Abstract
Spastic cerebral palsy is generally considered to result from cerebral cortical or pyramidal tract damage. Here, we precisely targeted the left pyramidal tract of 2-month-old Sprague-Dawley rats placed on a stereotaxic instrument under intraperitoneal anesthesia. Based on the rat brain stereotaxic map, a 1-mm hole was made 10 mm posterior to bregma and 0.8 mm left of sagittal suture. A microsyringe was inserted perpendicularly to the surface of the brain to a depth of 9.7 mm, and 15 μL of ethanol was slowly injected to establish a rat model of spastic cerebral palsy. After modeling, the rats appeared to have necrotic voids in the pyramidal tract and exhibited typical signs and symptoms of flexion spasms that lasted for a long period of time. These findings indicate that this is an effective and easy method of establishing a rat model of spastic cerebral palsy with good re-producibility. Ethanol as a chemical ablation agent specifically and thoroughly damages the pyramidal tract, and therefore, the animals display flexion spasms, which are a typical symptom of the disease.
Keywords: neural regeneration, brain injury, spastic cerebral palsy, animal models, ethanol, pyramidal tract, stereotaxic instrument, targeted injection, modeling methods, neuroregeneration
Research Highlights
-
(1)
Targeted ethanol injection is a rapid and easy method of selectively damaging the pyramidal tract for establishing a rat model of spastic cerebral palsy with gross morphological and pathological features typical of the disease.
-
(2)
Ethanol, as a chemical ablation agent for complete pyramidal tract damage in the rat brain, has less impact on other parts of the brain. Thus, the characteristic symptoms and signs of the disease can be observed for an extended period of time in the model.
INTRODUCTION
Cerebral palsy is a syndrome caused by non-progressive brain injury and developmental defects during pregnancy, during childbirth or after birth up to infancy. Its main manifestations include motor dysfunction and abnormal posture[1]. Surveillance of Cerebral Palsy in Europe has shown that 2–3‰ of newborns per year suffer from cerebral palsy, and 40–100‰ of preterm babies and babies with low birth weight are found to have cerebral palsy at birth[2]. In developed countries, the rate of cerebral palsy is 1–2.5‰[3]. In recent years, neonatal mortality and the stillbirth rate have decreased significantly with the development of obstetric techniques and neonatal medicine; however, the incidence of cerebral palsy is gradually increasing[4]. Spastic cerebral palsy is the most common type of cerebral palsy, accounting for 45–60% of cerebral palsy patients. Generally, spastic cerebral palsy is considered to be caused by cerebral cortical or pyramidal tract damage. Patients with spastic cerebral palsy usually appear to have increased muscle tone, hyperreflexia, pathological reflex and other signs, with varying degrees of limb paralysis[5]. The pathogenesis of cerebral palsy is still unclear, and there is still no effective treatment[4,6,7,8]. Therefore, the clinical treatment of cerebral palsy is in need of significant advancement. The establishment of a stable and reliable animal model has become an important aim in the study of spastic cerebral palsy[9,10,11,12].
There are numerous studies on the use of animal models of spastic cerebral palsy. These include models generated by infection, ischemia and hypoxia, and bilirubin-induced brain injury[13,14,15,16,17,18,19,20]. Sun and Li[21] successfully prepared a rabbit model of cerebral palsy similar to human kernicterus by intraperitoneal injection of 300 mg/kg bilirubin. Mallard et al[22] performed unilateral uterine artery ligation in pigs at 30 days of pregnancy to prepare a pig model of intrauterine ischemia and hypoxia. Zhang and colleagues[23] removed part of the cerebral cortex and medulla via a skull opening to generate a model in rats. Du[24] exposed the motor cortex and inserted a microsyringe to a depth of 2 mm for injection of 10 μL ethanol to damage the motor cortex. Dixon et al[25] rapidly injected saline into the rat brain to increase intracranial pressure and damage brain tissue. Wu[26] and Yu[27] established a model of spastic cerebral palsy by electrical pyramidal tract damage. Studies from Delcour[28] and Vottier[29] showed that prenatal ischemia can cause white matter injury, thereby leading to cerebral palsy symptoms in rats. Repeated intraperitoneal or intrauterine injection of mucopolysaccharides into pregnant rats can stimulate the production of inflammatory cytokines that damage oligodendrocytes, promote the synthesis of other cytokines, and increase nitric oxide synthesis, neutrophil infiltration, and adhesion molecule expression, thereby inducing white matter lesions in the immature brain tissue[30,31]. Intraperitoneal injection of lipopolysaccharide induces localized endotoxin-mediated damage to the white matter, which is closely related to the local concentration of the lipoglycan in the tissue[32]. Neonatal ischemia-hypoxia can cause cerebral palsy in neonatal rats, with neuropathological features similar to humans[33]. Another study showed that intrauterine injection of mucopolysaccharides via the vagina, performed in rats at 15 days of pregnancy, produces behavior changes in the neonatal rats 1–21 days after birth[34]. Some studies showed that neonatal rats were subject to intrauterine injection of mucopolysaccharides, and then placed in a hypoxic environment to establish a cerebral palsy model. In addition, prenatal and perinatal hypoxia-ischemia is used by many scholars to establish a rat model of cerebral palsy[35]. Currently, the most common method of establishing a cerebral palsy model is subjecting neonatal rats to unilateral carotid artery ligation, followed by placement in an anoxic environment[36]. A combination method is used by some researchers for establishing cerebral palsy models. For example, Girard and colleagues[37] combined mucopolysaccharide injection with carotid artery ligation in pregnant rats, which were placed in a hypoxic environment postoperatively to create the cerebral palsy model.
