Abstract
The proprietary Chinese medicine preparation Kaiyu Granule is made of bupleurum, nutgrass galingale rhizome, szechwan lovage rhizome, turmeric root tuber, white peony alba, cape jasmine fruit, fried semen ziziphi jujubae, and prepared liquorice root. It is a common recipe for the clinical treatment of depression in China. In this study, after 21 days of unpredictable stress exposure, Wistar rats exhibited similar behavioral changes to patients with depression. Moreover, G-protein-coupled inwardly rectifying K+ channel 1 mRNA and protein expression were significantly reduced in rat hippocampal CA1 and CA3 regions. However, G-protein-coupled inwardly rectifying K+ channel 1 mRNA, protein expression, and rat behavior were clearly better after administration of 12, 8, or 4 g/kg of Kaiyu Granule when depression model rats underwent stress. 12 g/kg of Kaiyu Granule had the most obvious effects on the increased expression of G-protein-coupled inwardly rectifying K+ channel 1 mRNA and protein in rat hippocampal CA1 and CA3 regions. These results suggested that Kaiyu Granule improved depression by affecting G-protein-coupled inwardly rectifying K+ channel 1 expression in the rat hippocampus.
Keywords: neural regeneration, chronic stress, hippocampus, fluoxetine hydrochloride capsules, depression, neuropeptide, G-protein-coupled inwardly rectifying K+ channel 1, in situ hybridization, grants-supported paper, neuroregeneration
INTRODUCTION
Chaihu Shugan Powder is a representative recipe for dispersing depressed liver-energy and regulating vital energy in Chinese medicine. Zhu and colleagues[1] confirmed that Chaihu Shugan Powder diminished P-endorphin levels in rat plasma and hypothalamus and indicated that Chaihu Shugan Powder regulated hypothalamic-pituitary-adrenal axis in rat models of chronic restraint stress. Xiong and Zhu[2] verified that Chaihu Shugan Powder partially antagonized stress-induced slow increases in body weight, elevated adrenocorticotropic hormone levels, and increased sucrose water intake in stressed rats, indicating that Chaihu Shugan Powder adjusts disordered function in rat models of chronic restraint stress. However, previous studies mainly focused on the effects of Chinese drugs on neurotransmitters (serotonin, noradrenalin, dopamine and acetylcholine) in animal models of depression, and have not studied neuropeptide protein expression levels in the nerve conduction pathway. Kaiyu Granule is a recipe after addition and subtraction of Chaihu Shugan Powder, prepared by a Hospital Affiliated to Changchun University of Chinese Medicine, China. Nevertheless, the mechanism of action of Chaihu Shugan Powder on depression, especially effects on depression-related protein expression levels remain unclear.
The G-protein-coupled inwardly rectifying K+ channel (GIRK) is a member of inwardly rectifying K+ channel family. It can be directly activated by G-protein β- and γ-subunits, and it regulates presynaptic membrane neurotransmitter release and neuronal excitability. A previous study verified that GIRK directly coupled to many neurotransmitters and neuropeptide receptors, and is a direct effector between the heart and many neurotransmitters[3]. GIRK is a member of a seven member subfamily of inwardly rectifying K+ channel and is composed of five subunits (i.e., GIRK 1–5). Of these subunits, GIRK1 protein is expressed in hippocampal CA1, CA2 and CA3. GIRK exists as a tetramer. At present, in situ hybridization results show that GIRK is extensively distributed in various tissues, couples to various receptors in the central nervous system, and play important roles in maintaining resting potentials and slow postsynaptic inhibition[4]. A study of G-protein-coupled ion channels has been performed on the acetylcholine potassium channel, which has been shown to be constructed by GIRK 1–4[5]. Acetylcholine-induced GIRK opening and K+ efflux through the G protein induced hyperpolarization. Another study found that each subunit of GIRK1–3 was widely distributed in the brain, such as in