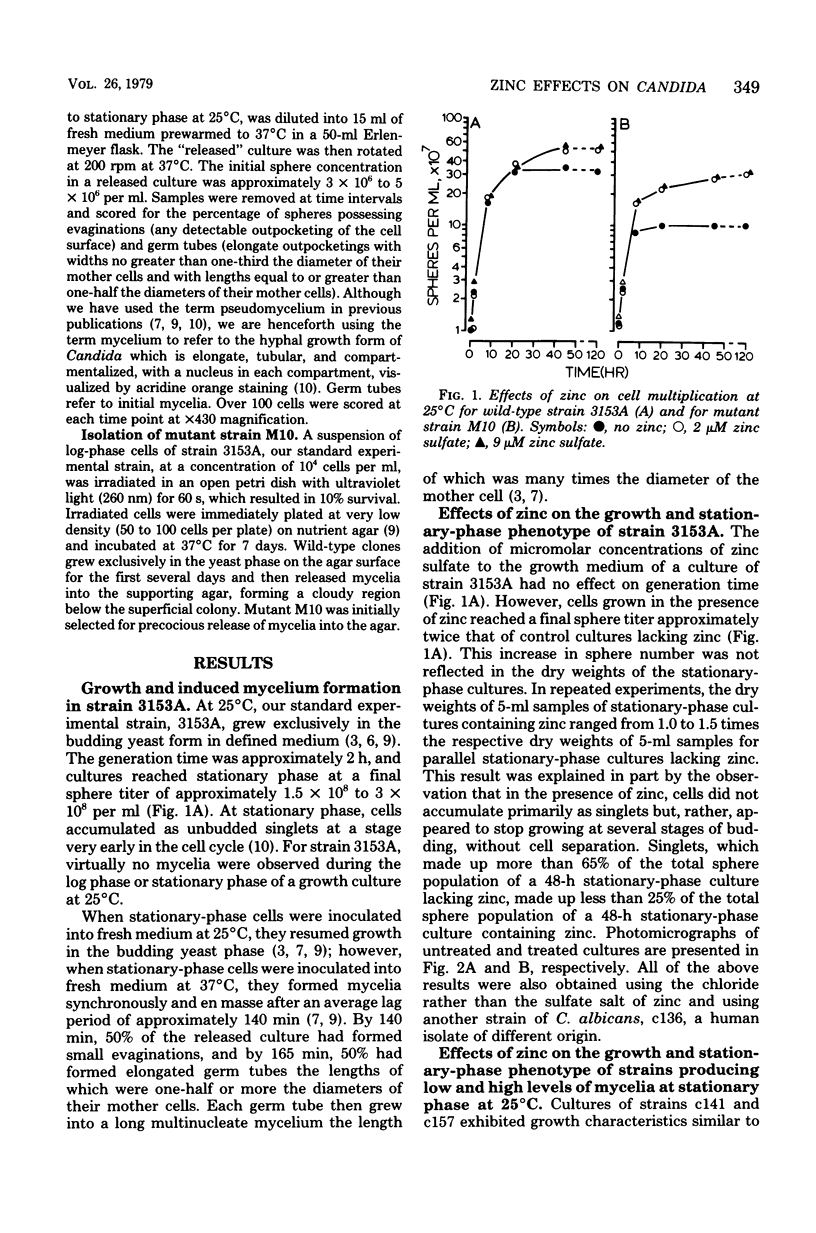
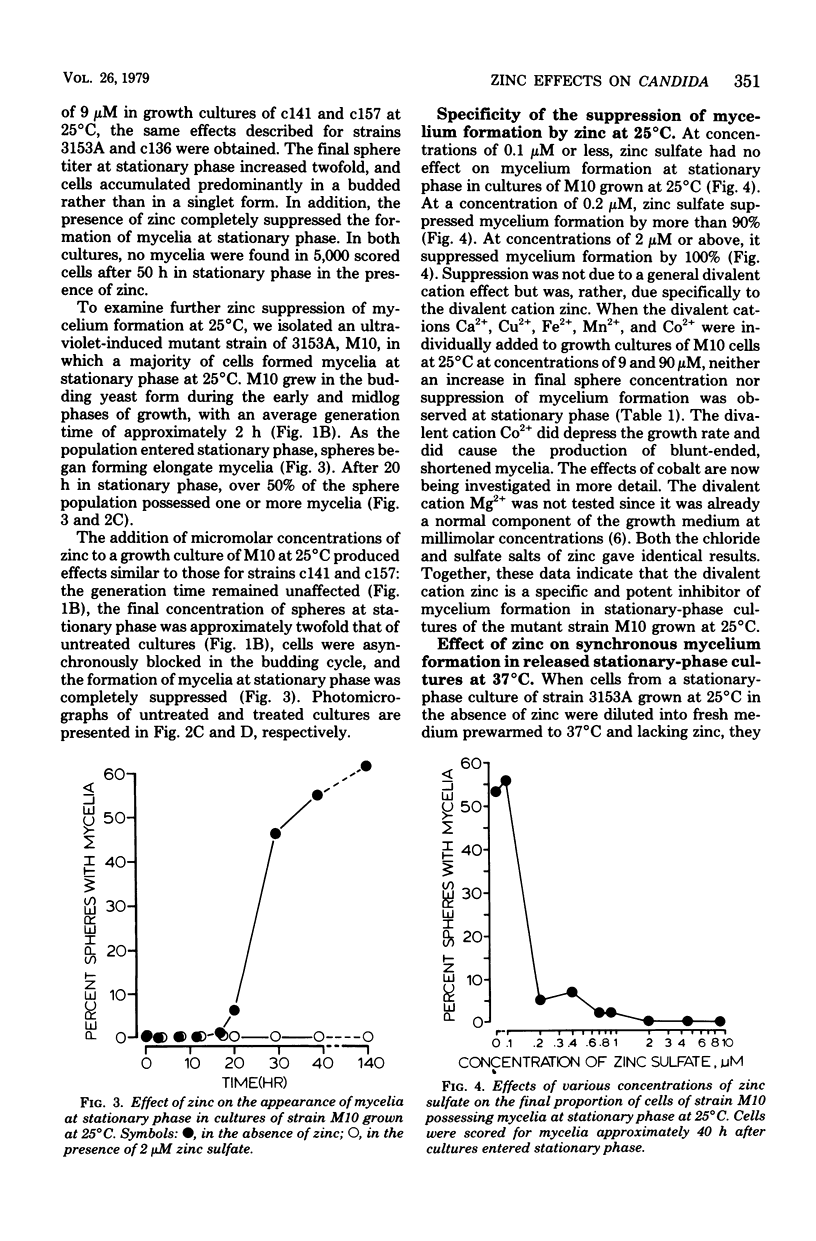
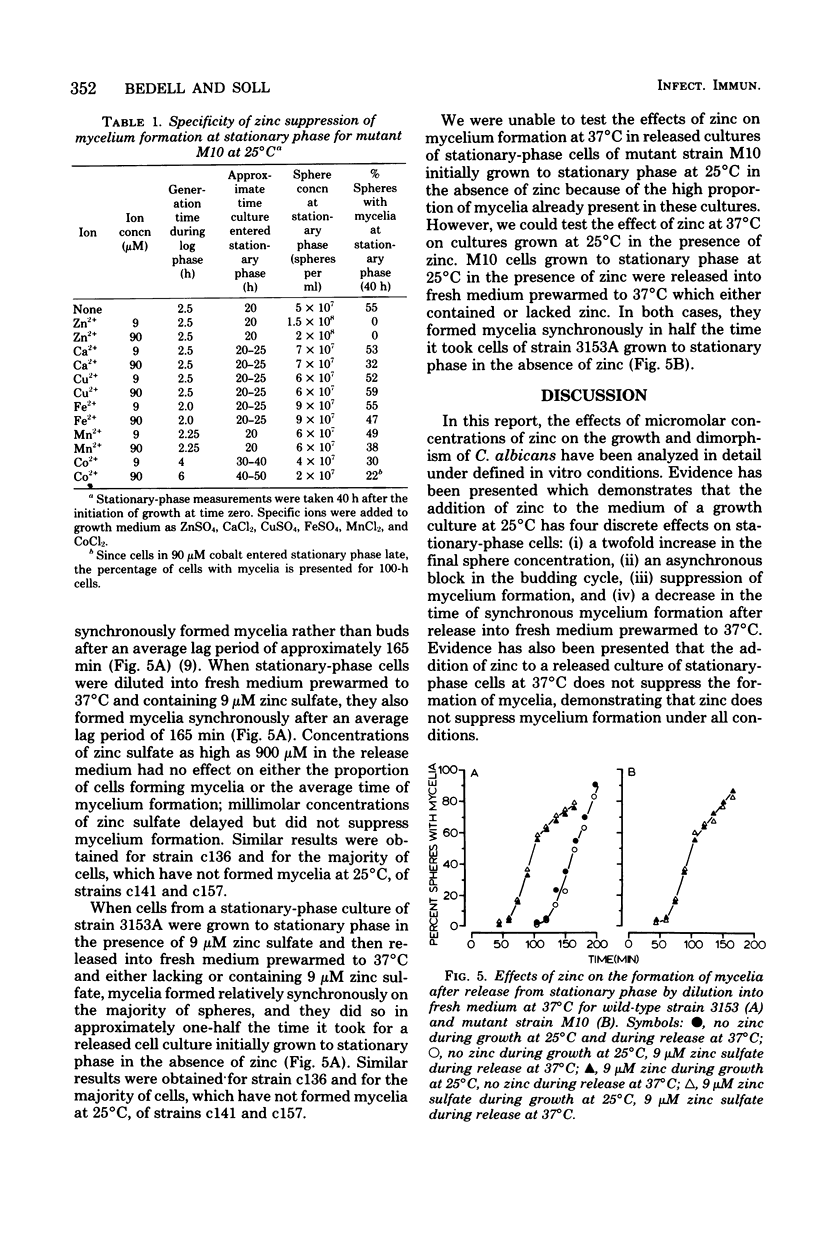
Abstract
In this analysis we have examined in detail the effects of low concentrations of zinc on the growth and dimorphism of Candida albicans. Evidence is presented that micromolar concentrations of zinc added to growth cultures grown at 25 degrees C (i) cause a twofold increase in the final concentration of spheres at sationary phase, (ii) result in an asynchronous block in the budding cycle at stationary phase, (iii) completely suppress mycelium formation in two independently isolated human strains which produce low but significant levels of mycelia at stationary phase, and (iv) completely suppress mycelium formation in cultures of mutant M10, in which over 60% of the cells form mycelia at stationary phase. In contrast, micromolar concentrations of zinc do not inhibit mycelium formation induced by releasing cells from stationary-phase cultures into fresh medium at 37 degrees C. In addition, if zinc is present in the growth medium of the initial culture at 25 degrees C, the average time of subsequent mycelium formation after release into fresh medium at 37 degrees C is halved. It is demonstrated that the above effects are specific to zinc. The possibility of alterante pathways for mycelium formation is suggested, and the medical implications of this possibility are discussed.