The inflammatory response during cerebral hypoxic-ischemic reperfusion promotes secondary brain damage, which is one of the main causes of hypoxic-ischemic injury[38,39,40]. Interleukin-1β, a cytokine found in the early stage of hypoxic-ischemic brain injury, is a critical signaling molecule in the nerve-endocrine-immune system[41]. Zhang et al[42] found that interleukin-1β is involved in the pathogenesis of brain injury in adult rats during cerebral ischemia-reperfusion. Intrauterine infection in pregnant rats can increase the mRNA levels of interleukin-1β and tumor necrosis factor in the brain of neonatal rats in a dose-dependent manner. Elevated expression of glial fibrillary acidic protein is found in the hippocampus and cortex, and the number of astrocytes increases. The levels of myelin basic proteindecrease and oligodendrocyte activity is altered[43]. Inflammatory cytokines can cause white matter lesions in the immature brain by stimulating other cytokines, and by promoting nitric oxide synthesis, neutrophil infiltration and adhesion molecule expression, as well as by killing oligodendrocytes[44]. Derrick et al[45] established an intrauterine ischemia and hypoxia model in pregnant rabbits to simulate birth injuries caused by placental abruption. However, atypical symptoms or symptoms that are of limited duration in these animal models produced by the above methods limit the in-depth study of spastic cerebral palsy. Therefore, we precisely targeted the pyramidal tract using a stereotaxic instrument and used a microsyringe for ethanol injection, aiming to establish a new rat model of spastic cerebral palsy.
RESULTS
Quantitative analysis of experimental animals
Twelve male Sprague-Dawley rats were randomly assigned into model and control groups, with six rats in each group. Rats in the model group were given an intracranial injection of ethanol, targeting the left pyramidal tract, to establish a model of spastic cerebral palsy. In the control group, only a microsyringe was inserted into the brain. At the end of observation, one rat died from anesthesia overdose in each of the two groups. In the end, five rats from each group were included in the final analysis.
Targeted injection of ethanol effectively established a model of spastic cerebral palsy in Sprague-Dawley rats
By 18 hours after modeling, rats in the model group had no activity or food intake, and exhibited listlessness, obvious flexion spasms of the right forepaw and hind limbs (supplementary Video 1 online), and increased muscle tension in the right upper and lower limbs. At 36 hours postoperatively, the rats had a small amount of active food intake and movement, and mental status also improved. During exercise, claudication of the right limbs was apparent, and active and passive activities were accompanied with a clockwise circling movement with a diameter of about 20 cm (supplementary Video 2 online), and flexion spasm of the right limbs persisted. After 48 hours, the diet in the model group was slightly improved, but still worse than in the control group. Greater activity was displayed by the model rats, and mental status continued to improve; however, flexion spasms still persisted. By 72 hours after modeling, the diet in the model rats had begun to normalize, and mental status and physical activity were nearly normal, and flexion spasms of the right limbs had stabilized (Figure 1A).
Figure 1.
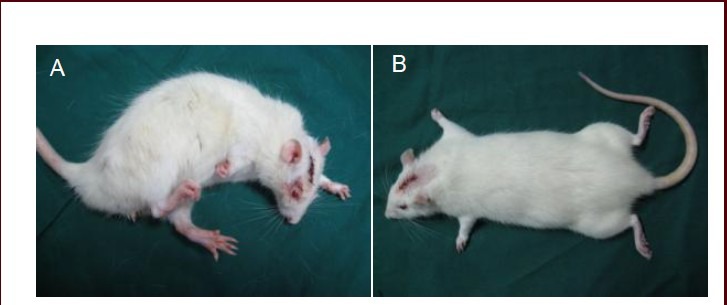
Rat behavior 72 hours postoperation.
(A) Spasms of the right limbs were visible in the model group. (B) No abnormalities were found in the limbs of rats in the control group.
In the control group, the rats had decreased activity and food intake, and poor mental status, but without flexion spasm of the right limbs, within 18 hours after surgery. Muscle tension was similar for the bilateral limbs, and their functioning was normal. After 36 hours, mental status, activities, and food intake were becoming normal. The rats in the control group completely recovered their activities, food intake and mental status 72 hours postoperatively (Figure 1B).
Targeted injection of ethanol produced a localized pyramidal tract lesion in Sprague-Dawley rats
Hematoxylin-eosin staining showed that at 72 hours postoperatively, the rats in the model group had necrotic voids in the pyramidal tract, but without injuries to other brain regions. In the control group, the pyramidal tract was normal (Figure 2).
Figure 2.
