Keywords: neural regeneration, brain injury, normobaric oxygen, cerebral ischemia, focal cerebral ischemia, blood-brain barrier, hypoxia-inducible factor-1α, grants-supported paper, neuroregeneration
Abstract
Oxygen inhalation has been shown to increase oxygen supply to tissues after cerebral ischemia/ reperfusion injury, protecting injured neural cells. However, hyperbaric oxygen may aggravate oxidative stress. By contrast, normobaric oxygen has the rapid and non-invasive characteristics and may have therapeutic effects on ischemic/hypoxic disease. Rats inhaled normobaric oxygen (95% O2) for 6 consecutive days, and then a rat model of focal cerebral ischemia was established. Nissl and 2,3,5-triphenyltetrazolium chloride (TTC) staining revealed that normobaric oxygen pretreatment improved neurological deficits and reduced infarct volume. Immunohistochemical staining and western blot assay revealed that the expression of hypoxia-inducible factor-1α, Notch-1, vascular endothelial growth factor and erythropoietin were increased. Behavioral studies also verified that neurological deficit scores increased. The hypoxia-inducible factor inhibitor 2-methoxyestradiol treatment at 1 hour before administration of normobaric oxygen could suppress the protective effect of normobaric oxygen. Given these observations, normobaric oxygen pretreatment may alleviate cerebral ischemic injury via the hypoxia-inducible factor signal pathway.
INTRODUCTION
Although the use of hyperbaric oxygen in early stroke was not successful in clinical practice[1], oxygen has some unique advantages. Oxygen diffuses across the blood-brain barrier easily to reach its target tissue. Some reports, however, have shown that hyperbaric oxygen might aggravate oxidative stress after stroke, demonstrating that only the combination of hyperbaric oxygen and free radical scavengers had a protective function[2]. The ideal therapeutic time window and pressure of hyperbaric oxygen are still being investigated in multicenter clinical trials. Nowadays, normobaric oxygen has been used extensively for its cheap, fast and non-invasive characteristics. Data in recent years showed that normobaric oxygen was able to act rapidly after the appearance of stroke symptoms[3]. Exposure to normobaric oxygen could not only increase oxygen dissolution in blood, but also enhance the combination of hemoglobin with oxygen, thus ensuring increased oxygen concentration for the body[4]. Because the pathophysiological process of most diseases is related to ischemia and anoxia, the treatment of normobaric oxygen is a good method to treat ischemic and anoxic diseases[5]. Normobaric oxygen inhalation could not only treat ischemic and anoxic disease, but also have a deep influence its prevention. As with ischemic and anoxic preconditioning, normobaric oxygen preconditioning could enhance the capacity of anti-oxidants, and prevent subsequent serious ischemic and anoxic injury. This hypothesis has been confirmed using a cerebral ischemia model, but the exact mechanism remains unknown. Several studies demonstrated that normobaric oxygen preconditioning had the ability to decrease the permeability of the blood-brain barrier, thus relieving brain edema[6]. Furthermore, normobaric oxygen could up-regulate glutamate transporters and decrease neuronal injury caused by increased excitatory glutamic acid and free radical aggregation[7]. Normobaric oxygen could also inhibit the activities of nicotinamide adenine dinucleotide phosphate oxidase and matrix metalloproteinase-9, and prolong the therapeutic time window of acute stroke[8,9,10,11].
Studies surrounding the protective effect of hypoxia-inducible factor-1α and its downstream genes now focus on neuronal anoxic preconditioning[12]. Preconditioning can protect post-ischemic neurons through inducing target gene expression such as vascular endothelial growth factor, erythropoietin, glucose transporter-1 and glucose synthase. The Notch signal transduction pathway plays a significant role in the recovery process after cerebral injury. Hypoxia-inducible factor is the housekeeper transcription factor and a key regulator of Notch-1[13,14,15]. Previous studies revealed that hypoxia-inducible factor-1α could combine with Notch in a low-oxygen environment, and together regulate downstream target genes and influence cellular proliferation and differentiation. Notch signaling is a highly evolutionarily conserved pathway that regulates many aspects of cellular differentiation in many organisms. It mediates cell to cell signaling between adjacent cells expressing Notch receptors and Notch ligands[16]. Experiments have demonstrated that hypoxia increases the half-life of Notch, an effect that requires the presence of hypoxia-inducible factor-1α[17]. Notch-1 signal pathway is started by ligand binding, which induces proteolytic cleavage of Notch-1 to liberate release the Notch intracellular domain (NICD), an intracellular domain. The NICD goes into the nucleus and then to inhibit transcriptional effectors by the interaction with a transcriptional activation complex. The NICD can stimulate the neuronal differentiation of a few ependymal cells in the case of focal ischemia as well[18]. Previous researches have suggested that hypoxia-inducible factor-1 potentiated Notch signal pathway in embryonic neural stem cells, P19 carcinoma[19] and medullolastoma precursors[20], but the signal pathway has not been illuminated yet. This study aimed to confirm that normobaric oxygen preconditioning may prevent and treat ischemic injury through activating hypoxia-inducible factor-1α and its target genes such as Notch-1, vascular endothelial growth factor and erythropoietin.
RESULTS
Quantitative analysis of experimental animals
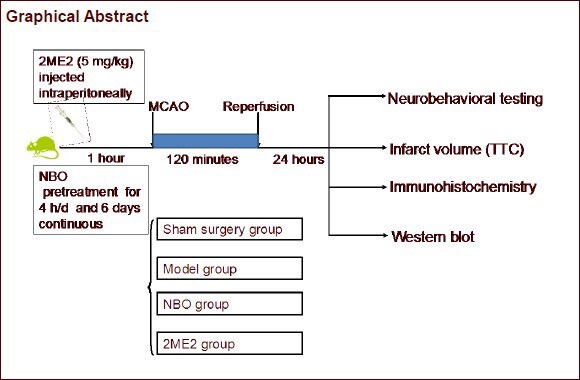
Sixty Sprague-Dawley male rats were equally and randomly assigned to the following four groups: sham surgery (control), model (middle cerebral artery occlusion/reperfusion), normobaric oxygen pretreatment (middle cerebral artery occlusion pretreated with normobaric oxygen), and 2-methoxyestradiol pretreatment (middle cerebral artery occlusion with normobaric oxygen injected with 2-methoxyestradiol). The ischemic model was established in the latter three groups. Sixty rats were included in the final analysis except for the twelve rats died in the study (n = 0 in sham surgery group; n = 5 in model group; n = 3 in normobaric oxygen pretreatment group; n = 4 in 2-methoxyestradiol pretreatment group). Approximately two-thirds of the deaths were induced by subarachnoid hemorrhage, as confirmed by autopsy. Neurological deficits were observed in all rats at 24 hours after reperfusion. Rats were then sacrificed and samples were processed for 2,3,5-triphenyltetrazolium chloride (TTC) staining, western blot assay, and Nissl and immunohistochemical staining. The number of rats for each analysis technique was five.