the hippocampus, neocortex, olfactory system, diencephalon, and cerebellum[6]. GIRK4 is mainly distributed to the cerebellar granular cell layer, superior colliculus, lateral septal nuclei, and hippocampal CA3 pyramidal neurons[7]. Numerous inhibitory neurotransmitters act on their corresponding receptors and then activate GIRK by activating the pertussis toxin-sensitive G protein. GIRK suppresses neuronal excitability by producing slow, inhibitory postsynaptic potentials GIRK regulates neurotransmitter release via presynaptic inhibition in the brain. GIRK1 protein in the paraventricular hypothalamic nucleus exists presynaptically but not postsynaptically. Neurons projecting to the paraventricular nucleus are located in several nerve nuclei (such as the nucleus raphe dorsalis and parabrachial nuclei) of the hypothalamus, limbic system, and brain stem[8]. In situ hybridization results have confirmed that GIRK1 mRNA exists in these neurons, and these neurons contain dopamine, noradrenalin, and neuropeptide. Dopamine, noradrenalin, and neuropeptide receptor have been shown to couple to GIRK in neurons and heterogeneous cells[9]. In the hippocampal CA1 region, GIRK is connected to excitatory synapses but not to inhibitory synapse, indicating that these channels regulate excitatory synapse-mediated signal afference[10]. When noradrenergic neurons are in a persistent depolarized excitatory state, GIRKs are in a closed inhibitory state for a long time, and GIRK density on the cell membrane can decrease. This can result in GIRK1 mRNA expression in the hippocampus and parietal cortex that is noticeably lower in depression model subjects compared to controls[11]. In situ hybridization results demonstrate that GIRK1 mRNA expression is lower in the brain of depression model rats. However, GIRK reduced the frequency peak potentials of neurons, produces slow inhibitory postsynaptic potentials, exerts postsynaptic inhibitory effects, and finally suppresses neuronal excitability[12]. Therefore, GIRK1 mainly exerts postsynaptic inhibitory effects in neurons that can release inhibitory neurotransmitters, such as GABAergic neurons. Reduced GIRK1 expression leads to increased inhibitory effects of GABAergic neurons, possibly suppressing the excitability of aminergic neurons, and providing a biochemical basis for the onset of depression[13].
In this study, we sought to establish models of depression using classical chronic stress and isolation methods, which are different from pharmacological models and models of olfactory tubercle resection. We analyzed the the effects of Kaiyu Granule on GIRK1 protein expression in the hippocampal CA1 and CA3 regions of depression model rats using in situ hybridization, which has been rarely used previously to study traditional Chinese medicine. We determined the mechanisms of action of Kaiyu Granule for the treatment of depression at the molecular level in the neuroendocrine system.
RESULTS
Quantitative analysis of experimental animals
A total of 72 rats were equally and randomly assigned to six groups: normal, model, fluoxetine, low-, moderate- and high-dose Kaiyu Granule groups. Models of depression were established using chronic stress in the latter five groups. The rats in the high-, moderate-, and low-dose Kaiyu Granule groups were intragastrically administered 12, 8 or 4 g/kg Kaiyu Granule. The rats in the fluoxetine group were intragastrically administered fluoxetine hydrochloride capsules. All rats were included in the final analysis.
Kaiyu Granule improved the behavioral performance of depression model rats
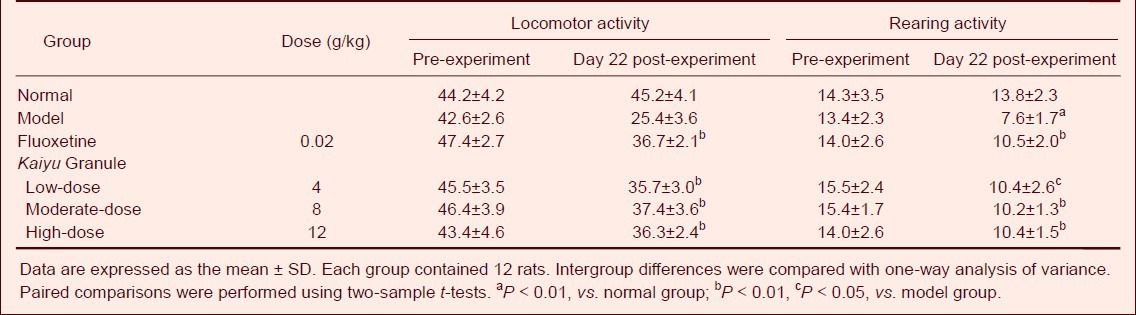
Body weight, sucrose water consumption, locomotor activity, and rearing activity significantly increased in depression rats at 22 days of the experiment (at 1 day after drug administration) in the Kaiyu Granule groups compared to those in the model group (P < 0.01, P < 0.05). Moreover, no significant difference in behavior was detected between the Kaiyu Granule groups and the fluoxetine group (P > 0.05; Tables 1, 2).
Table 1.