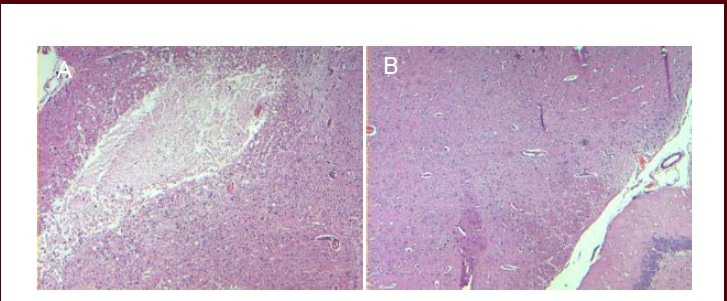
Morphology of the pyramidal tract 72 hours after surgery (hematoxylin-eosin staining, optical microscope, × 40).
(A) Rats in the model group appeared to have necrotic voids in the pyramidal tract. (B) No abnormalities were found in the pyramidal tract of control rats.
DISCUSSION
Advantages and disadvantages of targeted ethanol injection for establishing a rat model of spastic cerebral palsy
The stereotactic technology used in the present study provides for more accurate positioning, thereby minimizing surgical errors and significantly improving the reproducibility of the experiment. Neonatal and pregnant rats have been used to establish models of cerebral palsy. However, confounding factors can influence the pathophysiology in neonatal and pregnant rats. Additionally, neonatal and pregnant rats are much less resistant and tolerant to surgery than adult rats. Moreover, adult rats are inexpensive, easy to feed, and easy to test behaviorally. In particular, the large brain volume in adult rats facilitates pathological and biochemical analysis of the brain tissues[46]. Therefore, adult rats were preferred over neonatal and pregnant rats in the present study. Ethanol, a chemical ablation agent used in our study, is a better alternative to electrical stimulation to destroy the pyramidal tract. The brain is the body's nerve center, and nerve impulse conduction is an electrochemical process[47]. Although the electric current is only discharged from the tip of the insulated needle, the current conduction path when discharging is uncontrolled. Although the current damages the pyramidal tract, we cannot control or monitor its impact on the other parts of the brain. In comparison, ethanol destroys the pyramidal tract completely without damaging the other parts of the brain, thereby helping to maintain the specific signs and symptoms of spastic cerebral palsy for a long period of time. The chemical damage by ethanol is fast and direct, and requires no complex operations or cumbersome experimental procedures. Previous studies mostly focused on methodology or examination of microscopic morphological changes, and some scholars emphasized the histological changes. Spiegler et al[48] occluded four uterine arteries in maternal rats at 18 weeks of pregnancy for 45 minutes, and then collected the brain tissue of neonatal rats for histological examination. Periventricular leukomalacia is the major pathological feature of neonatal hypoxic-ischemic brain damage, and is the main cause of cerebral palsy[49]. Numerous experimental studies have focused on periventricular leukomalacia, oligodendrocyte loss and glial cell proliferation[50,51]. Riddle et al[52] observed changes to the sheep brain after acute hypoxia and ischemia reperfusion by measuring the blood flow distribution, oxygen saturation and pathological indexes in fetal sheep brain tissue, and found that periventricular white matter injury in the animal model was pathologically similar to white matter lesions in preterm infants. In a study by Wang et al[53], 5-day-old postnatal rats underwent intracranial injection of 3-nitropropionic acid. The pathological changes in the brain tissues included widespread damage to the white matter and cerebral cortex, corpus callosum atrophy and ventricular dilatation.
In our experiment, the microsyringe was vertically inserted into the pyramidal tract with no damage to other parts of the brain. Postoperative signs of flexion spasms were very obvious, but there were other symptoms as well. The control group was established to verify that ethanol is the prime agent destroying the pyramidal tract, rather than the microsyringe needle. In addition, we conducted preliminary experiments with many reagents, such as normal saline and hypertonic saline, to determine whether ethanol destroys the pyramidal tract by simply occupying volume. However, we found that there were no signs of flexion spasm in adult rats administered normal saline, hypertonic saline or other liquid reagents after recovery from anesthesia, indicating that liquid reagents induce no substantial damage to the brain. Moreover, the injected ethanol could also be absorbed after destroying the pyramidal tract. We also attempted to destroy the pyramidal tract using heavy metals such as mercury, but the rats died postoperatively before recovery from anesthesia. After the injection of ethanol, the drilling hole was sealed with wax, which not only effectively stopped the bleeding, but also prevented blood from entering the drilling hole and prevented normal saline from entering the needle tract during postoperative rinsing, helping to avoid dilution of the ethanol. It is very important to rigorously conduct the experiments, especially in studies on dose-effect and time-dependent relationships. Furthermore, the needle insertion sites and injection zones were positioned in reference to The Rat Brain in Stereotaxic Coordinates (3rd edition), precisely using a stereotaxic instrument, thereby ensuring targeting accuracy during injection.
Precautions during targeted injection of ethanol for establishment of the rat model of spastic cerebral palsy
We should closely observe the rat's breathing and heart beat throughout the experimental procedures, including anesthesia. A 20 mL syringe and a urinary catheter are necessary for suctioning to avoid suffocation in rats in case there are signs of airway obstruction[53]. Inaccurate three-dimensional coordinates could make the microsyringe needle deviate from the target site, thereby resulting in a failed experiment or unsatisfactory experimental results. In addition, stable skull fixation, without penetration of the bilateral tympanic membranes, is required. However, this was relatively poor in our experiment. Therefore, researchers should be careful and gentle during the operation to avoid loosening and dislocation of the skull. In particular, excessive force during the targeted injection will not only increase experimental errors, but will also damage the microsyringe, thus causing experimental failure. For the three-dimensional coordinates, the target site should not be selected 0.7 mm anterior to the line between the ears and 1 mm to the midline, so as not to damage the sinus and cause bleeding. However, we inserted the microsyringe 1 mm distal to the sagittal suture, which resulted in more bleeding during the experiment. In this experiment, we had to carry out the targeted injection of ethanol after hemostasis.
