat cerebrospinal fluid on oxygen-glucose deprivation-induced injury of cultured hippocampal neurons in neonate rats and possible mechanism. Sheng Li Xue Bao. 2011;63(6):491–497. [PubMed] [Google Scholar]
- [15].Zhang YB, Lu GW, Yang MF, et al. Effect of hypoxic preconditioning on hippocampal Bcl-2 and Caspase-3 expression in mice. Zhonghua Shenjing Ke Zazhi. 2007;40(8):553–555. [Google Scholar]
- [16].Zhang YB, Lu GW, Yang MF, et al. Changes in Bcl-2 and Caspase-3 expressions in cortex of hypoxic preconditioning mice. Sheng Li Xue Bao. 2008;60(2):249–253. [PubMed] [Google Scholar]
- [17].Niu JZ, Zhang YB, Yang MF, et al. Protective effect of hypoxic preconditioning against cerebral ischemic injury induced by acute cerebral infarction in mice. Zhonghua Shenjing Yixue Zazhi. 2009;8(8):777–780. [Google Scholar]
- [18].Yang MF, Zhang YB, Sun BL, et al. Effect of brain homogenate from hypoxia-preconditioned mice on rat embryonic hippocampal neurons with hypoxia/reoxygenation injury. Zhonghua Shenjing Yixue Zazhi. 2009;8(11):1094–1097. [Google Scholar]
- [19].Zhang YB, Lu GW, Yang MF, et al. Changes in the bcl-2 expression and caspase-3 activity in mouse ependyma during hypoxic preconditioning. Zhonghua Shenjing Yixue Zazhi. 2007;6(10):986–988. [Google Scholar]
- [20].Zhang YB, Yang MF, Sun BL, et al. Hypoxic preconditioning stimulates proliferation of endogenous neural stem cells in brain hippocampus. Zhongguo Zuzhi Gongcheng Yanjiu yu Linchuang Kangfu. 2010;14(36):6699–6702. [Google Scholar]
- [21].Hayashi T, Deguchi K, Nagotani S, et al. Cerebral ischemia and angiogenesis. Curr Neurovasc Res. 2006;3(2):119–129. doi: 10.2174/156720206776875902. [DOI] [PubMed] [Google Scholar]
- [22].Rosell-Novel A, Montaner J, Alvarez-Sabín J. Angiogenesis in human cerebral ischemia. Rev Neurol. 2004;38(11):1076–1082. [PubMed] [Google Scholar]
- [23].Wei L, Fraser JL, Lu ZY, et al. Transplantation of hypoxia preconditioned bone marrow mesenchymal stem cells enhances angiogenesis and neurogenesis after cerebral ischemia in rats. Neurobiol Dis. 2012;46(3):635–645. doi: 10.1016/j.nbd.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Zhang P, Li J, Liu Y, et al. Human embryonic neural stem cell transplantation increases subventricular zone cell proliferation and promotes peri-infarct angiogenesis after focal cerebral ischemia. Neuropathology. 2011;31(4):384–391. doi: 10.1111/j.1440-1789.2010.01182.x. [DOI] [PubMed] [Google Scholar]
- [25].Zan L, Wu H, Jiang J, et al. Temporal profile of Src, SSeCKS, and angiogenic factors after focal cerebral ischemia: correlations with angiogenesis and cerebral edema. Neurochem Int. 2011;58(8):872–879. doi: 10.1016/j.neuint.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Zhang A, Liang L, Niu H, et al. Protective effects of VEGF treatment on focal cerebral ischemia in rats. Mol Med Report. 2012;6(6):1315–1318. doi: 10.3892/mmr.2012.1069. [DOI] [PubMed] [Google Scholar]
- [27].Yang J, Guo L, Liu R, et al. Neuroprotective effects of VEGF administration after focal cerebral ischemia/ reperfusion: dose response and time window. Neurochem Int. 2012;60(6):592–596. doi: 10.1016/j.neuint.2012.02.020. [DOI] [PubMed] [Google Scholar]
- [28].Herz J, Reitmeir R, Hagen SI, et al. Intracerebroventricularly delivered VEGF promotes contralesional corticorubral plasticity after focal cerebral ischemia via mechanisms involving anti-inflammatory actions. Neurobiol Dis. 2012;45(3):1077–1085. doi: 10.1016/j.nbd.2011.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Emerich DF, Silva E, Ali O, et al. Injectable VEGF hydrogels produce near complete neurological and anatomical protection following cerebral ischemia in rats. Cell Transplant. 2010;19(9):1063–1071. doi: 10.3727/096368910X498278. [DOI] [PubMed] [Google Scholar]
- [30].Chi OZ, Hunter C, Liu X, et al. Effects of deferoxamine on blood-brain barrier disruption and VEGF in focal cerebral ischemia. Neurol Res. 2008;30(3):288–293. doi: 10.1179/016164107X230135. [DOI] [PubMed] [Google Scholar]
- [31].Hu X, Zheng H, Yan T, et al. Physical exercise induces expression of CD31 and facilitates neural function recovery in rats with focal cerebral infarction. Neurol Res. 2010;32(4):397–402. doi: 10.1179/016164110X12670144526309. [DOI] [PubMed] [Google Scholar]
- [32].Biswas S, Charlesworth PJ, Turner GD, et al. CD31 angiogenesis and combined expression of HIF-1alpha and HIF-2alpha are prognostic in primary clear-cell renal cell carcinoma (CC-RCC), but HIFalpha transcriptional products are not: implications for antiangiogenic trials and HIFalpha biomarker studies in primary CC-RCC. Carcinogenesis. 2012;33(9):1717–1725. doi: 10.1093/carcin/bgs222. [DOI] [PubMed] [Google Scholar]
- [33].Melinte R, Jung I, Georgescu L, et al. VEGF and CD31 expression in arthritic synovium and cartilage of human knee joints. Rom J Morphol Embryol. 2012;53(4):911–915. [PubMed] [Google Scholar]