Effects of Kaiyu Granule on body weight (g) and sucrose water consumption (mL) in depression model rats
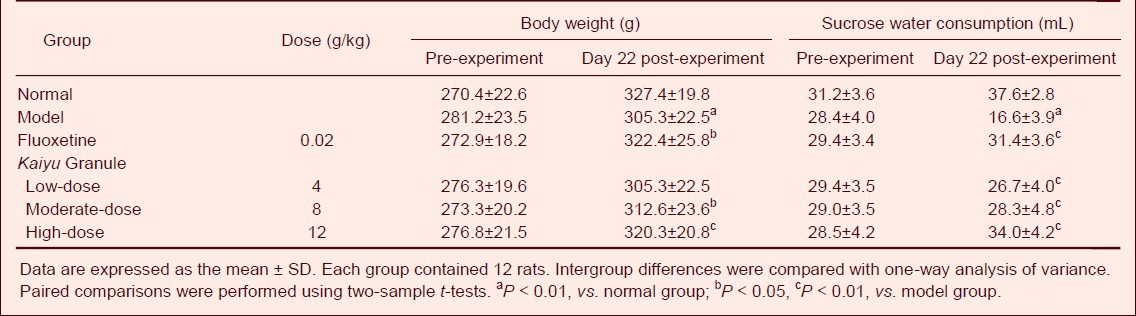
Table 2.
Effects of Kaiyu Granule on locomotor activity (point/5 minutes) and rearing activity (point/5 minutes) in depression model rats
Kaiyu Granule increased GIRK1 expression in the hippocampal CA1 and CA3 regions of depression model rats
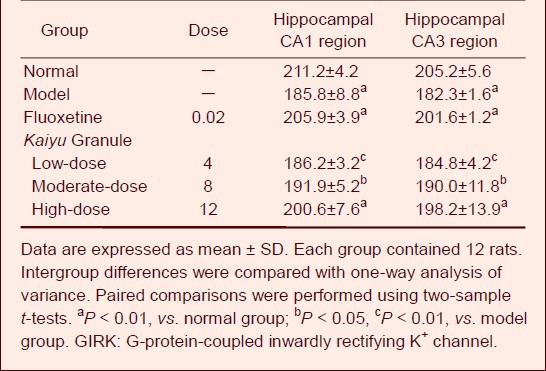
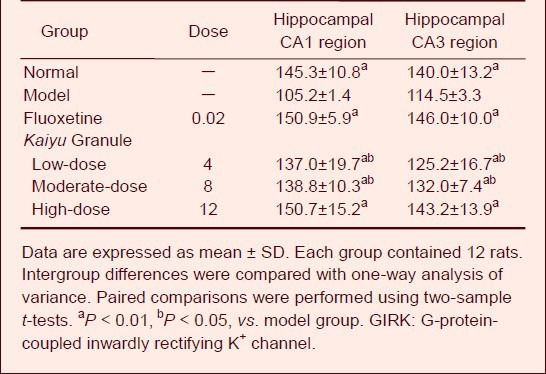
GIRK1 expression increased in the hippocampal CA1 and CA3 regions in depression model rats as compared with the normal group. At 21 days after drug administration, no significant alteration in GIRK1 expression was detected in the low-dose Kaiyu Granule group (P > 0.05), but GIRK1 expression significantly increased in the moderate- and high-dose Kaiyu Granule groups and fluoxetine group (P < 0.05, P < 0.01). It is worthwhile to note that GIRK1 expression in the hippocampal CA1 and CA3 regions was similar in the moderate- and high-dose Kaiyu Granule groups and fluoxetine group (P > 0.05; Figure 1, Table 3).
Figure 1.
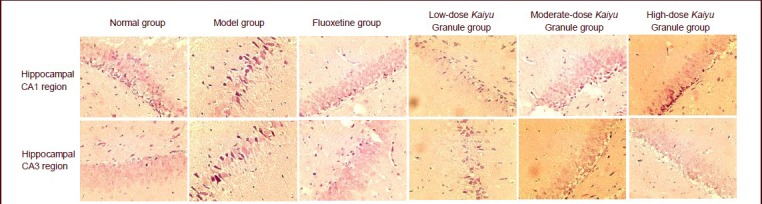
GIRK1 expression in the rat hippocampal CA1 and CA3 regions (immunohistochemistry, × 40).
Moderate- and high-dose Kaiyu Granule evidently increased GIRK1 expression in the hippocampal CA1 and CA3 regions of depression model rats, and their effects were similar to those of fluoxetine. GIRK1-positive expression was brownish red color. GIRK: G-protein-coupled inwardly rectifying K+ channel.
Table 3.