The rat model of spastic cerebral palsy prepared using the method described in this study exhibits obvious signs of limb spasms, which continue to manifest for an extended period of time. The operating procedure is simple and standardized with perfect repeatability and accurate positioning. The experimental results are described qualitatively. Further studies are required to determine the optimal ethanol dose and the dose-effect and time-effect relationships.
Characteristics and application of the targeted injection of ethanol for establishment of the rat model of spastic cerebral palsy
The animal model described in the present study shows obvious symptoms of flexion spasms and typical signs that endure for a long term. The modeling method is simple and standardized with good repeatability. Based on this model, we can carry out in-depth studies of the pathology of cerebral palsy and assess treatment strategies for the disease. For example, the model can allow us to examine the changes in the central nervous system and peripheral nerves during cerebral palsy. We can also investigate whether end-to-end anastomosis of the nerves that control the flexor and extensor muscles can alleviate the flexion spasms. Using this model, we can also investigate whether nerve growth factor treatment or partial transplantation of the pyramidal tract can accelerate the disappearance of flexion spasms when the most typical symptoms appear, allowing researchers to explore new approaches and methods for the treatment of cerebral palsy and other brain injuries. In addition, while the symptoms are maintained for an extended period in this model, they gradually disappear. Therefore, we can explore the mechanisms underlying recovery from the flexion spasms. Such studies may provide insight that will help accelerate recovery from cerebral palsy.
Although there has been significant progress in our understanding of disease pathogenesis and animal model preparation, it is necessary to identify better indicators of the disease and to generate novel animal models that better simulate spastic cerebral palsy[54]. In this study, we targeted the pyramidal tract to establish a rat model of spastic cerebral palsy. The method is rapid and easy, and produces a model with manifestations, pathological changes and symptoms typical of cerebral palsy.
MATERIALS AND METHODS
Design
A randomized, controlled, animal experiment.
Time and setting
All experiments were performed at the Experimental Animal Center, the Third Hospital of Hebei Medical University, China from September 2010 to March 2012.
Materials
A total of 12 male Sprague-Dawley rats of specific pathogen-free grade, aged 2 months and weighing 250 ± 12 g were provided by the Experimental Animal Center, the Third Hospital of Hebei Medical University, China (certificate No. SYXK (Ji) 2008-0026). The rats were housed in a feeding room, specific pathogen-free grade, at 22 ± 1°C, 50–70% humidity, with a 12-hour light/dark cycle (illumination: 150–200 lx). All experimental procedures were in accordance with the Guidance Suggestions for the Care and Use of Laboratory Animals, published by the Ministry of Science and Technology of China[55].
Methods
Animal anesthesia
The rats were anesthetized by intraperitoneal injection of 10% chloral hydrate (0.004–0.005 mL/g). Then, we closely observed the vital signs of anesthetized rats. The rats were anesthetized successfully if they exhibited the following: deeper and slower breathing, limb and muscle weakness, delayed corneal reflex, and no response to sharp stimulation of the tail[56].
Fixation of rats
First, the rats were placed in a stereotactic apparatus (Anhui Zhenghua Biologic Apparatus Facilities Co., Ltd., Huaibei, Anhui Province, China) with the root of the incisor hooked firmly with the incisor fixator. Then, the left and right ear bars were inserted into the rat ears, followed by appropriate pressure until the rats appeared to have slightly protruding eyes, and we tightened the knob of the ear bars. We adjusted the incisor to align the bregma with the lambda[57]. When the incisor bar was located at 3.9 ± 0.5 mm below the horizontal plane, the rat skull was in the horizontal position. To avoid loosening of the skull during surgery, we checked again whether each knob was tightened. After checking, the rat head should be stably and firmly fixed on the stereotactic apparatus.
Preoperative preparation
The rat skull was exposed and disinfected with iodine in alcohol and ethanol. The microsyringe (Zhenhai Glass Instrument Factory, Ningbo, Zhejiang Province, China) containing ethanol (Shanghai Nanxiang Reagent Co., Ltd., Shanghai, China) was fixed firmly in the stereotactic apparatus.