Full text
PDF



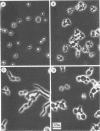
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beisel W. R. Zinc metabolism in infection. Prog Clin Biol Res. 1977;14:155–179. [PubMed] [Google Scholar]
- Campo A. G., Jr, McDonald C. J. Treatment of acrodermatitis enteropathica with zinc sulfate. Arch Dermatol. 1976 May;112(5):687–689. [PubMed] [Google Scholar]
- Chaffin W. L., Sogin S. J. Germ tube formation from zonal rotor fractions of Candida albicans. J Bacteriol. 1976 May;126(2):771–776. doi: 10.1128/jb.126.2.771-776.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwell L. H., Culotti J., Pringle J. R., Reid B. J. Genetic control of the cell division cycle in yeast. Science. 1974 Jan 11;183(4120):46–51. doi: 10.1126/science.183.4120.46. [DOI] [PubMed] [Google Scholar]
- Johnston G. C., Singer R. A. RNA synthesis and control of cell division in the yeast S. cerevisiae. Cell. 1978 Aug;14(4):951–958. doi: 10.1016/0092-8674(78)90349-5. [DOI] [PubMed] [Google Scholar]
- Lee K. L., Buckley H. R., Campbell C. C. An amino acid liquid synthetic medium for the development of mycelial and yeast forms of Candida Albicans. Sabouraudia. 1975 Jul;13(2):148–153. doi: 10.1080/00362177585190271. [DOI] [PubMed] [Google Scholar]
- Mitchell L. H., Soll D. R. Commitment to germ tube or bud formation during release from stationary phase in Candida albicans. Exp Cell Res. 1979 Apr;120(1):167–179. doi: 10.1016/0014-4827(79)90547-0. [DOI] [PubMed] [Google Scholar]
- Saltarelli C. G. Growth stimulation and inhibition of Candida albicans by metabolic by-products. Mycopathol Mycol Appl. 1973 Sep 28;51(1):53–63. doi: 10.1007/BF02141285. [DOI] [PubMed] [Google Scholar]
- Soll D. R., Stasi M., Bedell G. The regulation of nuclear migration and division during pseudo-mycelium outgrowth in the dimorphic yeast Candida albicans. Exp Cell Res. 1978 Oct 1;116(1):207–215. doi: 10.1016/0014-4827(78)90077-0. [DOI] [PubMed] [Google Scholar]
- WIDRA A. PHOSPHATE DIRECTED Y-M VARIATION IN CANDIDA ALBICANS. Mycopathol Mycol Appl. 1964 Sep 30;23:197–202. doi: 10.1007/BF02068455. [DOI] [PubMed] [Google Scholar]
- Yamaguchi H. Control of dimorphism in Candida albicans by zinc: effect on cell morphology and composition. J Gen Microbiol. 1975 Feb;86(2):370–372. doi: 10.1099/00221287-86-2-370. [DOI] [PubMed] [Google Scholar]