Normobaric oxygen pretreatment reduced the neurological deficits of rats with focal cerebral ischemia
The neurological deficit scores were detected 24-hour after cerebral ischemia/reperfusion. Neurological function in rats of model group decreased significantly compared with the sham surgery group (P < 0.05). However, normobaric oxygen pretreatment improved neurological function in rats with focal cerebral ischemia (P < 0.05). However, no significant effect was observed between 2-methoxyestradiol pretreatment and the model groups (P > 0.05; Figure 1).
Figure 1.
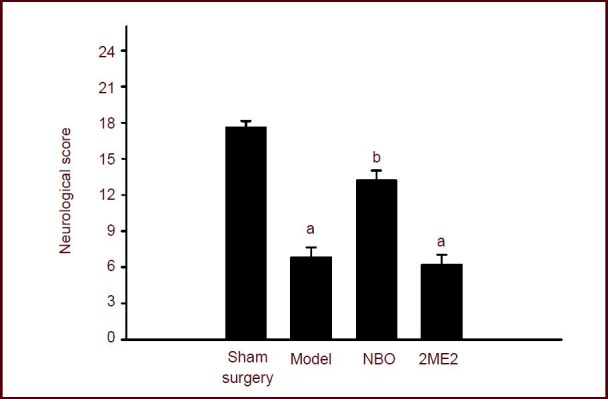
Effect of normobaric oxygen pretreatment on neurological function in rats with focal cerebral ischemia reperfusion.
Maximum possible score is 18 points and minimum score is 3 points. The lower the score, the more severe the neurologic injury. Data were expressed as mean ± SEM. Statistical significance was determined using one-way analysis of variance followed by the Tukey post hoc test for multiple comparisons. aP < 0.05, vs. sham surgery group; bP < 0.05, vs. model group. NBO: Normobaric oxygen; 2ME2: 2-methoxyestradiol pretreatment.
Normobaric oxygen pretreatment decreased infarction size in rats with focal cerebral ischemia
TTC staining showed a white infarction in the cortex and basal ganglia after ischemic injury. After normobaric oxygen pretreatment, the infarct area was confined to the basal ganglia, which is the ischemic core in this model. The infarct volume was significantly decreased in rats with middle cerebral artery occlusion pretreated with normobaric oxygen compared with the model group (P < 0.05), while 2-methoxyestradiol injection did not decrease the infarct volume in rats with middle cerebral artery occlusion (P > 0.05; Figure 2).
Figure 2.
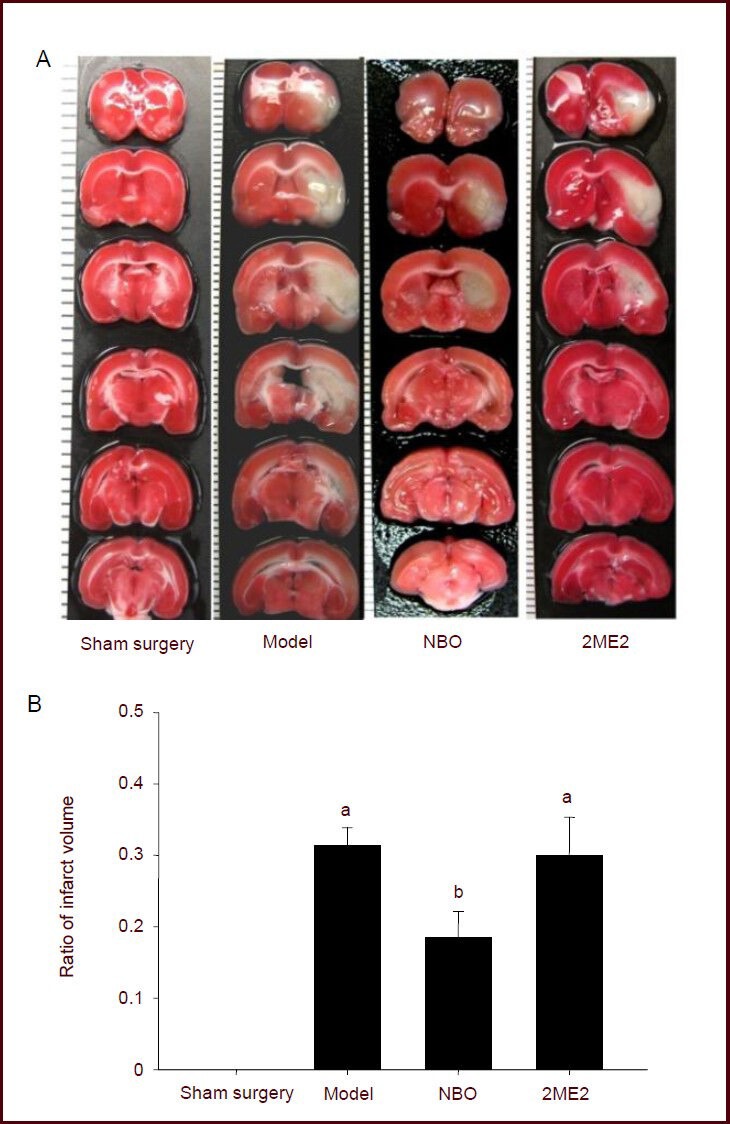
Effect of NBO on infarct volume in focal cerebral ischemia rats.