Preparing the model of spastic cerebral palsy
A parietal incision about 2-cm-long was made over the midline through the skin, subcutaneous tissue, deep fascia and periosteum, layer by layer, with the cut skin fixed on the stereotactic apparatus. After removal of the periosteum, the bregma and sagittal suture were exposed (Figure 3A). Based on The Rat Brain in Stereotaxic Coordinates (3rd edition)[57], the stereotaxic coordinates were determined: a hole, about 1 mm in diameter, was drilled 10 mm posterior to bregma, 0.8 left of sagittal suture, and 9.7 mm ventral to dura (Figure 3B). Then, we determined whether there were deviations from the stereotaxic coordinates (Figure 3C). If bleeding, cotton balls with saline were used for hemostasis by compression rather than repeated rubbing. The drilling was stopped when the bit reached the dura mater. A 5 mL syringe needle was used to determine whether the hole was drilled through the skull to avoid damage to the microsyringe needle during insertion. Then, the microsyringe needle was vertically inserted into the cranium to a depth of 9.7 mm (Figure 3D) for intracranial injection of 15 μL ethanol. After injection, the microsyringe was removed and brain cottons were used for adequate hemostasis by compression. Afterwards, the drilled hole was sealed with bone wax and the wound was rinsed with normal saline and sewed (Figure 3E). In the control group, the microsyringe was inserted into the brain without ethanol injection.
Figure 3.
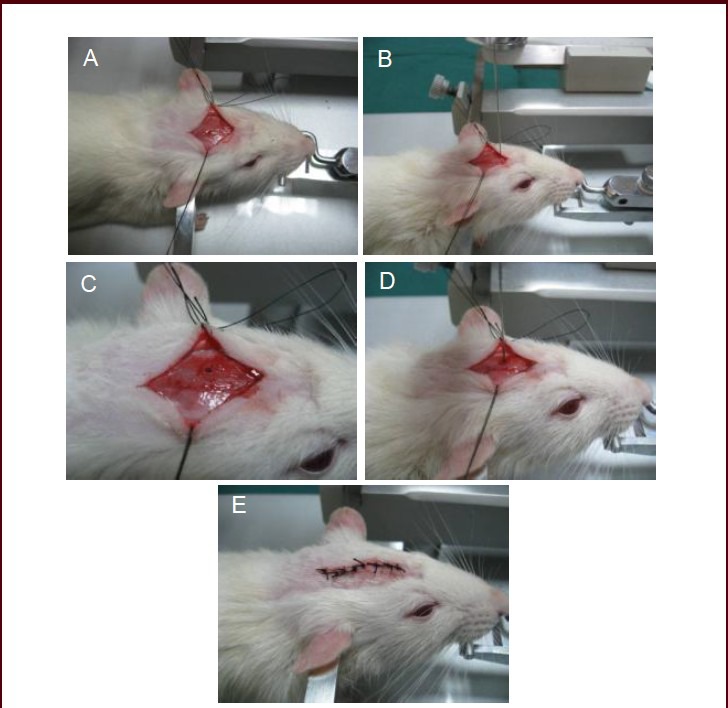
Process of preparing a rat model of spastic cerebral palsy.
(A) Expose the bregma and sagittal suture. (B) Accurate orientation. (C) Drill the parietal bone. (D) Insert the microsyringe into the skull. (E) Sew up the incision.
Postoperative treatment
The rats were removed from the stereotactic apparatus and placed in a 25–28°C environment. Before awakening, the rats were monitored for general conditions. Generally, the rats would recover from anesthesia at postoperative 2 hours.
The symptoms and signs were compared between the model and control groups within 24 hours postoperatively. Rats were only given feeding without postoperative dressing or antibiotic treatment.
Gross observation
Detailed observations were done for food intake, activity, mental state, and spasticity in rats at 18, 36, 48 and 72 hours postoperatively.
Pathological observation of the pyramidal tract using hematoxylin-eosin staining
Approximately 72 hours after surgery, one rat was randomly selected from each of the two groups. After anesthetization with 10% chloral hydrate (0.004–0.005 mL/g), the rats were placed in a sterile operating table to open the parietal incision and clear the wound and subcutaneous hematoma. The original incision was extended from the tip of the rat nose to the neck to expose the entire skull clearly. The bone wax filling the drilled hole was removed. A transverse incision was made using a wire saw from the eyebrow area through the temporal line to the external occipital protuberance, which was not too deep. Then, the skull was opened along the transverse incision to separate the bone marrow from the spinal canal at the foramen magnum using a small cutting bit. A micro scissor was used to cut off the olfactory bulb and optic nerve, and then, a knife was inserted into the sella to isolate the pituitary gland.
Finally, the brain with the pituitary gland was taken out. After removal of the dura mater, the brain was immersed in formalin for 3 days to fix the brain tissue[58]. After fixation, the brain tissue was dehydrated, cleared and immersed in paraffin. Then the tissue specimen was placed in melted paraffin wax until solidified. The paraffin block was trimmed, placed on the wooden holder and cut into slices, 5 μm in thickness. The slices were immersed in 40°C water, then baked in a 65°C oven for 30 minutes, and finally stained with hematoxylin-eosin. An optical microscope (Shanghai Bimu Instrument Co., Ltd., Shanghai, China) was used for pathological and morphological observation of the rat brain tissues.
Footnotes
Conflicts of interest: None declared.
Ethical approval: The study was approved by the Experimental Animal Center of Hebei Medical University, China and the Experimental Animal Center of the Third Hospital of Hebei Medical University, China.
Supplementary information: Supplementary data associated with this article can be found, in the online version, by visiting www.nrronline.org.