Effects of Kaiyu Granule on GIRK1 expression (absorbance) in hippocammpal CA1 and CA3 regions of depression model rats
Kaiyu Granule increased GIRK1 mRNA expression in hippocampal CA1 and CA3 regions of depression model rats
GIRK1 mRNA expression increased in the hippocampal CA1 and CA3 regions of depression model rats as compared with the normal group. At 21 days after drug administration, GIRK1 mRNA expression significantly increased in various Kaiyu Granule groups and the fluoxetine group (P < 0.05, P < 0.01). It is worthwhile to note that GIRK1 mRNA expression in the hippocampal CA1 and CA3 regions of depression rats was similar between the high-dose Kaiyu Granule and fluoxetine groups (P > 0.05; Figure 2, Table 4).
Figure 2.
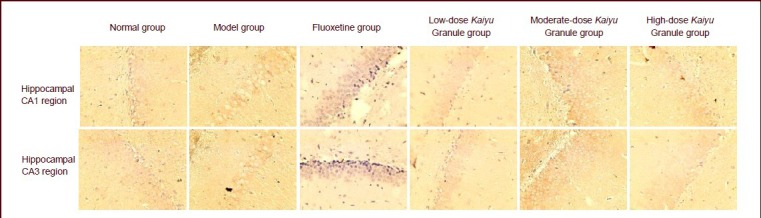
GIRK1 mRNA expression in the rat hippocampal CA1 and CA3 regions (in situ hybridization, × 40).
Various doses of Kaiyu Granule evidently increased GIRK1 mRNA expression in the hippocampal CA1 and CA3 regions of depression model rats, and their effects were similar to those of fluoxetine. GIRK1 mRNA-positive expression was brownish red color. GIRK: G-protein-coupled inwardly rectifying K+ channel.
Table 4.
Effects of Kaiyu Granule on GIRK1 mRNA expression (gray scale) in the hippocammpal CA1 and CA3 regions of depression model rats
DISCUSSION
This study demonstrated that GIRK1 expression in the hippocampal CA1 region was significantly higher in the high-dose Kaiyu Granule and fluoxetine groups compared to the untreated depression model group. GIRK1 expression in the hippocampal CA1 region was also high in the low- and moderate-dose Kaiyu Granule groups, but apparently reduced in the model group. GIRK1 expression in the hippocampal CA3 region was also evidently increased in the high- and moderate-dose Kaiyu Granule groups and fluoxetine group. GIRK1 expression in the hippocampal CA3 region was also high in the low-dose Kaiyu Granule group, but obviously diminished in the model group. Kaiyu Granule obviously reduced the decrease in body weight in depression model rats and increased sucrose water consumption, which suggested that Kaiyu Granule improved their behavior, increased locomotor activity and rearing activity, and improved depression symptoms. Increased GIRK1 mRNA expression reduced postsynaptic inhibitory effects, increased neuronal excitability, diminished the number of neurons that could release inhibitory neurotransmitters such as GABAergic neurons, and decreased postsynaptic inhibitory effects.
Increased GIRK1 expression reduced the inhibitory effects of GABAergic neurons and possibly increased monoaminergic neuronal excitability[14] decreased depression-like symptoms. In summary, Kaiyu Granule could increase GIRK1 expression in hippocampal CA1 and CA3 regions of depression rats, indirectly increase the secretion of monoamine neurotransmitters, and finally reduce depression-like symptoms.
MATERIALS AND METHODS
Design
A randomized controlled animal study.
Time and setting
Experiments were performed in the College of Pharmacy, Jilin University, China, from June 2006 to March 2007.
Materials
Animals
A total of 72 clean adult male Wistar rats aged 2 months and weighing 180–220 g were provided by Changchun Gaoxin Animal Center, China, animal license No. SCXK-(Ji) 2007-0003. Animals were allowed free access to food and water (except during fasting diet and water deprivation stress). They were housed at 18–22°C (except during hot and cold stress) and at a humidity of 60–70%. The protocols were conducted in accordance with the Guidance Suggestions for the Care and Use of Laboratory Animals, formulated by the Ministry of Science and Technology of China[15].
Drugs
Kaiyu Granule was provided by Department of Pharmacy, Changchun University of Chinese Medicine, China. Kaiyu Granule (nosocomial preparation) was made of bupleurum, nutgrass galingale rhizome, szechwan lovage rhizome, turmeric root tuber, white peony alba, cape jasmine fruit, fried semen ziziphi jujubae, and prepared liquorice root. All materials were decocted three times with an eight-times volume of distilled water, with the first decoction for 2 hours, second decoction for 2 hours, and third decoction for 1 hour. Decoction solutions were mixed and filtered. Filtrate was condensed to clear paste, which was dried and triturated. The preparation was mixed with sucrose, treated with 75% alcohol, granulated, dried at 80°C, and made into granules[16]. 1 mL herb liquor containing 1 g crude drug was ensured, and then the preparation was stored at 4°C.