(A) 2,3-5-Triphenyltetrazolium chloride (TTC) staining of cerebral infarction. Normal tissue stained deep red and infarct tissue with loss of mitochondrial enzyme activity did not stain, but appeared white[21]. The border between stained and unstained tissues was well demarcated and could be identified easily by visual inspection[22].
(B) Infarct volume. The ratio of infarct volume = infarct area of the ipsilateral hemisphere/total area of the ipsilateral hemisphere. Data were expressed as mean ± SEM. Statistical significance was determined using one-way analysis of variance followed by Tukey post hoc test for multiple comparisons. aP < 0.05, vs. sham surgery group; bP < 0.05, vs. model group. NBO: Normobaric oxygen; 2ME2: 2-methoxyestradiol pretreatment.
Normobaric oxygen pretreatment improved the morphology of the cerebral cortex in rats with focal cerebral ischemia
Nissl staining showed that in the sham surgery group, normal cortical layers of cells were arranged in rows. The neurons remained intact and showed no features of edema. Neurons in the basal ganglia were also normal. Cell bodies of cortical neurons were large, displayed a pale cytoplasm, and contained large, round vesicular nuclei. The Nissl bodies could be seen in the cytoplasm. In the model group, neural cells were poorly arranged and cell bodies were swollen. Nuclear pyknosis and fusion were observed. Some of the neurons appeared triangular and the Nissl bodies decreased or disappeared. Cell shape was also abnormal in the normobaric oxygen pretreatment group. However, cortical neuronal structure was clear. The degree of dissolution of the nucleus was smaller than that in the model group. The use of hypoxia-inducible factor inhibitor 2-methoxyestradiol improved cell deformation compared with the model group (Figure 3).
Figure 3.
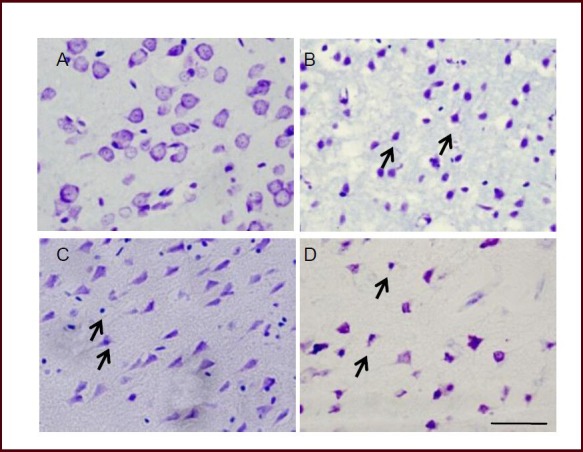
Effect of normobaric oxygen pretreatment on the pathological features in tissue obtained from focal cerebral ischemia rats (Nissl staining).
(A) Intact neuronal cells could be found in sham operated animals. (B) After severe ischemia, cells with aberrant morphology (triangular in shape exhibiting dark staining due to condensation of cytoplasm and karyoplasms) in the rat of model group were seen in the penumbral region.
Arrows indicate the deformed neurons. More surviving cells in the penumbral area were seen in the normobaric oxygen pretreatment group (C), and 2-methoxyestradiol pretreatment had little effect on damaged cellular morphology (D). Scale bar: 20 μm.
Normobaric oxygen pretreatment increased expression of hypoxia-inducible factor-1α, Notch-1, vascular endothelial growth factor and erythropoietin in rats with focal cerebral ischemia
Immunohistochemistry revealed the expression of hypoxia-inducible factor-1α, vascular endothelial growth factor, erythropoietin and Notch-1 in the penumbral area of the ipsilateral hemisphere.
No immunoreactivity or positive staining was observed in the sham-operated rats. Massive immunoreactivity of hypoxia-inducible factor-1α, vascular endothelial growth factor and erythropoietin and Notch-1 was localized in infarct regions in the model group after 24 hours of reperfusion. However, the extent of hypoxia-inducible factor-1α, vascular endothelial growth factor, erythropoietin and Notch-1 immunostaining was increased in normobaric oxygen pretreated rats and decreased in 2-methoxyestradiol treated rats (Figure 4).
Figure 4.
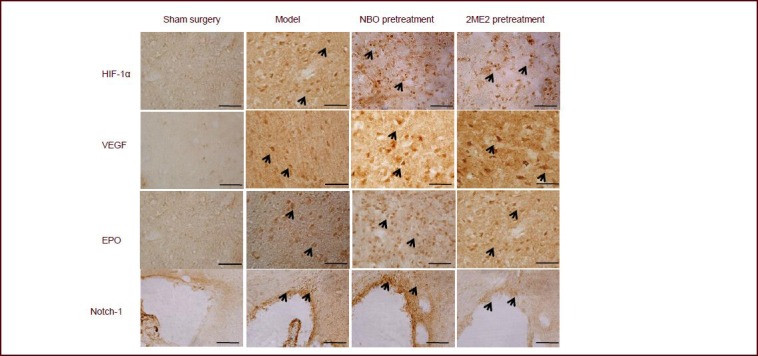
Immunohistochemistry showing the effect of normobaric oxygen (NBO) pretreatment on the expression of hypoxia-inducible factor (HIF)-1α, vascular endothelial growth factor (VEGF), erythropoietin (EPO) and Notch-1 in the penumbral area of the ipsilateral hemisphere.
No immunoreactivity for HIF-1α VEGF or EPO was observed in the sham-operated rats. Massive immunoreactivity for HIF-1α, VEGF and EPO were found in cells of the infarct region at 24 hours after reperfusion and NBO inhalation. However, the extent of immunostaining decreased in the 2-methoxyestradiol (2ME2) treated rats. No immunoreactivity for Notch-1 was observed in the sham-operated rats. Notch-1 was found to be robustly expressed in cells from the model and NBO pretreatment groups in the subventricular zone, but only mildly expressed in the 2ME2 pretreatment group. Arrows indicate positive staining in cells. Scale bars: 50 μm.
Western blot assay of the cerebral cortex including infarct area showed a strong up-regulation of hypoxia-inducible factor-1α, Notch-1, vascular endothelial growth factor and erythropoietin at 24 hours after reperfusion. Markers were also increased by normobaric oxygen preconditioning (P < 0.05). However, the level of hypoxia-inducible factor-1α, Notch-1, vascular endothelial growth factor and erythropoietin was decreased by 2-methoxyestradiol treatment (P < 0.05; Figure 5).