(Reviewed by Patel B, Raye W, Wei HT, Sun XC)
(Edited by Yu J, Wang L, Li CH, Song LP, Liu WJ, Zhao M)
REFERENCES
- [1].Chinese Association of Rehabilitation Medicine. The definition, typing and diagnosis of cerebral palsy in children. Zhonghua Wuli Yixue yu Kangfu Zazhi. 2007;29(5):309. [Google Scholar]
- [2].Surveillance of Cerebral Palsy in Europe. Surveillance of cerebral palsy in Europe: a collaboration of cerebral palsy surveys and registers. Surveillance of Cerebral Palsy in Europe (SCPE) Dev Med Child Neurol. 2000;42(12):816–824. doi: 10.1017/s0012162200001511. [DOI] [PubMed] [Google Scholar]
- [3].Reid SM, Carlin JB, Reddihough DS. Rates of cerebral palsy in Victoria, Australia, 1970 to 2004: has there been a change? Dev Med Child Neurol. 2011;53(10):907–912. doi: 10.1111/j.1469-8749.2011.04039.x. [DOI] [PubMed] [Google Scholar]
- [4].Liu W, Chen G, Chi GM. Current treatment of cerebral palsy (review) Zhongguo Kangfu Lilun yu Shijian. 2007;13(12):1118–1120. [Google Scholar]
- [5].Xu SD, Ge BF, Xu YK, et al. Beijing: People's Military Medical Press; 2005. Practical Orthopedics. [Google Scholar]
- [6].Wen LB, Chen G, Chi GM. Prospects for surgical treatment of cerebral palsy. Guoji Shenjing Bing Xue Shenjing Waike Xue Zazhi. 2009;36(1):48–52. [Google Scholar]
- [7].Li HM, Sun XH, Ye XX. The status of the diagnosis and treatment of children with cerebral palsy. Xiandai Yiyao Weisheng. 2007;23(16):2437. [Google Scholar]
- [8].Park KI, Hack MA, Ourednik J, et al. Acute injury directs the migration, proliferation, and differentiation of solid organ stem cells: evidence from the effect of hypoxia-ischemia in the CNS on clonal “reporter” neural stem cells. Exp Neurol. 2006;199(1):156–178. doi: 10.1016/j.expneurol.2006.04.002. [DOI] [PubMed] [Google Scholar]
- [9].Seledtsov VI, Kafanova MY, Rabinovich SS, et al. Cell therapy of cerebral palsy. Bull Exp Biol Med. 2005;139(4):499–503. doi: 10.1007/s10517-005-0330-2. [DOI] [PubMed] [Google Scholar]
- [10].Liu RW, Huang HY, Xi HT, et al. Embryo olfactory ensheathing cell transplantation for cerebral palsy: a result report of 4 cases 4 weeks after transplantation. Zhongguo Zuzhi Gongcheng Yanjiu yu Linchuang Kangfu. 2007;11(28):5645–5648. [Google Scholar]
- [11].Luan Z, Qu XQ, Liu WP, et al. Treatment of heteroptics after cerebral palsy with transplantation of human neural stem cells into cerebral ventricle in infants: 7 case report. Zhongguo Kangfu Lilun yu Shijian. 2007;13(12):1103–1105. [Google Scholar]
- [12].Wright J, Rang M. The spastic mouse. And the search for an animal model of spasticity in human beings. Clin Orthop Relat Res. 1990;253:12–19. [PubMed] [Google Scholar]
- [13].Li J, Chen G. Research of cerebral palsy animal models. Zhongguo Kangfu Yixue Zazhi. 2007;22(11):1134–1136. [Google Scholar]
- [14].Chen G, Li J, Liu W, et al. Construction and stability of hypoxic-ischemic encephalopathy models in neonatal rats. Zhongguo Zuzhi Gongcheng Yanjiu yu Linchuang Kangfu. 2008;12(37):7326–7329. [Google Scholar]
- [15].Wen LB, Liu W, Chen G, et al. Effect of hypoxic-ischemic injury to neonatal rat brain tissue. Zhongguo Linchuang Lilun yu Shijian. 2009;15(1):35–37. [Google Scholar]
- [16].Uehara H, Yoshioka H, Kawase S, et al. A new model of white matter injury in neonatal rats with bilateral carotid artery occlusion. Brain Res. 1999;837(1-2):213–220. doi: 10.1016/s0006-8993(99)01675-3. [DOI] [PubMed] [Google Scholar]
- [17].Toso L, Poggi S, Park J, et al. Inflammatory-mediated model of cerebral palsy with developmental sequelae. Am J Obstet Gynecol. 2005;193(3 Pt 2):933–941. doi: 10.1016/j.ajog.2005.05.072. [DOI] [PubMed] [Google Scholar]
- [18].Li XJ, Gao J, Sun ZR. A study on establishing a new preterm animal model with cerebral palsy and identifying the animal model. Zhongguo Kangfu Yixue Zazhi. 2004;19(12):885–889. [Google Scholar]
- [19].Aydin A, Genç K, Akhisaroglu M, et al. Erythropoietin exerts neuroprotective effect in neonatal rat model of hypoxic-ischemic brain injury. Brain Dev. 2003;25(7):494–498. doi: 10.1016/s0387-7604(03)00039-1. [DOI] [PubMed] [Google Scholar]