Fluoxetine hydrochloride capsules (C17H18F3NO·HCl) were produced by Apotex Inc., Canada, repacked by Liaoyuan Yadong Pharmaceutical Factory, specification: 20 mg/granule; permission number of import drugs H20040354.
Methods
Preparation of depression model rats
Depression model rats were established by a chronic stress method[17,18]. Each rat was isolated in an individual cage, and underwent various stressors over 21 days, including swimming in cold water (4°C, 5 minutes), water deprivation (24 hours), a high temperature environment (45°C, 5 minutes), fasting diet (24 hours), tail-clamping (1 minute), weaving (once a second, 5 minutes), electric shock on a footplate (5 mV, one stimulus was done every 30 seconds, each for 10 seconds, totally 15 times), and daytime or nighttime inversion. Over these 21 days, one stressor was pseudorandomly chosen and given each day. Each kind of stressor was given 2–3 times. The same stressor was not given 2 days in a row. Thus, the rats could not predict which stressor they would receive. In the normal group, six rats were housed in each cage and allowed free access to food and water, but they did not receive any stressors.
Intragastric administration of Kaiyu Granule
The rats in the high-, moderate-, and low-dose Kaiyu Granule groups were intragastrically administrated 12, 8, or 4 g crude drugs/kg Kaiyu Granule during chronic stress, separately at 50, 20, and 5 times the clinical dose[1,19]. The rats in the fluoxetine group were given 2 mg/kg fluoxetine hydrochloride capsules, which is seven times the clinical dose (0.333 mg/kg). The outer coating of the capsule was removed, and the drugs were dissolved in distilled water. The rats were intragastrically administered the drug once at 8 a.m. every day for 21 consecutive days[20]. The rats in the normal and model groups were given 10 mL/g distilled water by intragastric administration at 8 a.m., once a day, for 21 consecutive days.
Behavior detection
Body weight: The body weights of the rats in each group were determined with an electronic balance before the experiment and after 21 days of stress.
Sucrose preference test: At 10:00 a.m., 1% sucrose water and plain water were separately laid around the sides of the cage in each group. The position of the water container was exchanged every 12 hours over 48 hours to acclimate the subjects to the container. After 24 hours of water deprivation and fasting, all rats were administered bottles of 1% sucrose water and plain water. The sucrose consumption percentage within 3 hours was calculated as: sucrose consumption percentage (%) = sucrose consumption/(sucrose + plain water consumption) × 100%.
Open field test: The open-field box was made of wood, with a length, width, and height of 80 cm × 80 cm × 40 cm, and contained 25 squares of equal area. The inner wall and undersurface were black. The test was conducted after model induction and after drug administration. The rats were placed in the center of an open-field box under quiet conditions at room temperature with soft light. The frequency of crossing the square was used to determine locomotor activity. Three or four claws entering the square was scored as 1. The frequency of rearing was determined by rearing activity. Removing two forelimbs from the ground (> 1 cm) once was scored 1. Each rat was measured once each session and the additional rat was not assessed until the box was cleared.
Preparation of specimens
After 21 days of daily stress, the rats were anesthetized with sodium pentobarbital 1 mL/200 g (Tianjin Pharmaceutical Group Xinzheng Co., Ltd., Xinzheng, Henan Province, China). By left ventricular intubation, the rats were fixed with saline and 4% paraformaldehyde (prepared with PBS). The brain was obtained by decapitation, postfixed in 4% paraformaldehyde for further use[21], and then serially sliced into 6–8 μm-thick coronal sections with a cryostat. The brain sections containing the hippocampus[22] were placed in 0.1 mol/L PBS[23,24].
Immunocytochemical staining of GIRK1 expression in rat hippocampus
The sections were dewaxed, treated with 3% H2O2 at room temperature for 10 minutes to deactivate endogenous enzymse, washed three times with distilled water, immersed in 0.01 mol/L citrate buffer (pH 6.0), and heated with a microwave oven. The power supply was cut off until boiling, with an interval of 5–10 minutes, repeated for 1–2 times. After cooling, the sections were washed twice with PBS (pH 7.2–7.6) and blocked with fetal bovine serum at room temperature for 20 minutes. Any unnecessary liquid was discarded. Without washing, the sections were incubated in mouse anti-rat GIRK1 monoclonal antibody (1:100; Shanghai Hengfei Biological Technology Co., Ltd., Shanghai, China) at 7°C for 1 hour, washed with PBS (2 minutes × 3), incubated with biotinylated goat anti-mouse IgG (1:50; Beijing Hongyuechuangxin Technology Co., Ltd., Beijing, China) at 37°C for 20 minutes, washed with PBS (2 minutes × 3). Subsequently, the sections were incubated in streptavidin-peroxidase complex at 37°C for 20 minutes, washed with PBS (5 minutes × 4), visualized with 3,3’-diaminobenzidine, lightly counterstained with hematoxylin, dehydrated, permeabilized, and mounted[25]. Images of the sections were analyzed using a light microscope (Olympus, Japan) and high-definition color pathological image analysis system (Huida Instruments Co., Ltd., Hubei Province, China). Absorbance values were measured after the gray scale was regulated. Three visual fields of each section was selected, and the average value was calculated[26,27].