Figure 5.
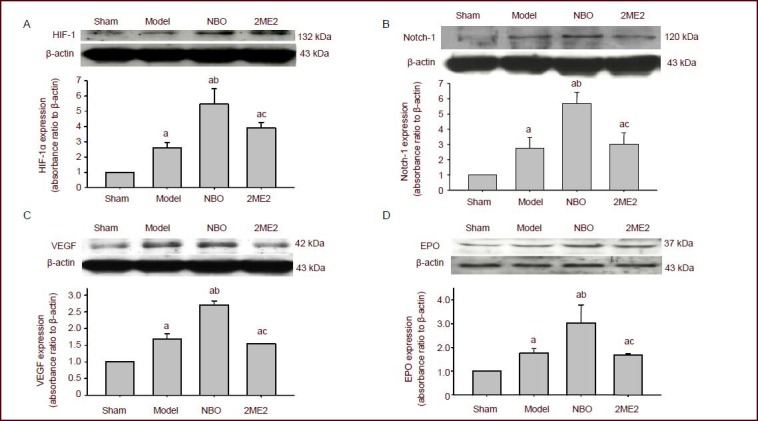
Western blot assay showing the effect of normobaric oxygen pretreatment (NBO) on the protein expression of hypoxia-inducible factor (HIF)-1α (A), vascular endothelial growth factor (VEGF; C), erythropoietin (EPO; D) and Notch-1 (B) in the penumbral area of the ipsilateral hemisphere.
Data were expressed as mean ± SEM. Statistical significance was determined using one-way analysis of variance followed by the Tukey post hoc test for multiple comparisons. aP < 0.05, vs. sham surgery (sham) group; bP < 0.05, vs. model group; cP < 0.05, vs. NBO group. 2ME2: 2-Methoxyestradiol pretreatment.
Normobaric oxygen pretreatment increased the number of nestin-positive cells in the subventricular zone of rats with focal cerebral ischemia
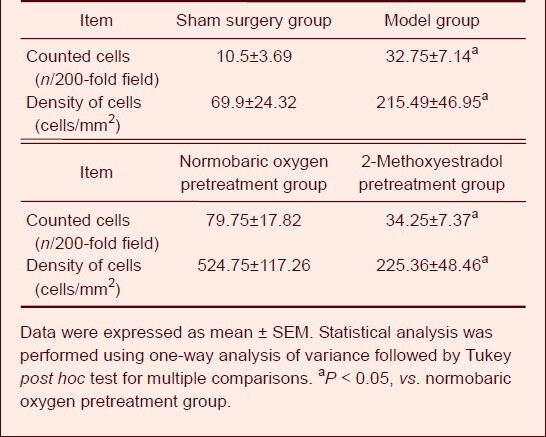
Immunohistochemistry staining demonstrated that the amount of nestin-positive cells in the subventricular zone after normobaric oxygen pretreatment was significantly higher than that in the model and 2-methoxyestradiol pretreatment group (P < 0.05; Table 1).
Table 1.
Effects of normobaric oxygen pretreatment on the number of nestin-positive cells in the subventricular zone of rats with focal cerebral ischemia
DISCUSSION
This study investigated the protective effect and the underlying mechanism of normobaric oxygen preconditioning in ischemic brain injury in a focal ischemia/ reperfusion model.
Hypoxia-inducible factor-1 is a transcription factor discovered by Semenza et al[23] in 1992 and cloned in 1995. Hypoxia-inducible factor-1 is closely related to hypoxia-induced specific response, and has a deep influence on gene induced by hypoxia. The activity and protein expression of hypoxia-inducible factor-1 in brain increased after ischemia, with the increase of its downstream genes Notch-1, vascular endothelial growth factor and erythropoietin. 2-Methoxyestradiol is a kind of estrogen derivative and is nowadays mainly used in phase II clinical trials for tumor suppression. Mabjeesh et al[24] believed that 2-methoxyestradiol could inhibit expression of hypoxia-inducible factor-1 at the transcription level, which would subsequently inhibit the expression of its downstream genes. In this study, 2-methoxyestradiol inhibited the protein expression of hypoxia-inducible factor-1, Notch-1, vascular endothelial growth factor and erythropoietin. Normobaric oxygen preconditioning enabled tolerance to brain ischemia that was shown by the reduced infarct area seen with TTC staining and improved neurological score by enhancing the expression of hypoxia-inducible factor-1 and the regulated genes Notch-1, vascular endothelial growth factor and erythropoietin. Thereby, these results confirmed that the protective effect of normobaric oxygen preconditioning on cerebral ischemia/reperfusion injury probably occurs via the upregulation of hypoxia-inducible factor-1 and its downstream genes.
Several studies revealed that the hypoxia-inducible factor-1 signal pathway regulates neurogenesis via an increase of vascular endothelial growth factor and erythropoietin[25]. It is well known that vascular endothelial growth factor, a critical factor in neurogenesis and angiogenesis, stimulates neural stem cell proliferation and the migration of neural progenitors in the subventricular zone in vitro[26]. Hypoxia-inducible factor-1 might be an important factor for maintaining the vascular niche environment and promoting angiogenesis through transcriptional modulation of vascular endothelial growth factor. Finally, hypoxia-inducible factor-1 represented an intrinsic regulator of differentiation and might ultimately be proven therapeutically useful for intensifying recovery and repair in the adult brain.