- [20].Li XJ, Jiang ZM, Sun YQ, et al. A study on brainstem auditory evoded potentials in animal model of cerebral palsy of rabbits induced by bilirubin. Xiandai Kangfu. 1999;3(2):164–165. [Google Scholar]
- [21].Sun YQ, Li XJ. A study on the animal model of bilirubin-induced cerebral palsy in newborn rabbits. Zhongguo Kangfu. 1999;14(2):65–67. [Google Scholar]
- [22].Mallard C, Loeliger M, Copolov D, et al. Reduced number of neurons in the hippocampus and the cerebellum in the postnatal guinea-pig following intrauterine growth-restriction. Neuroscience. 2000;100(2):327–333. doi: 10.1016/s0306-4522(00)00271-2. [DOI] [PubMed] [Google Scholar]
- [23].Zhang JD, Zhang X, Yu X. Establishment of a spastic cerebral palsy model in rats. Zhongguo Linchuang Kangfu. 2006;10(38):150–151. [Google Scholar]
- [24].Du ZM. Beijing: Science Press; 1987. Neuroanatomical Tracing Pathway. [Google Scholar]
- [25].Dixon CE, Lyeth BG, Povlishock JT, et al. A fluid percussion model of experimental brain injury in the rat. J Neurosurg. 1987;67(1):110–119. doi: 10.3171/jns.1987.67.1.0110. [DOI] [PubMed] [Google Scholar]
- [26].Wu SP, Luo YX, Xiong G. Medication of spastic cerebral palsy in rat. Zhongguo Kangfu. 1998;13(3):103–104. [Google Scholar]
- [27].Yu YZ, Deng YX, Gao YQ, et al. Study on methods of establishing spastic cerebral palsy model by electronic injury in rats and identifying animal model. Shiyong Erke Linchuang Zazhi. 2007;22(12):928–929. [Google Scholar]
- [28].Delcour M, Russier M, Xin DL, et al. Mild musculoskeletal and locomotor alterations in adult rats with white matter injury following prenatal ischemia. Int J Dev Neurosci. 2011;29(6):593–607. doi: 10.1016/j.ijdevneu.2011.02.010. [DOI] [PubMed] [Google Scholar]
- [29].Vottier G, Pham H, Pansiot J, et al. Deleterious effect of hyperoxia at birth on white matter damage in the newborn rat. Dev Neurosci. 2011;33(3-4):261–269. doi: 10.1159/000327245. [DOI] [PubMed] [Google Scholar]
- [30].Saliba E, Henrot A. Inflammatory mediators and neonatal brain damage. Biol Neonate. 2001;79(3-4):224–227. doi: 10.1159/000047096. [DOI] [PubMed] [Google Scholar]
- [31].Saadani-Makki F, Kannan S, Lu X, et al. Intrauterine administration of endotoxin leads to motor deficits in a rabbit model: a link between prenatal infection and cerebral palsy. Am J Obstet Gynecol. 2008;199(6):651.e1–7. doi: 10.1016/j.ajog.2008.06.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Girard S, Kadhim H, Beaudet N, et al. Developmental motor deficits induced by combined fetal exposure to lipopolysaccharide and early neonatal hypoxia/ischemia: a novel animal model for cerebral palsy in very premature infants. Neuroscience. 2009;158(2):673–682. doi: 10.1016/j.neuroscience.2008.10.032. [DOI] [PubMed] [Google Scholar]
- [33].Calvert JW, Yin W, Patel M, et al. Hyperbaric oxygenation prevented brain injury induced by hypoxia-ischemia in a neonatal rat model. Brain Res. 2002;951(1):1–8. doi: 10.1016/s0006-8993(02)03094-9. [DOI] [PubMed] [Google Scholar]
- [34].Poggi SH, Park J, Toso L, et al. No phenotype associated with established lipopolysaccharide model for cerebral palsy. Am J Obstet Gynecol. 2005;192(3):727–733. doi: 10.1016/j.ajog.2004.12.053. [DOI] [PubMed] [Google Scholar]
- [35].Stigger F, Felizzola AL, Kronbauer GA, et al. Effects of fetal exposure to lipopolysaccharide, perinatal anoxia and sensorimotor restriction on motor skills and musculoskeletal tissue: implications for an animal model of cerebral palsy. Exp Neurol. 2011;228(2):183–191. doi: 10.1016/j.expneurol.2011.01.001. [DOI] [PubMed] [Google Scholar]
- [36].Taniguchi H, Andreasson K. The hypoxic-ischemic encephalopathy model of perinatal ischemia. J Vis Exp. 2008;21 doi: 10.3791/955. pii: 955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Girard S, Kadhim H, Beaudet N, et al. Developmental motor deficits induced by combined fetal exposure to lipopolysaccharide and early neonatal hypoxia/ischemia: a novel animal model for cerebral palsy in very premature infants. Neuroscience. 2009;158(2):673–682. doi: 10.1016/j.neuroscience.2008.10.032. [DOI] [PubMed] [Google Scholar]
- [38].Drobyshevsky A, Derrick M, Wyrwicz AM, et al. White matter injury correlates with hypertonia in an animal model of cerebral palsy. J Cereb Blood Flow Metab. 2007;27(2):270–281. doi: 10.1038/sj.jcbfm.9600333. [DOI] [PubMed] [Google Scholar]