In situ hybridization of GIRK1 mRNA expression in rat hippocampus
Each rat hippocampus was dewaxed. The sections were immersed in 0.1 mol/L PBS at room temperature (5 minutes × 3), washed with 0.1 mol/L glycine and 0.1 mol/L PBS for 5 minutes, at room temperature, followed by a wash with 0.4%Triton X-100-PBS for 10 minutes at room temperature to induce protein denaturation and to increase penetration of the probe. The sections were washed with 0.1 mol/L PBS (5 minutes × 3) at room temperature, digested with 1 μg/mL proteinase K 37°C for 30 minutes. Detected mRNA was exposed. The reaction was terminated by 4% paraformaldehyde for 5 minutes at room temperature and washed in 0.1 mol/L PBS (5 minutes × 3). Excess fixative solution was removed. The specimens were treated with 0.25% acetic anhydride (prepared by 0.1 mol/L triethanolamine) for 10 minutes at room temperature, prehybridized in prehybridization solution (5 × standard saline citrate, 50% formamide) at 37°C for 2 hours, and hybridized in hybridization solution at 42°C for 12–18 hours (the probe concentration was 250 ng/mL). Subsequently, the sections were washed with 4 × with standard saline citrate at room temperature (10 minutes × 3), 2 × with standard saline citrate at room temperature (10 minutes × 3), 2 × with standard saline citrate + RNaseA (10 μg/mL) at 37°C for 30 minutes, 0.1 × with standard saline citrate at room temperature (10 minutes × 3), and washed with TSM1 at room temperature for 10 minutes. The sections were incubated with TSM2 containing anti-DIG antibody (1:1 000–3 000) at room temperature for 3 hours, washed with TSM1 at room temperature for 10 minutes, washed with TSM3 at room temperature for 5 minutes, and then visualized in TSM3 containing nitro blue tetrazolium and 5-bromo-4-chloro-3-indolyl phosphate for 3–6 hours in the dark. The reactions were terminated by adding TE buffer. The sections were placed on chrome-alum gelatin-coated slides at 37°C overnight, dehydrated, permeabilized, and mounted with neutral gum. All hybridization procedures were conducted after RNA enzyme was deactivated in accordance with a digoxin-labeled oligonucleotide probe in situ hybridization kit[28,29] (Boster, Wuhan, Hubei Province, China). The average gray value was calculated using a light microscope (Olympus) and high-definition color pathological image analysis system[30]. Three visual fields of each section was selected, and the average value was calculated[22].
Statistical analysis
The data are expressed as mean ± SD, and analyzed using SPSS 13.0 software (SPSS, Chicago, IL, USA). Intergroup differences were compared with one-way analysis of variance. Paired comparisons were performed using two-sample t-tests. A value of P < 0.05 was considered statistically significant.
Research background: GIRK has been shown to directly couple to many inhibitory transmitter receptors, and it is a direct effector between the heart and many neurotransmitters in the brain.
Research frontiers: The mechanisms of many traditional Chinese drug compound preparations are unclear. This study investigated the effects of GIRK1 mRNA expression in the hippocampus of rats undergoing chronic stress, and discovered the partial mechanisms of Kaiyu Granule in treatment of depression.
Clinical significance: Results confirmed that Kaiyu Granule could improve depression symptom by affecting the expression of GIRK1. Kaiyu Granule is an effective drug for depression.
Academic terminology: Inwardly rectifying K+ channels are a specific subset of potassium selective ion channels and allow K+ to move more easily into rather than out of the cell.
Peer review: This study established depression model rats, used fluoxetine as a positive control, and found that Kaiyu Granule obviously increased the expression of GIRK1 in the hippocampal CA3 and CA1 regions, and confirmed the effects of Kaiyu Granule against depression.