Hypoxia-inducible factor-1 may be beneficial in ischemic preconditioning
Bergeron et al[27] found that upregulation of hypoxia-inducible factor-1 and its related genes promoted vessel remodeling around the infarct area after acute focal cerebral ischemia, which was helpful for promoting neuronal survival in ischemic brain. Hypoxia-inducible factor-1 induces the expression of a series of genes such as vascular endothelial growth factor, erythropoietin, glucose transporter-1 and glycolytic enzyme, and this is important for cell survival in a low-oxygen environment. Mechanisms of the protective effect of hypoxia-inducible factor in cerebral ischemic injury may occur by promoting the reconstruction of the microcirculation. Hypoxia-inducible factor induces the increased expression of vascular endothelial growth factor, improves angiogenesis and augments perfusion and oxygen supplement in ischemic tissue, which aids and promotes recovery. Hypoxia-inducible factor can also ameliorate energy metabolism after cerebral ischemia. Ischemic preconditioning induces the expression of hypoxia-inducible factor-1 which causes an increased mRNA level and the protein expression of glucose transporter-1, aldolase A, lactic dehydrogenase A and phosphofructokinase protein level[28]. Thus, it promoted the transport and usage of glucose and glycolysis, and provided more energy for brain tissue allowing a reduction in cerebral injury. Hypoxia-inducible factor can also regulate erythropoietin level to protect the brain. The protective effect of hypoxia-inducible factor-1 on cerebral ischemia was mostly studied in the adaptive response after hypoxic preconditioning. Studies confirmed that preconditioning could alleviate brain edema, decrease permeability of the blood-brain barrier and ameliorate neuronal function post-reperfusion by expressing hypoxia-inducible factor-1. Increased expression of hypoxia-inducible factor-1 protein is one of the mechanisms in improving cerebral ischemic tolerance[29].
Effect of hypoxia-inducible factor-Notch signal pathway on brain injury
The Notch signal transduction pathway consists of receptors, ligands and DNA binding proteins. When Notch ligand binds to its receptor, it is activated and mediates some important physiological functions such as cellular proliferation, differentiation and apoptosis. Androutsellis-Theotokis et al[30] found, using in vivo and in vitro experiments, that activation of the Notch pathway regulated the quantity of stem cells, and exogenous Notch protein ligand injection improved neurological function of adult rats after ischemic injury.
In the recovery process after ischemia and reperfusion, there are two key factors. One is the recovery of injured neurons and necrotic neuron replacement, and the other is angiogenesis that provides enough nutrient substance for tissue. The study of ischemia/reperfusion injury model showed that Notch-1 played a key role in the process of inducing neuronal regeneration after ischemia. Therefore, Notch-1 might play an important role in the cerebral repair process after normobaric oxygen exposure. Hypoxia-inducible factor is the housekeeper transcription factor of hypoxic tolerance and also the key regulator of Notch-1[13,14,15].
The present study demonstrated that repeating normobaric oxygen exposure could prevent cerebral ischemic/reperfusion injury, and this protective effect might be exerted via regulating the hypoxia-inducible factor signaling pathway. This finding illustrates the beneficial effects of normobaric oxygen application as a potential treatment strategy.
MATERIALS AND METHODS
Design
A randomized, controlled animal trial.
Time and setting
This protocol was performed in the Department of Anatomy and Histoembryology in Peking University Health Science Center in China from March 2010 to July 2011.
Materials
Sixty male Sprague-Dawley rats weighing 260–280 g were used in the study and they were provided by the Center of Experimental Animals of Peking University Health Science Center, China (license No. SYXK (Jing) 2011-0039]. The animals were housed in individual cages in a specific pathogen free environment maintained at a constant temperature of 22–25°C and moisture of 55 ± 5% with free access to food and water before and after surgery. All experimental protocols were conducted according to the Guidance Suggestions for the Care and Use of Laboratory Animals, pressed by the Ministry of Science and Technology of China[31].
Methods
Establishment of focal cerebral ischemia model
Focal cerebral ischemia was conducted by intraluminal middle cerebral artery[32] blockage with a nylon suture, as previously described by Longa et al[33] with minor modification by Kawamura et al[34]. Briefly, the rats were anesthetized by 4% isoflurane with a mixture of 60% medical air and 40% oxygen and anesthesia was maintained with 2% isoflurane. Rats were placed in the supine position on a heated operating table with body temperature maintained at around 37 ± 0.5°C. Throughout the experiment, frequent checks were made to ensure that the animals were adequately anesthetized. This was performed by applying a painful stimulus to a paw and observing blood pressure responses.
The bifurcation of the right common carotid artery was exposed. The right middle cerebral artery was occluded for 2 hours by insertion of a 4.0 monofilament nylon suture with a slightly enlarged and round tip through the internal carotid artery from external carotid artery. The suture was withdrawn allowing reperfusion. A successful occlusion of the right middle cerebral artery is achieved when the left forelimb is paretic after filament introduction.
Normobaric oxygen treatment and intraperitoneal injection of hypoxia-inducible factor-1α inhibitor
The rats in the normobaric oxygen pretreatment group inhaled 95% O2 (Beijing ZG Special Gases Science & Technology Co., Ltd., Beijing, China) for 4 hours every day for 6 days prior to model induction. The rats in the 2-methoxyestradiol group were intraperitoneally injected with 5 mg/kg[24] 2-methoxyestradiol (BIOMOL Inc, Farmingdale, USA) at 1 hour before inhalation of normobaric oxygen.
Neurological deficits evaluated by neurological scores
The neurological scores, based on the scoring system of Garcia et al[35], were performed in a blinded fashion at 24 hours after reperfusion. The test scores included spontaneous activity, symmetry in the movement of four limbs, forepaw outstretching, climbing, body proprioception and response to vibrissae touch.
TTC staining and evaluation of infarction volume
TTC staining was performed at 24 hours after reperfusion to determine the infarction volume. Brain tissues were cut into 2-mm thick coronal sections and immersed in 2% solution of TTC (Sigma, St. Louis, MO, USA) for 30 minutes at 37°C. Then, the stained slices were fixed by immersion in 4% formaldehyde solution. The infarct area of each section was traced and measured using an image analysis system [Imaging-Pro-Plus (Olympus, Silver Spring, MD, USA)]. The ratio of infarct volume was then calculated with the following formula: infarct area of the ipsilateral hemisphere/total area of the ipsilateral hemisphere.
Nissl staining of the infarct area
After anesthesia, rats were transcardiac perfusd with 250 mL of 4% paraformaldehyde in 0.1 mol/L PBS (pH 7.4) at 24 hours after reperfusion. Brain was removed and fixed with 4% paraformaldehyde for another 48 hours. Sucrose in PBS (30%) was used for cryoprotecting brains for 48 hours at 4°C, and coronal brain sections (20-μm thick) were cut on a cryostat (Leica, Heidelberger, Germany). The sections were hydrated in 1% toluidine blue at 50°C for 20 minutes for Nissl staining. Then they were dehydrated and mounted with Permount (Fisher Scientific, Pittsburgh, PA, USA) after rinsing with distilled water. The slide was observed under an Olympus optical microscope.