- [34].Kasprzak A, Surdacka A, Tomczak M, et al. Expression of angiogenesis-stimulating factors (VEGF, CD31, CD105) and angiogenetic index in gingivae of patients with chronic periodontitis. Folia Histochem Cytobiol. 2012;50(4):554–564. doi: 10.5603/20324. [DOI] [PubMed] [Google Scholar]
- [35].Torzicky M, Viznerova P, Richter S, et al. Platelet endothelial cell adhesion molecule-1 (PECAM-1/CD31) and CD99 are critical in lymphatic transmigration of human dendritic cells. J Invest Dermatol. 2012;132(4):1149–1157. doi: 10.1038/jid.2011.420. [DOI] [PubMed] [Google Scholar]
- [36].Lin YF, Li YD, Zang DW. Effects of leukemia inhibitory factor on endogenous neural stem cell proliferation and glycoprotein-130 expression in a mouse model of cerebral infarction. Neural Regen Res. 2011;6(19):1452–1456. doi: 10.3969/j.issn.1673-5374.2012.19.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Koton S, Tanne D, Bornstein NM, et al. Ischemic stroke on awakening: patients’ characteristics, outcomes and potential for reperfusion therapy. Neuroepidemiology. 2012;39(3-4):149–153. doi: 10.1159/000341242. [DOI] [PubMed] [Google Scholar]
- [38].Taub E, Uswatte G, Bowman MH, et al. Constraint- induced movement therapy combined with conventional neurorehabilitation techniques in chronic stroke patients with plegic hands: a case series. Arch Phys Med Rehabil. 2013;94(1):86–94. doi: 10.1016/j.apmr.2012.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Yang Y, Li Q, Miyashita H, et al. Usefulness of postischemic thrombolysis with or without neuroprotection in a focal embolic model of cerebral ischemia. J Neurosurg. 2000;92(5):841–847. doi: 10.3171/jns.2000.92.5.0841. [DOI] [PubMed] [Google Scholar]
- [40].Yang Y, Li Q, Shuaib A. Enhanced neuroprotection and reduced hemorrhagic incidence in focal cerebral ischemia of rat by low dose combination therapy of urokinase and topiramate. Neuropharmacology. 2000;39(5):881–888. doi: 10.1016/s0028-3908(99)00248-8. [DOI] [PubMed] [Google Scholar]
- [41].Granger N, Blamires H, Franklin RJ, et al. Autologous olfactory mucosal cell transplants in clinical spinal cord injury: a randomized double-blinded trial in a canine translational model. Brain. 2012;135(Pt 11):3227–3237. doi: 10.1093/brain/aws268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Ding PS, Li L, Zhao ML, et al. Establishment of photochemical induced mouse model of acute cerebral infarction. Zhongguo Shiyan Dongwu Xuebao. 2001;9(4):193–195. [Google Scholar]
- [43].Lou JH, Wang J, Liu LX, et al. Measurement of brain edema by noninvasive cerebral electrical impedance in patients with massive hemispheric cerebral infarction. Eur Neurol. 2012;68(6):350–357. doi: 10.1159/000342030. [DOI] [PubMed] [Google Scholar]
- [44].Jiang J, Yang WW, Zhang N, et al. Hypoxic p reconditioning attenuated MCAO-induced brain ischemic injuries of mice. Jichu Yixue yu Linchuang. 2009;29(2):113–118. [Google Scholar]
- [45].Du JL, Liang J, Li MY, et al. MSK-1 and CREB are involved in HPC induced neuroprotection against cerebral ischemic in juries of mice. Jichu Yixue yu Linchuang. 2010;30(11):1143–1148. [Google Scholar]
- [46].Pichiule P, Agani F, Chavez JC, et al. HIF-1 alpha and VEGF expression after transient global cerebral ischemia. Adv Exp Med Biol. 2003;530:611–617. doi: 10.1007/978-1-4615-0075-9_60. [DOI] [PubMed] [Google Scholar]
- [47].Jin KL, Mao XO, Greenberg DA. Vascular endothelial growth factor: direct neuroprotective effect in in vitro ischemia. Proc Natl Acad Sci U S A. 2000;97(18):10242–10247. doi: 10.1073/pnas.97.18.10242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Palmer TD, Willhoite AR, Gage FH. Vascular niche for adult hippocampal neurogenesis. J Comp Neurol. 2000;425(4):479–494. doi: 10.1002/1096-9861(20001002)425:4<479::aid-cne2>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- [49].Jin K, Zhu Y, Sun Y, et al. Vascular endothelial growth factor (VEGF) stimulates neurogenesis in vitro and in vivo. Proc Natl Acad Sci U S A. 2002;99(18):11946–11950. doi: 10.1073/pnas.182296499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Sondell M, Lundborg G, Kanje M. Vascular endothelial growth factor has neurotrophic activity and stimulates axonal outgrowth, enhancing cell survival and Schwann cell proliferation in the peripheral nervous system. J Neurosci. 1999;19(14):5731–5740. doi: 10.1523/JNEUROSCI.19-14-05731.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Serrar A, Aznag H, Baudet B, et al. Pulmonary vascular endothelial growth factor and nitric oxide interaction during total cardiopulmonary bypass in neonatal pigs. J Thorac Cardiovasc Surg. 2003;125(5):1050–1057. doi: 10.1067/mtc.2003.402. [DOI] [PubMed] [Google Scholar]
- [52].The Ministry of Science and Technology of the People's Republic of China. Guidance Suggestions for the Care and Use of Laboratory Animals. 2006 Sep 30; [Google Scholar]