- [39].Basu A, Lazovic J, Krady JK, et al. Interleukin-1 and the interleukin-1 type 1 receptor are essential for the progressive neurodegeneration that ensues subsequent to a mild hypoxic/ischemic injury. J Cereb Blood Flow Metab. 2005;25(1):17–29. doi: 10.1038/sj.jcbfm.9600002. [DOI] [PubMed] [Google Scholar]
- [40].Liu XM, Zhang YL, Liu HS, et al. Effect of Xinnaoshutong capsule on IL-1β, TNF-α and neurocyte apoptosis in cerebral ischemia and reperfusion rats. Zhonghua Zhongyiyao Zazhi. 2008;23(10):870–873. [Google Scholar]
- [41].Aly H, Khashaba MT, El-Ayouty M, et al. IL-1beta, IL-6 and TNF-alpha and outcomes of neonatal hypoxic ischemic encephalopathy. Brain Dev. 2006;28(3):178–182. doi: 10.1016/j.braindev.2005.06.006. [DOI] [PubMed] [Google Scholar]
- [42].Zhang XZ, Liu XP, Liu WH, et al. The dynamic changes of cytokines and free radicals in cerebral ischemia reperfusion. Zhongguo Laonian Xue Zazhi. 2005;25(8):962–963. [Google Scholar]
- [43].Cai Z, Pan ZL, Pang Y, et al. Cytokine induction in fetal rat brains and brain injury in neonatal rats after maternal lipopolysaccharide administration. Pediatr Res. 2000;47(1):64–72. doi: 10.1203/00006450-200001000-00013. [DOI] [PubMed] [Google Scholar]
- [44].Saliba E, Henrot A. Inflammatory mediators and neonatal brain damage. Biol Neonate. 2001;79(3-4):224–227. doi: 10.1159/000047096. [DOI] [PubMed] [Google Scholar]
- [45].Derrick M, Drobyshevsky A, Ji X, et al. A model of cerebral palsy from fetal hypoxia-ischemia. Stroke. 2007;38(2 Suppl):731–735. doi: 10.1161/01.STR.0000251445.94697.64. [DOI] [PubMed] [Google Scholar]
- [46].Zhao CM, Lu LQ. Status and prospect of animal models for neonatal brain injury. Disan Junyi Daxue Xuebao. 2009;11(22):2167–2170. [Google Scholar]
- [47].Chen YZ. Beijing: People's Medical Publishing House; 1981. Electrophysiology of the Nervous System. [Google Scholar]
- [48].Spiegler M, Villapol S, Biran V, et al. Bilateral changes after neonatal ischemia in the P7 rat brain. J Neuropathol Exp Neurol. 2007;66(6):481–490. doi: 10.1097/01.jnen.0000263875.22306.3c. [DOI] [PubMed] [Google Scholar]
- [49].Chen RJ. Research progress of periventricular leukomalacia in premature. Shiyong Erke Linchuang Zazhi. 2004;19(2):83. [Google Scholar]
- [50].Gibson CL, Clowry GJ. The effect on motor cortical neuronal development of focal lesions to the sub-cortical white matter in the neonatal rat: a model for periventricular leukomalacia. Int J Dev Neurosci. 2003;21(4):171–182. doi: 10.1016/s0736-5748(03)00041-8. [DOI] [PubMed] [Google Scholar]
- [51].Robinson S, Petelenz K, Li Q, et al. Developmental changes induced by graded prenatal systemic hypoxic-ischemic insults in rats. Neurobiol Dis. 2005;18(3):568–581. doi: 10.1016/j.nbd.2004.10.024. [DOI] [PubMed] [Google Scholar]
- [52].Riddle A, Luo NL, Manese M, et al. Spatial heterogeneity in oligodendrocyte lineage maturation and not cerebral blood flow predicts fetal ovine periventricular white matter injury. J Neurosci. 2006;26(11):3045–3055. doi: 10.1523/JNEUROSCI.5200-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Wang T, Zhang L, Jiang L, et al. Neurotoxicological effects of 3-nitropropionic acid on the neonatal rat. Neurotoxicology. 2008;29(6):1023–1029. doi: 10.1016/j.neuro.2008.07.006. [DOI] [PubMed] [Google Scholar]
- [54].Yager JY, Ashwal S. Animal models of perinatal hypoxic-ischemic brain damage. Pediatr Neurol. 2009;40(3):156–167. doi: 10.1016/j.pediatrneurol.2008.10.025. [DOI] [PubMed] [Google Scholar]
- [55].The Ministry of Science and Technology of the People's Republic of China. Guidance Suggestions for the Care and Use of Laboratory Animais 2006-09-30 [Google Scholar]
- [56].Wang SJ, Sun YH, Zhao ZG, et al. Three methods of correcting the target site in the rat brain on stereotaxic coordinates. Hebei Yike Daxue Xuebao. 2001;22(2):75–77. [Google Scholar]
- [57].Zhuge QC. 3rd ed. Beijing: People's Medical Publishing House; 2005. The Rat Brain in Stereotaxic Coordinates. [Google Scholar]
- [58].Wang ZT, Hao LW, Li GS, et al. Jinan: Shandong Science & Technology Press; 2009. Anatomical Atlas of Wistar Rats. [Google Scholar]