Immunohistochemical staining for hypoxia-inducible factor-1α, Notch-1, vascular endothelial growth factor, erythropoietin and nestin expression in the rat cortex
Rats were transcardiacally perfused with 250 mL of 4% paraformaldehyde in 0.1 mol/L PBS (pH 7.4) after anesthesia at 24 hours after reperfusion. Then, the brains were removed and post-fixed with 4% paraformaldehyde for 48 hours. 30% sucrose was used for cryoprotecting brain for over 48 hours at 4°C, and coronal brain sections (20-μm thick) were cut on a cryostat. 3% Hydrogen peroxide (H2O2) was used for sections to prevent reaction with endogenous peroxidases. After 60 minutes of incubation with 3% normal serum in PBS, the sections were incubated with primary antibodies overnight at 4°C. The following antibodies were used: rabbit anti-hypoxia-inducible factor-1α polyclonal antibodies (1:200); rabbit anti-Notch1 polyclonal antibodies (1:200); goat anti-vascular endothelial growth factor polyclonal antibodies (1:200) and rabbit anti-erythropoietin polyclonal antibodies (1:200) and goat anti-Nestin polyclonal antibodies (1:200; Santa Cruz Biotechnology, Santa Cruz, CA, USA). After rinsing with PBS, the sections were then incubated with goat anti-rabbit IgG and rabbit anti-goat IgG (1:200; Santa Cruz Biotechnology) as a secondary antibody at room temperature for 30 minutes and were placed in avidin-peroxidase complex solution containing avidin-peroxidase conjugate for 30 minutes. Peroxidase activity was revealed by dipping the sections in a mixture containing 3,3’-diaminobenzidine (DAB) and H2O2 (ABC kit; Santa Cruz Biotechnology) at room temperature for 5 minutes. The sections were mounted, air-dried, dehydrated, and cover-slipped. Application of control serum, instead of the primary antibody, on another section of the same brain provided a negative control for each staining. Imaging-Pro Plus was used to count the number of nestin-positive cells in the subventricular zone of animals from different groups in accordance with the methods described previously[30]. We chose the subventricular zone as the observation zone for counting nestin-positive cell number. The density of positive cells was measured to compare the differences among groups. The semiquantitative morphometry was performed as reported previously[36]: Assessment and counting of positive cells in cortex utilized a transparent grid template containing a fixed number of squares with consistent dimensions (100 μm2). For comparison purposes, the number of cells per unit of cortical surface area was standardized to 1 mm2. These counts were made on a minimum of five sections per brain area per each of the five animals in each group. The 12 square grids were positioned on the computer monitor to overlay a predetermined and consistent area of the cerebral cortex that was observable on the monitor screen of each serial brain section under study. Over each section, the grid was positioned at 10 ×, and then magnified to 20 × to count cells. Initial positioning of the grid template was consistently the same between all brain sections and the treatment groups were blind to the observers. Following positioning, two independent investigators counted the number of positive neurons within 6 of the 12 squares using a systematic random sampling method of grid square selection[37].
Hypoxia-inducible factor-1α, Notch-1, vascular endothelial growth factor and erythropoietin protein level using the method of Western blot assay in the rat cortex
Brain tissue samples of rats (n = 5) from each group were used for western blot assay. After anesthesia, rats were sacrificed and brains were removed at 24 hours after reperfusion. The cortex, including the penumbral area, was dissected using the corpus callosum as a ventral landmark[38]. Samples were obtained from the cortex in the territory of the middle cerebral artery on the ischemic hemisphere. Tissues were homogenized in ice-cold lysis buffer (0.32 mol/L sucrose, 1 mmol/L ethylenediaminetetraacetate, 5 mmol/L Tris-HCl, pH 7.4, 0.1 mmol/L phenylmethylsulfonyl fluoride, 10 μmol/L eupepsin, 10 μmol/L pepstatin A, and 1 mmol/L β-mercaptoethanol). The protein content was first detected by Bio-Rad protein assay. Equal amounts of protein per lane (50 μg) were loaded onto an 8% polyacrylamide gel and separated by electrophoresis at 90 V for 30 minutes and then 120 V for 1.5 hours. Proteins were then transferred to nitrocellulose at 200 V for 2 hours and the membrane was blocked with 5% nonfat dry milk/0.5% Tween-20 in Tris-buffered saline for 2 hours. The nitrocellulose was then incubated with different antibodies overnight at 4°C: rabbit anti-hypoxia-inducible factor-1α polyclonal antibodies (1:800); rabbit anti-Notch-1 polyclonal antibodies (1:800); goat anti-vascular endothelial growth factor polyclonal antibodies (1:1 000) and rabbit anti-erythropoietin polyclonal antibodies (1:1 000; Santa Cruz Biotechnology). The membrane was then treated with horseradish peroxidase-conjugated goat anti-rabbit and rabbit anti-goat secondary antibody (1:3 000; Santa Cruz Biotechnology) for 60 minutes at 37°C. Immunoblots were probed and then exposed to X-ray film. The X-ray films were scanned and the absorbance was determined by Bio-Rad Image analysis (Bio-Rad, Hercules, CA, USA). As an internal control, the same nitrocellulose membrane was incubated with an antibody specifically for rabbit anti-β-actin (1:1 000; Santa Cruz Biotechnology) after being stripped.
Statistical analysis
Data were expressed as mean ± SEM and were analyzed using SPSS software 13.0 (SPSS, Chicago, IL, USA). Statistical significance was assured by one-way analysis of variance followed by Tukey post hoc test. A P value of < 0.05 was considered statistically significant.
Research background: Normobaric oxygen could be used to treat ischemic and hypoxic disease, and also could be used to prevent the ischemic damage
Research frontiers: Normobaric oxygen pretreatment promotes neural stem cell proliferation and differentiation in the subventricular zone and dentate gyrus. This process may occur through activation of hypoxia-inducible factor 1α and Notch signaling, and thus prevent cerebral ischemia/reperfusion injury.