Acknowledgments
We are very grateful to Sun B from College of Pharmacy of Jilin University in China for help in conducting immunohistochemistry and in situ hybridization, providing advice on technique for data collection, and assisting with the statistical analyses.
Footnotes
Funding: This study was supported by the New Drug Development Special-Purpose Foundation of State Administration of Traditional Chinese Medicine, No. 2005 DIX027A.
Conflicts of interest: None declared.
Ethical approval: This study was approved by the Animal Ethics Committee, College of Pharmacy of Jilin University in China.
(Reviewed by Murnane K, Pack M, Zhao JG, Zhu XJ)
(Edited by Yu J, Qiu Y, Li CH, Song LP, Liu WJ, Zhao M)
REFERENCES
- [1].Zhu QJ, Luo XL, Xiong ZF. Effect of Chaihu Shugan Powder on the regulation of hypothalamus-pituitary-adrenal axis in the rats with liver-depression syndrome. Hubei Zhongyi Zazhi. 2003;25(11):7–8. [Google Scholar]
- [2].Xiong ZF, Zhu QJ. Effect of Chaihu Shugan Powder on rats with liver-depression syndrome induced by chronic restrained stress. Zhongguo Zhongxiyi Jiehe Xiaohua Zazhi. 2004;12(4):220–221. [Google Scholar]
- [3].Mark MD, Herlitze S. G-protein mediated gating of inward-rectifier K + channels. Eur J Biochem. 2000;267(19):5830–5836. doi: 10.1046/j.1432-1327.2000.01670.x. [DOI] [PubMed] [Google Scholar]
- [4].Corey S, Clapham DE. Identification of native atrial G-protein-regulated inwardly rectifying K + (GIRK4) channel homomultimers. J Biol Chem. 1998;273(42):27499–27504. doi: 10.1074/jbc.273.42.27499. [DOI] [PubMed] [Google Scholar]
- [5].Jelacic TM, Kennedy ME, Wickman K, et al. Functional and biochemical evidence for G-protein-gated inwardly rectifying K + (GIRK) channels composed of GIRK2 and GIRK3. J Biol Chem. 2000;275(46):36211–36216. doi: 10.1074/jbc.M007087200. [DOI] [PubMed] [Google Scholar]
- [6].Lesage F, Guillemare E, Fink M, et al. Molecular properties of neuronal G-protein-activated inwardly rectifying K+ channels. J Biol Chem. 1995;270(48):28660–28667. doi: 10.1074/jbc.270.48.28660. [DOI] [PubMed] [Google Scholar]
- [7].Zhang H, He C, Yan X, et al. Activation of inwardly rectifying K + channels by distinct PtdIns(4,5)P2 interactions. Nat Cell Biol. 1999;1(3):183–188. doi: 10.1038/11103. [DOI] [PubMed] [Google Scholar]
- [8].Willner P. Validity, reliability and utility of the chronic mild stress model of depression: a 10-year review and evaluation. Psychopharmacology (Berl) 1997;134(4):319–329. doi: 10.1007/s002130050456. [DOI] [PubMed] [Google Scholar]
- [9].D’Aquila PS, Brain P, Willner P. Effects of chronic mild stress on performance in behavioural tests relevant to anxiety and depression. Physiol Behav. 1994;56(5):861–867. doi: 10.1016/0031-9384(94)90316-6. [DOI] [PubMed] [Google Scholar]
- [10].McEwen BS, De Kloet ER, Rostene W. Adrenal steroid receptors and actions in the nervous system. Physiol Rev. 1986;66(4):1121–1288. doi: 10.1152/physrev.1986.66.4.1121. [DOI] [PubMed] [Google Scholar]
- [11].Muma NA, Beck SG. Corticosteroids alter G protein inwardly rectifying potassium channels protein levels in hippocampal subfields. Brain Res. 1999;839(2):331–335. doi: 10.1016/s0006-8993(99)01754-0. [DOI] [PubMed] [Google Scholar]
- [12].Mirshahi T, Logothetis DE. Molecular determinants responsible for differential cellular distribution of G protein-gated inwardly rectifying K + channels. J Biol Chem. 2004;279(12):11890–11897. doi: 10.1074/jbc.M313322200. [DOI] [PubMed] [Google Scholar]
- [13].Katz RJ, Roth KA, Carroll BJ. Acute and chronic stress effects on open field activity in the rat: implications for a model of depression. Neurosci Biobehav Rev. 1981;5(2):247–251. doi: 10.1016/0149-7634(81)90005-1. [DOI] [PubMed] [Google Scholar]