Clinical significance: As normobaric oxygen is readily available it may be used in treatment and prevention of cerebral ischemia and reperfusion in the future.
Academic terminology: Normobaric oxygen refers to high-concentration high-flow oxygen obtained from an apparatus of inspired oxygen under normal pressure.
Peer review: The experiment extends the research into and clinical applications of high-pressure oxygen, and has important values for understanding the mechanisms of high-pressure oxygen treatment for ischemic brain injury.
Footnotes
Funding: This work was supported by the National Natural Science Foundation of China, No. 81000523 and the grant from Peking University Health Science Center for the New Teacher Funding, No. BMU20090463.
Conflicts of interest: None declared.
Ethical approval: This protocol was approved by the Animal and Ethics Review Committee of Peking University Health Science Center in China.
(Reviewed by Apricò K, Robens J, Zhan SQ, Yan JH)
(Edited by Yu J, Qiu Y, Li CH, Song LP, Liu WJ, Zhao M)
REFERENCES
- [1].Nighoghossian N, Trouillas P. Hyperbaric oxygen in the treatment of acute ischemic stroke: an unsettled issue. J Neurol Sci. 1997;150(1):27–31. doi: 10.1016/s0022-510x(97)05398-7. [DOI] [PubMed] [Google Scholar]
- [2].Acka G, Sen A, Canakci Z, et al. Effect of combined therapy with hyperbaric oxygen and antioxidant on infarct volume after permanent focal cerebral ischemia. Physiol Res. 2007;56(3):369–373. doi: 10.33549/physiolres.930907. [DOI] [PubMed] [Google Scholar]
- [3].Michalski D, Härtig W, Schneider D, et al. Use of normobaric and hyperbaric oxygen in acute focal cerebral ischemia - a preclinical and clinical review. Acta Neurol Scand. 2011;123(2):85–97. doi: 10.1111/j.1600-0404.2010.01363.x. [DOI] [PubMed] [Google Scholar]
- [4].Bernaudin M, Nedelec AS, Divoux D, et al. Normobaric hypoxia induces tolerance to focal permanent cerebral ischemia in association with an increased expression of hypoxia-inducible factor-1 and its target genes, erythropoietin and VEGF, in the adult mouse brain. J Cereb Blood Flow Metab. 2002;22(4):393–403. doi: 10.1097/00004647-200204000-00003. [DOI] [PubMed] [Google Scholar]
- [5].Mickel HS, Kempski O, Feuerstein G, et al. Prominent white matter lesions develop in Mongolian gerbils treated with 100% normobaric oxygen after global brain ischemia. Acta Neuropathol. 1990;79(5):465–472. doi: 10.1007/BF00296104. [DOI] [PubMed] [Google Scholar]
- [6].Bigdeli MR, Hajizadeh S, Froozandeh M, et al. Prolonged and intermittent normobaric hyperoxia induce different degrees of ischemic tolerance in rat brain tissue. Brain Res. 2007;1152:228–233. doi: 10.1016/j.brainres.2007.03.068. [DOI] [PubMed] [Google Scholar]
- [7].Bigdeli MR, Hajizadeh S, Froozandeh M, et al. Normobaric hyperoxia induces ischemic tolerance and upregulation of glutamate transporters in the rat brain and serum TNF-alpha level. Exp Neurol. 2008;212(2):298–306. doi: 10.1016/j.expneurol.2008.03.029. [DOI] [PubMed] [Google Scholar]
- [8].Henninger N, Fisher M. Normobaric hyperoxia-a promising approach to expand the time window for acute stroke treatment. Cerebrovasc Dis. 2006;21(1-2):134–136. doi: 10.1159/000090446. [DOI] [PubMed] [Google Scholar]
- [9].Liu W, Sood R, Chen Q, et al. Normobaric hyperoxia inhibits NADPH oxidase-mediated matrix metalloproteinase-9 induction in cerebral microvessels in experimental stroke. J Neurochem. 2008;107(5):1196–1205. doi: 10.1111/j.1471-4159.2008.05664.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Liu W, Hendren J, Qin XJ, et al. Normobaric hyperoxia reduces the neurovascular complications associated with delayed tissue plasminogen activator treatment in a rat model of focal cerebral ischemia. Stroke. 2009;40(7):2526–2531. doi: 10.1161/STROKEAHA.108.545483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Liu W, Hendren J, Qin XJ, et al. Normobaric hyperoxia attenuates early blood-brain barrier disruption by inhibiting MMP-9-mediated occludin degradation in focal cerebral ischemia. J Neurochem. 2009;108(3):811–820. doi: 10.1111/j.1471-4159.2008.05821.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Semenza GL. Expression of hypoxia-inducible factor 1: mechanisms and consequences. Biochem Pharmacol. 2000;59(1):47–53. doi: 10.1016/s0006-2952(99)00292-0. [DOI] [PubMed] [Google Scholar]
- [13].Zhu LL, Wu LY, Yew DT, et al. Effects of hypoxia on the proliferation and differentiation of NSCs. Mol Neurobiol. 2005;31(1-3):231–242. doi: 10.1385/MN:31:1-3:231. [DOI] [PubMed] [Google Scholar]
- [14].Mazure NM, Brahimi-Horn MC, Berta MA, et al. HIF-1: master and commander of the hypoxic world. A pharmacological approach to its regulation by siRNAs. Biochem Pharmacol. 2004;68(6):971–980. doi: 10.1016/j.bcp.2004.04.022. [DOI] [PubMed] [Google Scholar]
- [15].Magalhães J, Ascensão A, Soares JM, et al. Acute and chronic exposition of mice to severe hypoxia: the role of acclimatization against skeletal muscle oxidative stress. Int J Sports Med. 2005;26(2):102–109. doi: 10.1055/s-2004-817858. [DOI] [PubMed] [Google Scholar]
- [16].Pistollato F, Rampazzo E, Persano L, et al. Interaction of hypoxia-inducible factor-1α and Notch signaling regulates medulloblastoma precursor proliferation and fate. Stem Cells. 2010;28(11):1918–1929. doi: 10.1002/stem.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Cejudo-Martin P, Johnson RS. A new notch in the HIF belt: how hypoxia impacts differentiation. Dev Cell. 2005;9(5):575–576. doi: 10.1016/j.devcel.2005.10.001. [DOI] [PubMed] [Google Scholar]
- [18].Carlén M, Meletis K, Göritz C, et al. Forebrain ependymal cells are Notch-dependent and generate neuroblasts and astrocytes after stroke. Nat Neurosci. 2009;12(3):259–267. doi: 10.1038/nn.2268. [DOI] [PubMed] [Google Scholar]
- [19].Gustafsson MV, Zheng X, Pereira T, et al. Hypoxia requires notch signaling to maintain the undifferentiated cell state. Dev Cell. 2005;9(5):617–628. doi: 10.1016/j.devcel.2005.09.010. [DOI] [PubMed] [Google Scholar]
- [20].Pistollato F, Abbadi S, Rampazzo E, et al. Hypoxia and succinate antagonize 2-deoxyglucose effects on glioblastoma. Biochem Pharmacol. 2010;80(10):1517–1527. doi: 10.1016/j.bcp.2010.08.003. [DOI] [PubMed] [Google Scholar]
- [21].Bederson JB, Pitts LH, Germano SM, et al. Evaluation of 2,3,5-triphenyltetrazolium chloride as a stain for detection and quantification of experimental cerebral infarction in rats. Stroke. 1986;17(6):1304–1308. doi: 10.1161/01.str.17.6.1304. [DOI] [PubMed] [Google Scholar]
- [22].Isayama K, Pitts LH, Nishimura MC. Evaluation of 2,3,5- triphenyltetrazolium chloride staining to delineate rat brain infarcts. Stroke. 1991;22(11):1394–1398. doi: 10.1161/01.str.22.11.1394. [DOI] [PubMed] [Google Scholar]
- [23].Semenza GL, Jiang BH, Leung SW, et al. Hypoxia response elements in the aldolase A, enolase 1, and lactate dehydrogenase A gene promoters contain essential binding sites for hypoxia-inducible factor 1. J Biol Chem. 1996;271(51):32529–32537. doi: 10.1074/jbc.271.51.32529. [DOI] [PubMed] [Google Scholar]
- [24].Mabjeesh NJ, Escuin D, LaVallee TM, et al. 2ME2 inhibits tumor growth and angiogenesis by disrupting microtubules and dysregulating HIF. Cancer Cell. 2003;3(4):363–375. doi: 10.1016/s1535-6108(03)00077-1. [DOI] [PubMed] [Google Scholar]
- [25].Schölzke MN, Schwaninger M. Transcriptional regulation of neurogenesis: potential mechanisms in cerebral ischemia. J Mol Med (Berl) 2007;85(6):577–588. doi: 10.1007/s00109-007-0196-z. [DOI] [PubMed] [Google Scholar]
- [26].Zhang H, Vutskits L, Pepper MS, et al. VEGF is a chemoattractant for FGF-2-stimulated neural progenitors. J Cell Biol. 2003;163(6):1375–1384. doi: 10.1083/jcb.200308040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Bergeron M, Yu AY, Solway KE, et al. Induction of hypoxia-inducible factor-1 (HIF-1) and its target genes following focal ischaemia in rat brain. Eur J Neurosci. 1999;11(12):4159–4170. doi: 10.1046/j.1460-9568.1999.00845.x. [DOI] [PubMed] [Google Scholar]
- [28].Jones NM, Bergeron M. Hypoxic preconditioning induces changes in HIF-1 target genes in neonatal rat brain. Cereb Blood Flow Metab. 2001;21(9):1105–1114. doi: 10.1097/00004647-200109000-00008. [DOI] [PubMed] [Google Scholar]
- [29].Wacker BK, Perfater JL, Gidday JM. Hypoxic preconditioning induces stroke tolerance in mice via a cascading HIF, sphingosine kinase, and CCL2 signaling pathway. J Neurochem. 2012;123(6):954–962. doi: 10.1111/jnc.12047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Androutsellis-Theotokis A, Leker RR, Soldner F, et al. Notch signalling regulates stem cell numbers in vitro and in vivo. Nature. 2006;442(7104):823–826. doi: 10.1038/nature04940. [DOI] [PubMed] [Google Scholar]
- [31].The Ministry of Science and Technology of the People's Republic of China. Guidance Suggestions for the Care and Use of Laboratory Animals. 2006 Sep 30; [Google Scholar]
- [32].Badr AE, Yin W, Mychaskiw G, et al. Effect of hyperbaric oxygen on striatal metabolites: a microdialysis study in awake freely moving rats after MCA occlusion. Brain Res. 2001;916(1-2):85–90. doi: 10.1016/s0006-8993(01)02867-0. [DOI] [PubMed] [Google Scholar]
- [33].Longa EZ, Weinstein PR, Carlson S, et al. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20(1):84–91. doi: 10.1161/01.str.20.1.84. [DOI] [PubMed] [Google Scholar]
- [34].Kawamura S, Yasui N, Shirasawa M, et al. Rat middle cerebral artery occlusion using an intraluminal thread technique. Acta Neurochir (Wien) 1991;109(3-4):126–132. doi: 10.1007/BF01403007. [DOI] [PubMed] [Google Scholar]
- [35].Garcia JH, Wagner S, Liu KF, et al. Neurological deficit and extent of neuronal necrosis attributable to middle cerebral artery occlusion in rats. Statistical validation. Stroke. 1995;26(4):627–635. doi: 10.1161/01.str.26.4.627. [DOI] [PubMed] [Google Scholar]
- [36].Neese SL, Sherill LK, Tan AA, et al. Vagus nerve stimulation may protect GABAergic neurons following traumatic brain injury in rats: An immunocytochemical study. Brain Res. 2007;1128(1):157–163. doi: 10.1016/j.brainres.2006.09.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Elias H, Hyde DM. An elementary introduction to stereology (quantitative microscopy) Am J Anat. 1980;159(4):412–446. doi: 10.1002/aja.1001590407. [DOI] [PubMed] [Google Scholar]
- [38].Ashwal S, Tone B, Tian HR, et al. Core and penumbral nitric oxide synthase activity during cerebral ischemia and reperfusion. Stroke. 1998;29(5):1037–1047. [PubMed] [Google Scholar]