- [14].Oitzl MS, Fluttert M, Sutanto W, et al. Continuous blockade of brain glucocorticoid receptors facilitates spatial learning and memory in rats. Eur J Neurosci. 1998;10(12):3759–3766. doi: 10.1046/j.1460-9568.1998.00381.x. [DOI] [PubMed] [Google Scholar]
- [15].The Ministry of Science and Technology of the People's Republic of China. Guidance Suggestions for the Care and Use of Laboratory Animals 2006-09-30 [Google Scholar]
- [16].Spauschus A, Lentes KU, Wischmeyer E, et al. A G-protein-activated inwardly rectifying K + channel (GIRK4) from human hippocampus associates with other GIRK channels. J Neurosci. 1996;16(3):930–938. doi: 10.1523/JNEUROSCI.16-03-00930.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Schoots O, Voskoglou T, Van Tol HH. Genomic organization and promoter analysis of the human G-protein-coupled K + channel Kir3.1 (KCNJ3/HGIRK1) Genomics. 1997;39(3):279–288. doi: 10.1006/geno.1996.4495. [DOI] [PubMed] [Google Scholar]
- [18].Hu WP, Li XM, Hu SW, et al. Effect of chronic stress on spatial learning and memory and nitric oxide in hippocampus of rats. Zhongguo Xinli Weisheng Zazhi. 2003;17(2):75–76. [Google Scholar]
- [19].Zhang L, Jiang Y, Wang TW, et al. Effect of XiaoYu Anshen capsules on morphology of hippocampal neurons in chronic stress rats. Zhongyao Yaoli yu Linchuang. 2006;22(2):56–57. [Google Scholar]
- [20].Tu Y, JIa BH, Shi YJ, et al. China Association of Acupuncture-Moxibustion, ed. Acupuncture Technical Specifications and Academic Development Symposium; 2005. Effect of electroacupuncture on Baihui and Yintang in the glucocorticoid and its receptors in chronic stress depression rats. [Google Scholar]
- [21].Shi RX, Wu Q, Qin LN, et al. Effect of electroacqupuncture Baihui and Yintang on the body weight and HPA axis in the rats with chronic stress. Zhenjiu Linchuang Yanjiu. 2007;23(1):50–53. [Google Scholar]
- [22].Lu JH, Jiang JF, Wang LL, et al. Rapid-effect mechanism on BDNF-TrkB in hippocampus of depressive model rats by electro-acupuncture. Zhenjiu Linchuang Zazhi. 2011;27(5):51–54. [Google Scholar]
- [23].Willner P, Towell A, Sampson D, et al. Reduction of sucrose preference by chronic unpredictable mild stress, and its restoration by a tricyclic antidepressant. Psychopharmacology (Berl) 1987;93(3):358–364. doi: 10.1007/BF00187257. [DOI] [PubMed] [Google Scholar]
- [24].Sun DW, Wang L, Sun ZR. Influence of acupuncture on hpa axis in a rat model of chronic stress-induced depression. Shanghai Zhenjiu Zazhi. 2007;26(2):32–34. [Google Scholar]
- [25].Liu HY, Wang YH, Hou HH, et al. Effect of intensive training on nitric oxide synthase positive neurons in rat hippocampus. Zhongguo Yundong Yixue Zazhi. 2006;25(1):98–100. [Google Scholar]
- [26].Qin LN, Shi RX, Tu Y. The effect of electroacupuncture on brain-gut peptides in rats with chronic stress induced depression. Anhui Zhongyi Xueyuan Xuebao. 2007;26(1):25–26. [Google Scholar]
- [27].Porsolt RD. Animal models of depression: utility for transgenic research. Rev Neurosci. 2000;11(1):53–58. doi: 10.1515/revneuro.2000.11.1.53. [DOI] [PubMed] [Google Scholar]
- [28].Zhou CC, Zeng YS, Qin YJ, et al. Effect of Valerlan on number of p-CREB positive neurons in cerebral hippocampus of depression-model rats induced by chrinoc mild stress. Jiepou Xue Zazhi. 2010;32(2):81–87. [Google Scholar]
- [29].Xie LS, Tang J, Wang L, et al. Effects of moxibustion on the expression of nitricoxide synthase in the hippocampus and dentate gyrus of rheumatoid arthritis rats. Xibu Yixue. 2009;21(5):710–712. [Google Scholar]
- [30].Huang XB, Mu N, Mei F, et al. Efficacy and brain-derived neurotrophic factor in serum of treatment of acupuncture combined drug on senile depression. Shiyong Yixue Zazhi. 2011;27(1):127–129. [Google Scholar]


